Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery
2014-10-18RoziMOHAMEDPhaiLeeJONGAbdKudusKAMZIAH
Rozi MOHAMED • Phai Lee JONG • Abd Kudus KAMZIAH
Introduction
Aquilaria malaccensis (family Thymelaeaceae) is a major agarwood producer in Malaysia. The high market demand for agarwood has seriously affected natural sources of A. malaccensis.The species is now listed as endangered in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES 2010). Agarwood is used as incense in religious ceremonies, perfumes in the Arab market,ingredients in Chinese medicine, and also as ornamental materials (Okudera and Ito 2009, Kakino et al. 2010). Agarwood can fetch as much as US$100,000 per kilogram for superior pure wood material or US$ 100 per kilogram for low quality (Naef 2011).
To meet the demand for sustainable agarwood production,Aquilaria trees are now being planted on a large scale and investments have been made into formulating effective artificial inoculation methods for agarwood inducement in young plantation trees. Cultivation projects are increasing and are being carried out by individuals, local communities, larger enterprises,and governments, as well as scientists and foreign businessmen.
Agarwood is a resinous wood substance that is produced by the tree as a non-specific host response to wounding, insects, or microbial invasion. The resin contains tree extractives with aromatic terpenes. The main active compounds in agarwood are sesquiterpenes and 2-(2-phenylethyl) chromone derivatives(Chen et al. 2011, Naef 2011). Aquilaria trees are considered valuable when they can produce agarwood; this attracts investors who are interested at this lucrative business (Barden et al. 2000,Pojanagaroon and Kaewrak 2005).
Traditional methods for agarwood induction used in many countries include deliberate wounding of trees with large knives and hammering nails into tree trunks. Over the years, the practice has expanded to include the use of certain chemicals and microorganisms, and the creation of modern inducement kits. Several commercial inducement techniques are available in the market today including methods known as the CA Kit and the Taiwan and Pheerapan methods (Chang et al. 2011). These approachesuse microbes to accelerate the production of agarwood in standing trees. Using chemicals such as sodium chloride as an inducing agent have also been developed but they have yielded mixed results in regard to the quantity and quality of agarwood (Chen et al. 2011).
More recently, using a chemical solution to induce agarwood in the whole tree (Agar-Wit), has been shown to yield highquality agarwood within 20 months after treatment (Zhang et al.2012). GCMS profiles of essential oils from agarwood using this technique show high percentages of major sesquiterpene compounds, such as agarospirol and eudesmol. Although time is a major factor influencing agarwood quality, other factors include the age of the tree (young vs. mature), season (wet vs. dry),geographical location, and genetic (species) (Ng et al. 1997). In this study, we examined the effect of fungi and time on agarwood induction in young polybag-grown A. malaccensis trees in the nursery.
Materials and methods
Plant materials and inoculation
Five species of fungi (F1 to F5) belonging to the Deuteromycetes and Ascomycetes groups were tested. Fungi cultures were maintained in slant bottles on potato dextrose agar (PDA, OXOID Ltd., England) and were used for preparing spore suspension at a concentration of 107spores/ml in sterile distilled water. Fouryear-old A. malaccensis trees were grown in gallon size polybags in the nursery of the Faculty of Foresty, Universiti Putra Malaysia, Serdang, Malaysia. The trees were about 5 m high with a diameter between 4 to 4.5 cm.
Trees were inoculated by making wounds in a zig-zag manner along the trunk using an electric drill with a bit size of 4.8 mm in diameter. The first wound was inflicted 20 cm above the ground and the next wounds were made above the precedent wound at 10 cm intervals until we reached 20 wounds. The wounds were drilled into three different depths: 1.5 cm, 1.0 cm and 0.7 cm following the decreasing size of the tree diameter from bottom to top. Then, fungal spores (1 ml for the 1.5 cm depth and 0.5 ml for the other two depths) were injected into the drilled wounds,using a syringe, and the wounds were sealed with parafilm. This was replicated four times in each tree and a total of four trees were treated. Each wound contained propagules of a single fungus species, and five different species were tested, for a total of 80 wounds. The trees were left in the nursery and two were harvested each time at three and six months after the fungal application.
Measurement of discoloration zone
The extent of the discoloration zone at wound sites was measured in longitudinal (L) direction with L= LU+ LL, in which LUis the extension of upper part of discoloration and LLis the lower part of discoloration (Fig.1) (Nobuchi and Siripatanadilok 2008).In addition, quantitative readings on the intensity of the discolorations were captured under a Nikon Light Microscope (Japan)and measured directly using the software (NIS Elements BR 3.0,Nikon, Japan).
Statistical analysis
A two-factorial experiment by randomized complete block design (RCBD) was conducted in this trial. The discoloration zone(L) was measured and the means were calculated. Data for discoloration zone and color intensity were analyzed using SAS (version 9.1 for windows).
Results and discussion
Wood samples were harvested after the determined time of incubation. The bark was first removed from the stem to ensure a proper observation on the discoloration length that had formed surrounding the wounds. Discoloration zones (L) were measured longitudinally using a slide caliper because vertical discoloration appeared to be larger when compared to the horizontal. We observed that the length of discoloration was longer at the lower parts (LL) compared to the upper parts (LU) (Fig.1). This is consistent with Nobuchi and Siripatanadilok (2008). A total of 80 wounds were measured from the four trees. ANOVA analysis showed there was a significant difference between the months as the value of Pr > F (0.0001) is less than 0.05. Application of mean comparisons through a Duncan Multiple Range test showed that the six-month period of incubation yielded a higher mean when compared to the three-month period.
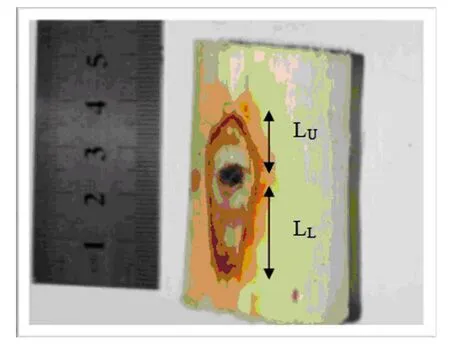
Fig.1. Naked eye measurement of the discoloration zone formed after artificial inoculation in young A. malaccensis tree. The discoloration length (L) was measured in longitudinal direction (L= LU + LL).
On average, the discoloration zone after six months’ inoculation (1.70 cm) was significantly longer than three months post inoculation (1.17 cm) (Fig.2a). To further examine the discoloration zone, the wood block was viewed under a light microscope and the image was captured. The intensity was directly read using the camera software: for example, a low intensityindicates a darkened zone. Microscopic observations revealed that wood samples with intensity values between 48 to 70 appeared to be darker than those categorized in the range of 80 to 100 (Fig.3). The mean intensity level for the 6-month sample was 48, while the 3-month sample had a significantly higher mean intensity of 90, suggesting that longer incubation time yields darker discoloration zones (Fig.2b).

Fig.2. Effects of artificial fungal inoculation on the (a) discoloration length (L), and (b) intensity value of the discoloration zones, formed in young A. malaccensis tree after three (3M) and six (6M) months incubation period. Each bar represents a single tree and a total of 80 wounds were examined from the four trees. Error bars represent standard deviations. A low intensity value indicates darkened zone.
When analyzing the effect of fungi species on the length of discoloration zones (Fig.4) and their intensities (data not shown),no significant differences were detected. It could be implied that these five species of fungi had no influence on the length and intensity of the discoloration zones. The darkened area surrounding the wounding site contains agarwood substances. This has been observed using histochemical methods where brownish droplets were captured in the parenchyma cells of agarwood chips from A. malaccensis trees that had been wounded (Wong et al. 2012). Sensory evaluation detected the unique aroma of agarwood when the discolored wood was burnt.

Fig.3 Discoloration zones observed on wood samples harvested three and six months after fungal inoculation. The darkened line(arrow) surrounding the drilled wound appeared broader and darker in 6-month-old sample compared to 3-month-old. Each picture is a representative from 40 wood samples collected at each harvest time.

Fig.4. Effects of different fungi species (F1 to F5) on the discoloration length (L) formed in young A. malaccensis tree. Bars represent means from a single fungus species. Each fungus species was applied four times per tree and two trees were harvested after three (3M) and six (6M)months of incubation. A total of 80 wounds were examined from the four trees. Error bars represent standard deviations.
Crude extracts from the 6-month old sample were subjected to GCMS analysis. The chemical profiles showed that fungiinoculated wood contained major compounds of agarwood such as benzylacetone, benzaldehyde, guaiene, palustrol, anisylacetone and chromone derivatives (Jong 2012). Our results showed that fungi inoculation was able to induce agarwood formation in young A. malaccensis trees. Similar results were obtained from artificially inoculated Aquilaria sinensis with the fungus Melanotus flavolivens when samples were harvested six months and one year post-inoculation (Lin et al. 2010). Major agarwood compounds— specifically benzaldehyde, benzenepropanoic acid,anisylacetone, and a chromone, 8-methoxy-2-(2-phenylethyl)-4H-1-benzopyran-4-one—were detected. However, less promising results for artificial fungal induction method were also reported (Tamuli et al. 2005, Bhuiyan et al. 2009).
We demonstrated that the five fungi species tested in this study produced similar effects on the length and intensity of the discoloration zones. A clear relationship was observed between the incubation time and the extent of discoloration. At the early stage of three months post-inoculation, the discoloration seemed to be weak. The intensity values revealed that the 3-month sample appeared lighter in color when compared to the 6-month sample. The amount and intensity of discoloration progressively increased over time. Our results implied that longer incubation time before harvesting, not the fungi species tested, would produce darker wood and subsequently better form of agarwood.Nevertheless, more trials should be conducted using similar or different fungi species and in a more controlled environment as environmental factors are known to directly affect agarwood induction (Ng et al. 1997).
Acknowledgements
This work was supported by the Universiti Putra Malaysia Research University Grant Scheme (Project No. 03-03-11-1438RU).
Barden A, Anak NA, Mulliken T, Song M. 2000. Heart of the matter: Agarwood use and trade and CITES implementation for Aquilaria malaccencis.Available at: www.traffic.org. [May 3, 2006].
Bhuiyan MNI, Begum J, Bhuiyan MNH. 2009. Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. Bangladesh Journal Pharmacology, 4: 24–28.
Chang YS, Nor Azah MA, Abdul Rashid AM. 2011. Inducement of gaharu and potentials of gaharu oils. In: A.M. Abdul Rashid and Y. Ahmad Zuhaidi (eds), Tapping the wealth from karas (Aquilaria malaccensis) tree. Malayan Forest Records, 50, pp. 48-62.
Chen HQ, Yang Y, Jian X, Wei JH, Zhang Z, Chen HJ. 2011. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.)Gilg trees. Molecules, 16: 4884-4896.
CITES. 2010. Appendix II of Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available at:http://www.cites.org/eng/app/appendices.php [7 August 2010].
Jong PL. 2012. Effects of mechanical wounding and infection patterns of Fusarium solani on gaharu formation in Aquilaria malaccensis Lam. Master Dissertation, Faculty of Forestry, Universiti Putra Malaysia.
Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M,Hara H. 2010. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complementary Alternative Medicine, 10: 68-75.
Lin F, Mei WL, Wu J, Dai HF. 2010. GC-MS analysis of volatile constituents from Chinese eaglewood produced by artificial methods. Journal Chinese Medicinal Materials, 33: 222-225.
Naef R. 2011. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species. Flavour Fragrance Journal, 26:73-87.
Ng LT, Chang YS, Kadir AA. 1997. A review on agar (gaharu) producing Aquilaria species. Journal of Tropical Forest Products, 2: 272-285.
Nobuchi T, Siripatanadilok SA. 2008. The formation of wood in tropical forest trees. In: T. Nobuchi and S. Mohd Hamami (eds), Cytological observations of Aquilaria crassna wood associated with the formation of aloeswood. Malaysia: UPM Press, pp. 147-160.
Okudera Y, Ito M. 2009. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnology, 26:307-315.
Pojanagaroon S, Kaewrak C. 2005. Mechanical methods to stimulate aloes wood formation in Aquilaria crassna Pierre Ex H. LEC. (Kristsana) trees.In: A. Jatisatienr, T. Paratasilpin, S. Elliott, V. Anusarnsunthorn, D. Wedge,L.E. Craker, Z.E. Gardner (eds), III WOCMAP Congress on medicinal and aromatic plants - Volume 2: Conservation, cultivation and sustainable use of medicinal and aromatic plants. ISHS Acta Horticulturae 676, Chiang Mai, Thailand, pp.161–166.
Tamuli P, Boruah P, Nath SC, Leclercq P. 2005. Essential oil of eaglewood tree: a product of pathogenesis. Journal Essential Oil Research, 17:601-604.
Wong MT, Siah CH, Faridah QZ, Mohamed R. 2012. Characterization of wound-responsive genes in Aquilaria malaccensis. Journal Plant Biochemistry Biotechnology, DOI: 10.1007/s13562-012-0144-z
Zhang Z, Yang Y, Meng H, Sui CH, Wei JH, Chen HQ. 2010. Advances in studies on mechanism of agarwood formation in Aquilaria sinensis and its hypothesis of agarwood formation induced by defense response. Chinese Traditional Herbal Drugs, 41: 156–160.
Zhang XL, Liu YY, Wei JH, Yun Y, Zhang Z, Huang JQ, Chen HQ, Liu YJ.2012. Production of high quality agarwood in Aquilaria sinensis trees via whole-tree agarwood induction technology. Chinese Chemical Letters, 23:727–730.
杂志排行
Journal of Forestry Research的其它文章
- Effects of selective logging on tree diversity and some soil characteristics in a tropical forest in southwest Ghana
- Long-term vegetation development on a wildfire slope in Innerzwain(Styria, Austria)
- Herbaceous species diversity in relation to fire severity in Zagros oak forests, Iran
- The impact of land afforestation on carbon stocks surrounding Tehran,Iran
- Spatial pattern analysis for quantification of landscape structure of Tadoba-Andhari Tiger Reserve, Central India
- Development and evaluation of local communities incentive programs for improving the traditional forest management: A case study of Northern Zagros forests, Iran
