Solvothermal Synthesis, Crystal Structure and Luminescent Property of a New Zn(II) Coordination Polymer With a Six-fold Interpenetrating Diamondoid Net①
2015-01-07DUJie
DU Jie
Solvothermal Synthesis, Crystal Structure and Luminescent Property of a New Zn(II) Coordination Polymer With a Six-fold Interpenetrating Diamondoid Net①
DU Jie②
(550005)
Under solvothermal conditions, a new Zn(II) coordination polymer, namely [(CH3)2NH]2[Zn(bpea)(bib)]·2H2O (1, H2bpea = biphenylethene-4,4΄-dicarboxylate and bib = 1,4-bis(1-imidazolyl) benzene), has been synthesized with mixed organic ligands. Complex 1 is of monoclinic system, space group2/with= 17.4680(13),= 6.0489(5),= 18.8144(10) Å,= 1753.9(2) Å3,= 2,M= 668.05,D= 1.265 g/cm3,(000) = 700 and= 0.749 mm-1. The final refinement gave= 0.0519 and= 0.0667 for 3266 reflections with> 2(). Single-crystal X-ray analysis reveals that complex 1 features a three-dimensional structure with a six-fold interpenetrating diamondoid net. Furthermore, the solid state luminescent property of 1 was investigated at room temperature in the solid state.
Zn(II), coordination polymer, crystal structure;
1 INTRODUCTION
Assembly of coordination polymers (CPs) has attracted great attention, and CPs with various network topologies have been prepared from the metal ions and organic ligands[1-4]. They have the potential applications to gas storage, catalysis, mag- netism, luminescence and so on[5-12]. The topolo- gical matrix of diamondoid net is well-defined and realized in the field of CPs. It is of interest to build diamondoid networks with big cavities. Interpene- trating diamondoid networks with open structures including counterions and guest molecules have been demonstrated[13-16]. Generally speaking, the degree of interpenetration mainly relied on the distance of metal ions, which is strongly related to the length of organic spacers. To some extent, the bigger separation of metal ions will bring higher interpenetrating number. And yet, in some cases this verdict is void, because so many factors can influence the degree of interpenetration, such as the configuration of organic spacers, the size of coun- terions, and so on[18-20]. Thereby, based on the current state of arts, it is still very difficult to rationally design, predict, and fully interpret the interpenetrating degree of diamondoid net.
Considering the above mention, herein, we chose a long flexible dicarboxylate ligand and rigid 1,4-bis(1-imidazolyl) benzene to assemble with Zn(II) ion under solvothermal conditions. As a result, we successfully obtained [(CH3)2NH]2[Zn(bpea)(bib)]·2H2O (1) which features a six-fold diamondoid topology.
2 EXPERIMENTAL
2. 1 General materials and methods
All reagents and solvents were commercially available and used as received. C, H and N analyses were carried out with a Perkin-Elmer 240C elemen- tal analyzer. FT-IR spectra were recorded with a Bruker Equinox 55 FT-IR spectrometer (KBr pellet) from 400 to 4000 cm-1. Solid-state fluorescence spectra were recorded with a Hitachi F-4600 equipped with a xenon lamp and a quartz carrier at room temperature. The powder X-ray diffraction (XRPD) measurements were performed with a Bruker D8 diffractometer operating at 40 kV and 40 mA using Curadiation (k = 0.15418 nm).
2. 2 Synthesis of complex [(CH3)2NH]2[Zn(bpea)(bib)]·2H2O (1)
A mixture of Zn(NO3)2·6H2O (14.9 mg, 0.05 mmol), H2bpea (13.4 mg, 0.05 mmol), bib (10.9 mg, 0.05 mmol) and DMF/H2O (4 mL, 3:1) was added to a 10 mL vial which was sealed, held at 120 ℃ for 48 h, and then cooled to room temperature. Colorless crystals of 1 were obtained in 68% yield based on Zn. Anal. Calcd. for 1: C, 57.53; H, 5.73; N, 12.58%. Found: C, 57.49; H, 5.72; N, 12.56%. IR(cm-1/KBr): 3406 w, 3026 w, 2865w, 2218 w, 1638 s, 1608 m, 1533 s, 1493 m, 1259 m, 1087 m, 1024 m, 938 m, 806 m, 759 m, 654m.
2. 3 X-ray crystallography
A suitable colorless block crystal was mounted on a glass fiber and the data were collected on a Bruker APEX2 CCD area-detector diffractometer with a Mo-radiation(= 0.71073 Å) at 293(2) K by using an-scan mode in the range of 2.17<<27.51° (–22≤≤22, –7≤≤7, –22≤≤24). For complex 1, a total of 11759 reflections with 4029 unique ones (int= 0.030) were measured, of which 3266 were observed with> 2() to the final= 0.0519 and= 0.0667 (= 1/[2(F)2+ (0.0936)2+ 0.772], where= (F2+ 2F2)/3),= 1.081 and (Δ/)max= 0.000. All non-hydrogen atoms were located by successive difference Fourier syntheses and refined with anisotropic thermal parameters on2. The hydrogen atoms were located by geometrical cal- culations, and their positions and thermal parameters were fixed during the structure refinement. The highest and lowest residual peaks in the final difference Fourier map are 0.546 and-0.397 e/Å3, respectively. The structure was solved by direct methods with SHELXS 97 and Fourier techniques and refined by full-matrix least-squares techniques on2with SHELXL 97 program[19, 20]. The selected bond lengths and bond angles are listed in Table 1.
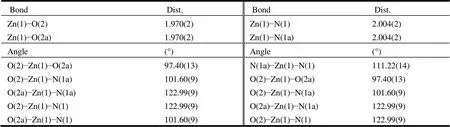
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
Symmetry code for 1: (a): −,, −+1/2
3 RESULTS AND DISCUSSION
3. 1 Structure description of [(CH3)2NH]2[Zn(bpea)(bib)]·2H2O
Single-crystal X-ray diffraction analysis shows that complex 1 crystallizes with monoclinic system, space group2/. In the structure of complex 1, the asymmetric unit consists of one crystallographically independent Zn(II) atom, one bpea2-anion, a half of bib ligand, one dimethylamine molecule and one free water molecule. As shown in Fig. 1, the Zn(II) atom adopts a tetrahedral geometry defined by two carboxylate oxygen atoms (Zn(1)–O(2) = 1.970(2) Å) and two nitrogen atoms (Zn(1)–N(1) = 2.004(2) Å). Each Zn(II) is connected to four adjacent Zn(II) atoms through two bpea2-and two bib ligands to result in a three-dimensional diamondoid framework with Schläfli symbol 66and the long symbol 62.62. 62.62.62. 62. with large adamantanoid cages, which is a typical of class Iatopology (Figs. 2 and 3). The Zn···Zn distances separated by bpea2-and bib ligands are 17.5822and 13.5599 Å, respectively. The Zn in the adamantanoid cage exhibits maximum dimen- sions (the longest intracage distances) of 36.293Å × 34.936Å × 18.814Å. Such a large cavity causes six independent equivalent cages to inter- penetrate with each other (Fig. 4). Despite the six- interpenetration and the free molecules filled in the cavity, the total void value is estimated to be 118.7 Å3, approximately 6.8% Å3of the total crystal volume of 1753.9 Å3by platon.
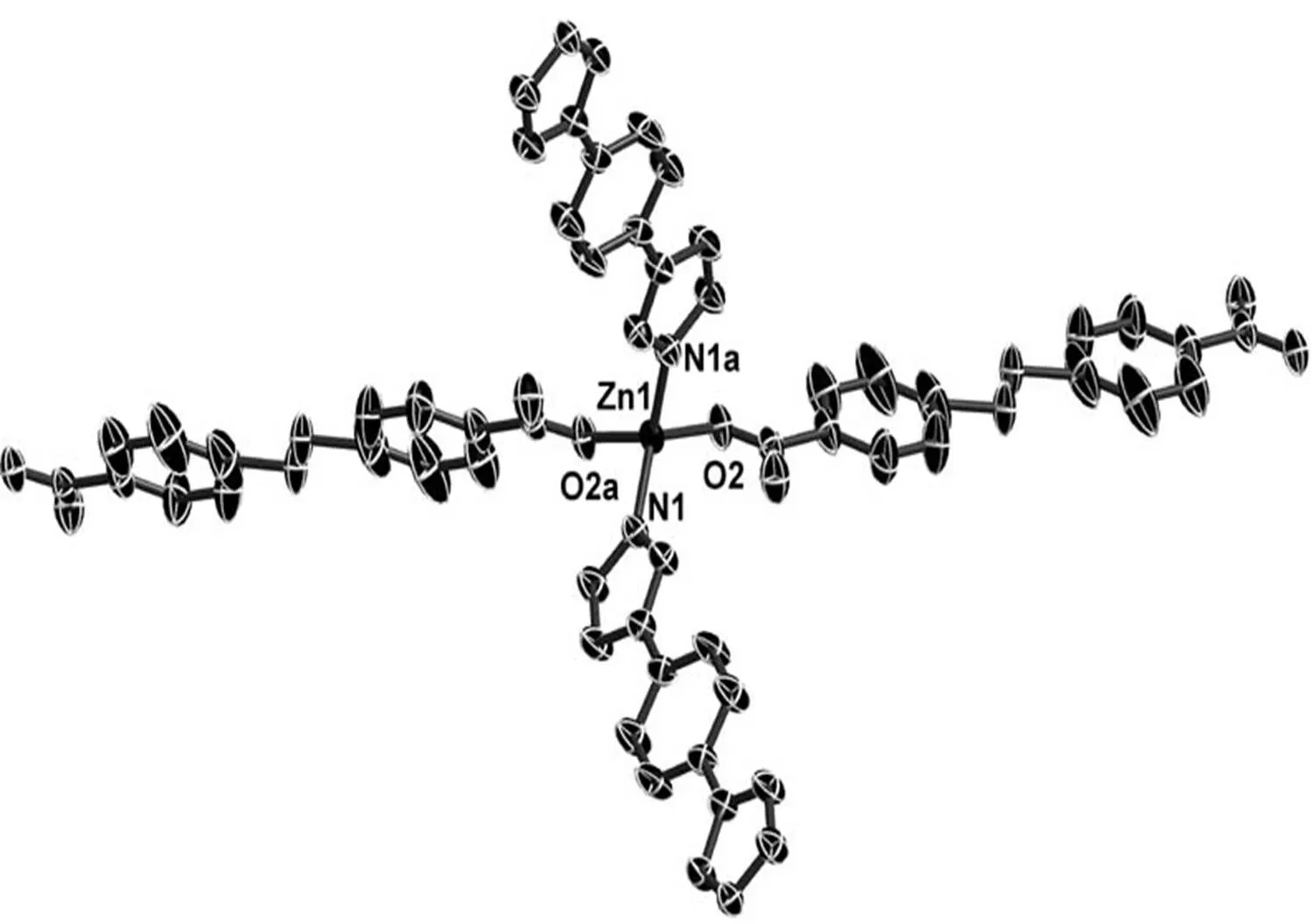
Fig. 1. A view of the local coordination of the ZnIIion, showing the atom-numbering scheme
Fig. 2. View of the three-dimensional structure of 1

Fig. 3. A singleunit cage in 1
Fig. 4. Schematic representation of six-fold interpenetratingtopology for 1
3. 2 Luminescence property
In order to substantiate the phase purity of the as-synthesized complex 1, powder XRD was per- formed before their photoluminescent property was measured (Fig. 5). The experimental powder XRD patterns are in good agreement with the corres- ponding simulated ones.
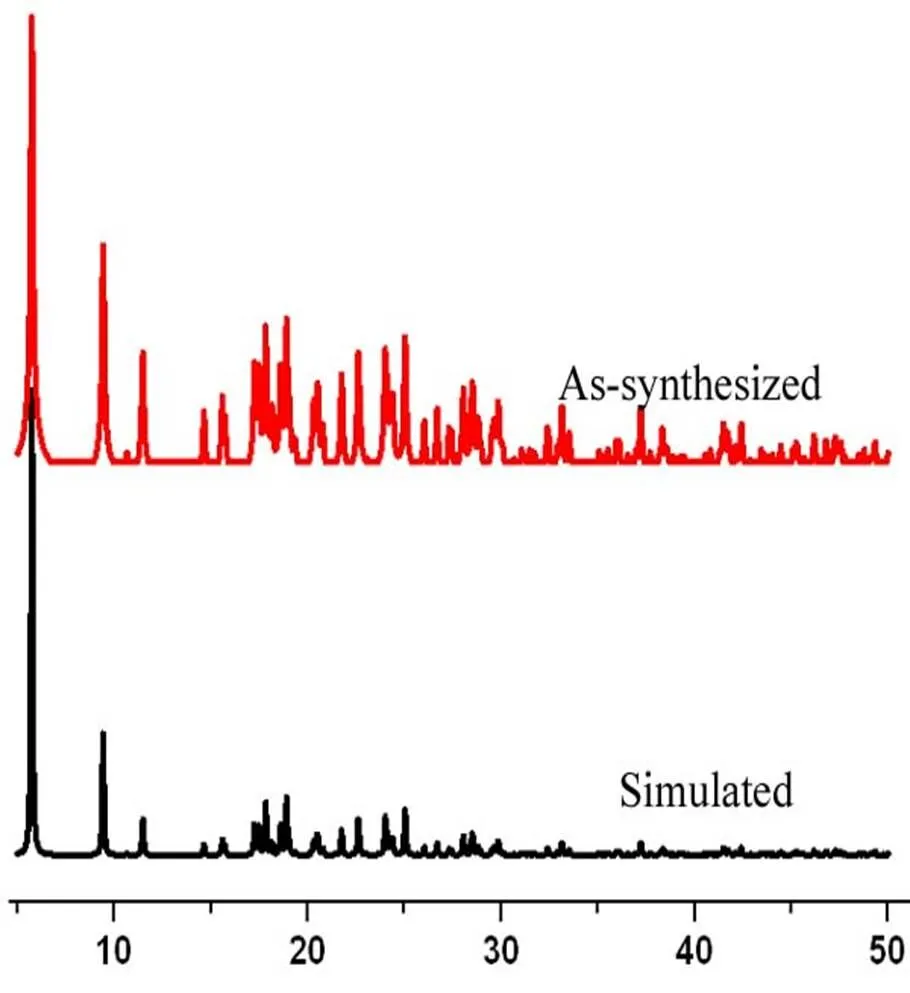
Fig. 5. PXRD for complex 1
The photoluminescent property of complex 1was investigated at room temperature. As shown in Fig.6, complex 1 shows the emission maximum at 412 nm (ex= 309 nm). The free bib displays fluorescent emission at 456 nmin the solid state at room temperature. The emission maximum of H2bpea is at 466 nm[21]. The emission bands of free ligands may be assigned to the π*-or*-n transitions[22, 23]. Compared to the emission of organic ligands, the significant blue-shit of the emission band of com- plex 1 is observed. These situations may be attri- buted to the coordination of organic ligand to the Zn(II) center, which significantly increases the rigidity and asymmetry of the ligand and reduces the loss of energy by radiationless decay.
采用叠代法剔除大于X±3S的计算方法求取20个元素平均值及标准离差,作为计算异常下限及划分地球化学色区的依据,计算公式为T=X+2S[8]。下限见表3。
4 CONCLUSION
In this paper, we obtained one new Zn(II) coor- dination polymer based on flexible dicarboxylate and imidazole-containing ligands. Complex 1 fea- tures a 4-connected six-fold interpenetrated 3Dtopology with the point symbol 66. Furthermore, complex 1 exhibits emission in the solid state at room temperature.
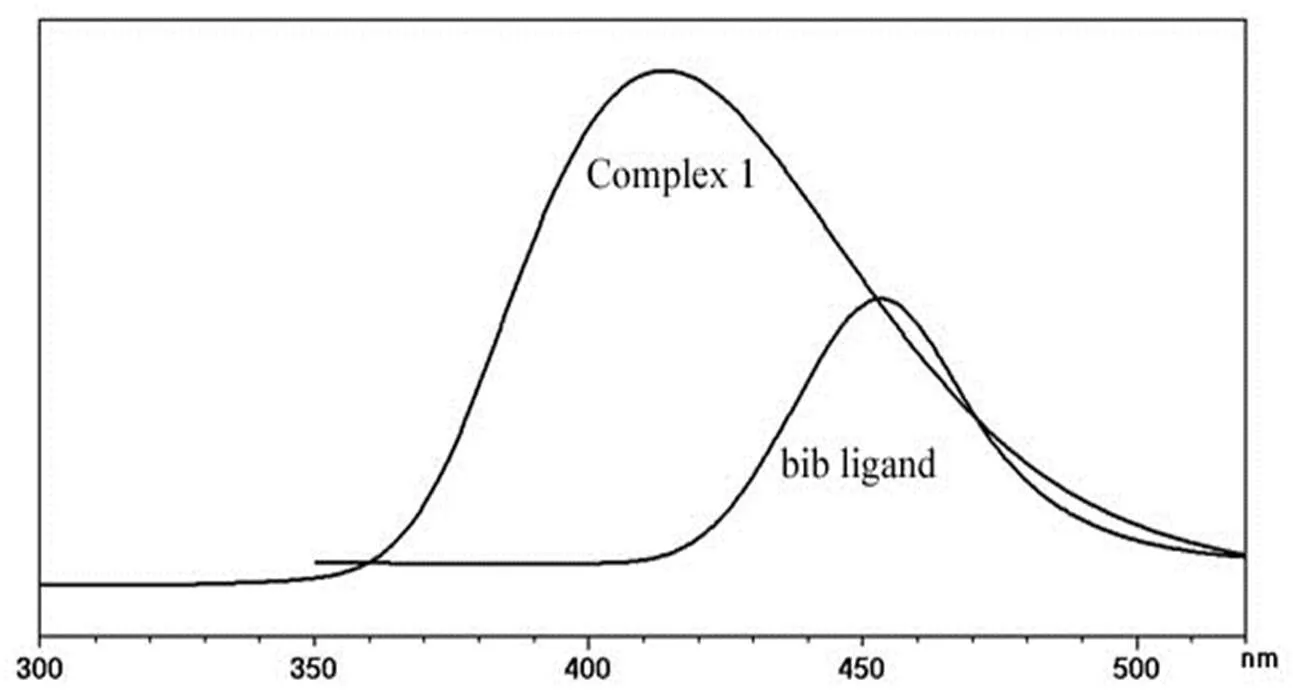
Fig. 6. Luminescent spectra for bib ligand and complex 1 in the solid state at room temperature
(1) Jiang, H. L.; Makal, T.; Zhou, H. C. Interpenetration control in metal-organic frameworks for functional applications.. 2013, 257, 2232–2249.
(2) Wei, Y. S.; Lin, R. B.; Wang, P.; Liao, P. Q.; He, C. T.; Xue, W.; Hou, L.; Zhang, W. X.; Zhang, J. P.; Chen, X. M. New porous coordination polymers based on expanded pyridyl-dicarboxylate ligands and a paddle-wheel cluster.2014, 16, 6325–6330.
(3) Mukherjee, S.; Samanta, D.; Mukherjee, P. S. New structural topologies in a series of 3metal complexes with isomeric phenylenediacetates and 1,3,5-tri(1-imidazolyl)benzene ligand: syntheses, structures, and magnetic and luminescence properties.. 2013, 13, 5335–5343.
(4) Zheng, S. T.; Wu, T.; Zuo, F.; Chou, C. T.; Feng, P.; Bu, X. Development of composite inorganic building blocks for metal-organic frameworks.. 2012, 134, 4517–4520.
(5) Wang, F.; Fu, H. R.; Kang, Y.; Zhang, J. New approach towards zeolitic tetrazolate-imidazolate frameworks (ZTIFs) with uncoordinated N-heteroatom sites for high CO2uptake.2014, 50, 12065–12068.
(7) Ma, L. F.; Wang, L. Y.; Hu, J. L.; Wang, Y. Y.; Batten, S. R.; Wang, J. G. Dicarboxylate anion-dependent assembly of Ni(II) coordination polymers with 4,4΄-dipyridyl sulfide.2009, 11, 777–783.
(8) Guo, F.; Zhu, B. Y.; Liu, M. L.; Zhang, X. L.; Zhang, J.; Zhao, J. P. Turning structural topologies of four Ni(II) coordination polymer through modifying the substitute group of organic ligand.2013, 15, 6191–6198.
(9) Kanoo, P.; Mostafa, G.; Matsuda, R.; Kitagawa, S.; Maji, T. K. A pillared bilayer porous coordination polymer with a 1D channel and a 2D interlayer space, showing unique gas and vapor sorption.2011, 47, 8106–8108.
(10) Lalonde, M. B.; Farha, O. K.; Scheidt, K. A.; Hupp, J. T. N-Heterocyclic carbene-like catalysis by a metal-organic framework (MOF) material.2012,, 1550–1554.
(11) Chen, W. T.; Liu, J. H.; Yang, S. M.; Liu, D. S.; Kuang, H. M. Hydrothermal synthesis, crystal structure and physical properties of {[Gd(H2O)4(2-C6NO2H5)2Gd(H2O)4](3-C6NO2H4)2(ZnCl3)2}·2H2O·2Cl.. 2009, 12, 811–814.
(12) Chen, W. T.; Hu, R. H.; Wang, Y. F.; Zhang, X.; Liu, J. A Tb–Zn tetra(4-sulfonatophenyl) porphyrin hybrid: preparation, structure, photophysical and electrochemical properties.. 2014, 213, 218–223.
(13) Dong, L. L.; Li, J. Q.; Liu, S. J.; Luo, M. B.; Luo, F. A new acylamide MOF showing uncommon ten-fold interpenetration.2014, 45, 30–32.
(14) Yang, E.; Ding, Q. R.; Kang, Y.; Wang, F. A photoluminescent metal-organic framework with threefold interpenenetrated diamond topological net.. 2014, 1072, 228–231.
(15) Kim, H.; Suh, M. P. Flexible eightfold interpenetrating diamondoid network generating 1D channels: selective binding with organic guests.. 2005, 44, 810–812.
(16) Mu, Y. J.; Han, G.; Ji, S. J.; Hou, H. W.; Fan, Y. T. Coordination polymers based on a flexible bis(triazole) ligand and aromatic polycarboxylate anions: syntheses, topological structures and photoluminescent properties.2011, 13, 5943–5950.
(17) Wang, F.; Tan, Y. X.; Yang, H.; Zhang, H. X.; Kang, Y.; Zhang, J. A new approach towards tetrahedral imidazolate frameworks for high and selective CO2uptake.. 2011, 47, 5828–5830.
(18) He, C. T.; Liao, P. Q.; Zhou, D. D.; Wang, B. Y.; Zhang, W. X.; Zhang, J. P.; Chen, X. M. Visualizing thedistinctly different crystal-to-crystal structural dynamism and sorption behavior of interpenetration-direction isomeric coordination networks.. 2014, 5, 4755–4762.
(19) Qin, L.; Jia, H. L.; Guo, Z. J.; Zheng, H. G. Molecular tectonics of four-connected network topologies by regulating the ratios of tetrahedral and square-planar building units..2014, 14, 6607–6612.
(20) Carlucci, L.; Ciani, G.; Proserpio, D. M.; Rizzato, S. Three novel interpenetrating diamondoid networks from self-assembly of 1,12-dodecanedinitrile with silver(I) salts..2002, 8, 1519–1526.
(21) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(22) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(23) Huang, K. L.; He, Y. T.; Huang, M. [Cd(SDC)(H2O)]: a 3D hybrid open framework with an interpenetrated (4,4) topology and double-strand helicates (SDC = 4,4΄-stilbenedicarboxylate).. 2008, 61, 2735–2742.
(24) Song, J.; Wang, B. C.; Hu, H. M.; Gou, L.; Wu, Q. R.; Yang, X. L.; Shangguan, Y. Q.; Dong, F. X.; Xue, G. L.hydrothermal syntheses, crystal structures and luminescent properties of two novel zinc(II) coordination polymers based on tetrapyridyl ligand.2011, 366,134−140.
(25) Chen, Y. Q.; Tian, Y. A zinc(II) coordination framework based on terpyridine and 5-nitroisophthalic acid mixed ligands: synthesis, crystal structure and fluorescence property.. 2014, 33, 1597–1592.
① Supported by the Joint Foundation between GZST and GZMU (LKM201305) and the Natural Science Foundation of China (No. 21363004)
. E-mail: scesgzmu@126.com
10.14102/j.cnki.0254-5861.2011-0649
23 January 2015; accepted10 March 2015 (CCDC 1042353)
猜你喜欢
杂志排行
结构化学的其它文章
- Syntheses, Structures, and Luminescent Properties of the ZnII and CdII 1-D Chain Polymers Assembled by SalicylhydroxamicAcid①
- Geometry, Electronic Properties and Magnetism of BemOn (m + n = 3, 4, 5) Clusters①
- New Dispersants for the Preparation of Y2O3 Spherical Powders by Homogeneous Precipitation at High Temperature①
- Syntheses and Crystal Structures of Three Ag(I) Complexes with Chloro-phenylacetic Acid and Nitrogen Heterocyclic Ligand①
- Synthesis and Characterization of a New 3D Pillared Bilayer Cd(II) Coordination Polymer Based on 6,6΄-Dinitro-2,2΄,4,4΄-biphenyltetracarboxylic Acid①
- Synthesis, Crystal Structure and Properties of Barium Coordination Compound Based on 1,3,5-Benzenetricarboxylic Acid Ligand①
