The impact of land afforestation on carbon stocks surrounding Tehran,Iran
2014-10-18SaeidVarameshSeyyedMohsenHosseiniFarshadKeivanBehjouEbrahimFataei
Saeid Varamesh • Seyyed Mohsen Hosseini • Farshad Keivan Behjou Ebrahim Fataei
Introduction
Carbon sequestration through afforestation is one of the most appropriate methods to balance the CO2emissions (Davis and Condron 2002; Smith 1999) to prevent global warming (Watson 2000; IPCC 2001). Many studies of afforestation and its influence on ecosystem carbon stocks have been reported in recent years (Grunzweig et al. 2003; Wauters et al. 2008; Stevens and Wesemael 2008; Varamesh et al. 2010).
Expanding plant coverage through afforestation results in greater absorption of atmospheric CO2through photosynthesis; it separates its carbon and oxygen atoms, releases the oxygen back to the atmosphere and uses the carbon to produce the biomass,namely root, stem, branch, and leaf (Kerckhoffs and Reid 2007).Apart from aboveground biomass, tree roots, litter, and soil also contain measurable carbon stocks (Johnson et al. 2003; Oliver et al. 2004).
Although the effect of afforestation on carbon sequestration of biomass is clear (Arevalo et al. 2009, Redondo-Brenes and Montagnini 2006; Mendham et al. 2003), the effect of afforestation on soil carbon stocks is not significant (Jackson et al. 2002; Varamesh et al. 2010). Scott (2000) stated that the most important factors that determine changes in soil carbon due to afforestation are the soil type and its previous use. Davis and Condron (2002) also indicated that total carbon stock of soil depends on the balance between input and output of carbon.Changes in the quantity and quality of microorganisms in thedetrital layer can also affect soil carbon stock (Lemma et al.2007).
The limits of our knowledge are due to our lack of understanding of survival, biology of fine roots, microorganism's reactions, and availability of nutrients and coefficients of various echo-physiological processes such as carbon and nitrogen cycles and their below-ground component (Ceullemans et al. 1999).Selecting the species is a main decision in management that has an important effect on carbon stock in forest ecosystems (Vallet et al. 2009).
We chose to focus on two species, C. arizonica and F.Rotundifolia, that are common species in afforestation of vast areas of Iran. The purpose of this research is to estimate the carbon sequestration of the 40-year old stands of C. arizonica and F. rotundifolia that were planted on degraded lands surrounding Tehran and to determine the share of each ecosystem component in carbon sequestration. Furthermore, the most important physical and chemical factors influencing soil organic carbon will be defined and the economical values of this afforestation in carbon sequestration will be estimated.
Materials and methods
Study area
The study area is located west of Tehran with an area of 900 hectares (along the Tehran-Karaj highway) in Chitgar forest park,located between 51°10′ and 51°15′ eastern longitudes and 35°42′and 35°45′ northern latitudes (Fig.1). This park was established in 1969 to reduce air pollution, create a green belt around Tehran,clear the air, provide entertainment and recreational facilities,and prevent the undesirable expansion of the city.

Fig.1. The location of study site
The area has an arid Mediterranean climate. The altitude is 1300 m a.s.l. and the mean annual precipitation is 232 mm. The soil texture is loamy-sandy. Out of the total, 6% of the planted area is covered with C. arizonica and 10% is covered with F.rotundifolia. Due to low annual rainfall, the forest stands have been under irrigation at the rate of 5000 m3per hectare for 6 months of the year and almost every 20 days once irrigation has been done.
Sample processing
Two stands, including C. arizonica and F. rotundifolia species with 10 ha in area, were selected and the surrounding degraded lands were used as control. To decrease the boundary effects,some surrounding rows of stands were not considered for sampling. Then at each stand, nested plots were picked by systematic random sampling. At first, in 10 m ×10 m plots,several measurements were taken, including diameter at breast height (DBH), tree height (H), height to the base of the crown(Hc), and diameter of canopy or crown in two perpendicular directions, termed here for convenience “length” (L) and “width”(W). At each stand, 10 trees were randomly harvested and a 5 cm slice of wood was taken from the bottom of each 2 m bole section.
Branches were cut and their weight was measured. The branches were then cut into 5 cm pieces and 10 samples were picked randomly. In each 5 m × 5 m plot, the leaf litter layer was removed and soil samples were taken from two depths, 0-15 cm and 15−30 cm. To minimize error, the bulk sampling was done in this way so that 4 soil samples were taken from the four corners of the plot and then the samples were mixed together. At the end, all of the existing leaf litter was also collected and weighed from a 1 m ×1 m plot. Samples of each component were pooled, sealed in plastic bags, and transported to the laboratory(MacDicken 1997; Losi et al. 2003; Hernandez et al. 2004;Redondo 2007; Varamesh et al. 2011).
Calculation of biomass
To calculate tree biomass and to compute trunk, canopy and root volume followed steps were done according to prescription(Hernandez et al. 2004). First, the basal area of tree was computed using Eq. 1, and second, the tree volume was gained using Eq. 2. And third, the biomass of trunk (kilogram) was computed according to equation 3.

where: π= 3.1415927; and r is the radius of the tree at breast height (0.5 DBH).

where: Ab is the basal area; H is the height; and Kc is a sitedependent constant in standard cubing practice used in forest inventory (0.5463).

where, V= volume of the trunk, WD= wood density .
100% inventory of tree roots need time consuming, and is expensive, and degradative work, so the root volume was calculated using equation number 4 (Hernandez et al. 2004),

where, BGB = Belowground biomass; AGB = Aboveground biomass.
The canopy volume of C. arizonica with equation number 5 and the canopy volume of F. rotundifolia with equation number 6 were computed.
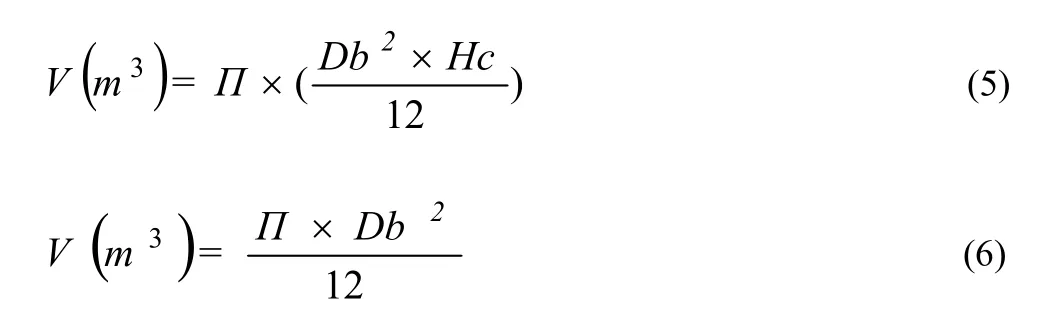
where, π = 3.141592; Db = diameter of the crown canopy (to calculate Db, the average of the field measurements L and W is taken and used as the diameter of the crown: Db = (L + W)/2);Hc = height from the ground to the highest point of the crown.
Laboratory methods
The trunk, branch, root, and litter samples were dried within 24hours at 105°C and then the percentage of organic carbon was measured by burning it in an electrical oven (MacDicken 1997;Birdsey et al. 2000; Losi et al. 2003). Density of the root, trunk,and branch samples was calculated using the dry weight density.Soil samples were dried in open air and broken into pieces.After separating the roots, stones, and other gross materials, the sampleswere ground and sieved through a 2mm sieve (mesh 20).The percentage of stones in the soil samples was calculated.
The soil texture was determined by the Bouyoucos hydrometer method (Bouyoucos 1962). The water content at saturation level(%) was also measured. Soil pH was measured using a pH meter(Orion Analyzer Model 901) in a suspension with a soil:water ratio of 1:2.5.
Total nitrogen was measured using a semi- Micro-Kjeldhal technique (Bremner and Mulvaney 1982). The bulk density was determined volumetrically (g/cm³) by using the cold method(Blake and Hartge 1986). To measure the organic matter and organic carbon, cold method based on organic carbon oxidation with potassium bichromate in a completely acidic environment(H2SO4) according to the following equation was done (Allison 1975):

Statistical analysis
First, normality was determined with through a Kolmogrov-Smirnow test and the homogeny of variances were investigated using the Levene test. For general comparison of the stands from the view point of soil characteristics, a one-way ANOVA test was conducted. To compare the means, aTukey test was applied.To define the most important soil factor that influences the soil organic carbon amount, stepwise regression was used. To compute the allometric equations of tree species, two variant and multi-variant regressions were used and appropriate equations with accepTablestatistical indicators were introduced.
Results
The results showed that the C. arizonica stand had 328.2 Mg·ha-1carbon sequestered over the 40-year period, with 56.5% of this amount stored in the trunk, 14. 6% in branches, 9.46% in roots,18.3% in soil, and 1.21% in the litter of this stand.
The F. rotundifolia stand sequestered 150.69 Mg·ha-1carbon over 40 years of which 50.1% is stored in trunk, 7.6% in branches, 8.2% in roots, 32.15% in soil and 1.1% in the litter of this stand. it had no plant cover and only 10.80 Mg ha-1carbon stored in the soil (Figs. 2 and 3).

Fig.2. Total carbon sequestration under various stands. C = C. arizonica;F = F. rotundifolia, B= degraded land
The stock of soil organic carbon at the two depths, 0−15 cm and 15−30 cm in the two stands showed that percentage of organic carbon at depth 0−15 cm of C. arizonica is higher (Fig.4).Results of regression analysis of organic carbon versus other tested soil properties showed that the ratio of carbon to nitrogen(C/N) and nitrogen content were respectively the most important factors affecting soil organic carbon. The other investigated characteristics had no significant effect on soil organic carbon(Table1).

Fig.4. Soil organic carbon (SOC) contents in tow depth under various vegetation types. C = C. arizonica; F = F. rotundifolia, B= degraded land
The results of regression analysis indicated that there is a linear relationship between soil organic carbon (dependent variable) and soil characters (Y= 0.34+2.3X2×10-2X1, R2=50.8).where: Y: the amount of Soil Organic Carbon, X1: C/N, X2:Nitrogen.
Investigation of some quantitative characteristics of the two planted stands also indicated that almost all of them showed significant difference (p <0.01). The values of DBH, basal area,total height, trunk volume, canopy volume, root volume,branches biomass, trunk biomass, and root biomass for the C.arizonica stand were higher than for the F. rotundifolia stand(Fig.5). The densities of the two stands were respectively 916 and 700 trees per hectare.
Comparison of carbon sequestration content in the two stands showed that the amount of carbon sequestered in soil, leaf litter,trunk, roots and branches of the two stands were significantly different (p<0.01); in the C. arizonica stand, the values were higher than in the F. rotundifolia stand (Fig.6).

Fig.5. General growth and productivity statistics of C. arizonica and F. rotundifolia stands at Chitgar forest park of Tehran, Iran

Fig.6. Carbon sequestration in above and belowground biomass of C.arizonica and F. rotundifolia stands
Related allometric equation to biomass
The most suiTableequations that were presented for C. arizonica and F. rotundifolia in the study area are shown in Tables 1 and 2.

Table1. Allometric equations for C. arizonica biomass

Table2. Allometric equations for F. rotundifolia biomass
Discussion
This study proved that reclamation of degraded lands with C.arizonica and F. rotundifolia species plantation has a high potential for carbon sequestration. Jackson et al (2002) and House et al (2002) also have underscored the importance of recultivation of degraded lands as an appropriate way to reduce the accumulation of atmospheric carbon. The more content of carbon sequestration in C. arizonica stand than F. rotundifolia can be attributed to more stock of this stand in area unit (Hoover et al.2000).
Distribution of total carbon sequestration also showed that carbon stock in aboveground biomass is more than ground biomass, which conforms to the results in Aradottir et al. (2000).In addition, the share of biomass in the carbon stock of trees changes over the forest stands‘ lifespan (Satoo and Madgwick 1982).
Honda et al. (2000) also state that almost all methods to estimate carbon sequestration are based on biomass inventory.As to the total carbon stock of different forest stands, there is a direct relationship between stored carbon and species, growth,site fertility, silvicultural and management activities (Redondo 2007). The higher volumes of carbon sequestration in the leaf litter in C. arizonica stand than F. rotundifolia may be due to the lower rate of decomposition in this stand (IPCC 2000). Leaf litter production depends on important factors such as species, climate,and growth site fertility and production ability in such a manner that various values are reported for different species in different growth conditions.
Furthermore because of lower production in the F.rotundifolia stand, the content of leaf litter in this stand was also lower than in the C. arizonica stand. Litter production and root decomposition, especially fine roots, are important processes that influence the soil carbon stock (Steele et al 1997). In this research, the low litter production and root volume in the F.rotundifolia stand can be counted as an accepTablereason for lower carbon sequestration in the F. rotundifolia stand than C.arizonica stand. Besides soil carbon sequestration has a great importance to reduce the climate changes (Rossi et al 2009).Dinakaran and Krishnayya (2008) stated that the type of plant coverage has significant effect on soil organic carbon, the prime example being soils with dense tree coverage that show high levels of organic carbon.
The results of our study showed that the effect of nitrogen on soil organic carbon(Alard et al. 2007). The soil texture and abundance of microlithic particles of soil are important characteristics of soil carbon changes in the of mentioned areas(Qing-Biao et al. 2009). The soil organic carbon changes are influenced by important factors like climate, plant coverage, and soil texture (Peng et al. 2004). The amount of carbon stock in various forest stands depends on species and the rate of crop.Commonly, the amount of carbon sequestered quickly increases with crop growth but, in the long term, the amount of carbon sequestered can not be highly dependent upon growth (Our results show that tree roots were one of the most important components of carbon sequestration in forest stands (Laclau 2003): this amount in the C. arizonica stand was greater than F.rotundifolia stand. Vedrova (2005) also reported that the root carbon stock in softwoods is greater than in hardwoods.The quantification of carbon stock information is very useful to define the value of important environmental services (Sandra 2000).
The results showed that about 54 ha (6%) of the total study area is covered with C. arizonica and 90 ha (10%) is covered with F. rotundifolia stand. Also, forest plantations with these two species demonstrated 317.4 and 140 t carbon sequestration in comparison with degraded land, respectively. Mentioned planted stands have respectively increased the carbon sequestration 17139.6 and 12600 tonnes in comparison with surrounding degraded land.
Considering that 27% of CO2weight is carbon, a tonne of sequestrated carbon equals 3.7 t of atmospheric CO2. Therefore the mentioned stands have respectively sequestered some 63416.52 and 46620 t of CO2.
Industrial air purification like filtering would demand highcosts (Cannell 2003). Cannell et al (1995) has reported this amount about $200–300 for each tonne of carbon in America.Luciuk et al. (2000) also estimated the value of carbon sequestration per tonne at about $348−790, counting the landrent expenses.
If we consider US $50 as the reasonable amount (Pablo et al.(2003) per tonne of carbon in the studied plantations, the economic value of carbon sequestration with C. arizonica and F.rotundifolia will be 875 and 625 thousand dollars, respectively.
The results of such plantings may differ as Schuman et al.(2002) explained due to climate, topography, soil characteristics,plant community composition, and various management activities. Therefore in order to increase the carbon sequestration the applicable alternatives of ecosystem management should be confirmed on three aspects of soil, biomass and litter.
Given that making changes to soil and leaf litter is not practical, the only direct change that is feasible is biomass management. For this reason, in many carbon sequestration projects, proper ecological management activities have been implemented to increase biomass production and to prevent land degradation.
Conclusion
In Iran, the carbon sequestration potential of forestry plantations has been an important issue during recent years. The results ofthis study have once again demonstrated that carbon stocks potential differs with plant species, location and management methods. The identification of the species with higher capability for carbon sequestration and implementation of the management activities that affect the sequestration process can assist with formulating land reclamation strategies. These considerations can potentially make the reclamation and re-cultivation of degraded lands an economically viable solution to mitigation of land degradation and climate change and finally provide an opportunity to achieve sustainable forestry.
Acknowledgement
It is with immense gratitude that we acknowledge the financial support and help of the Tarbiat Modares University.
Allard V, Soussana JF, Falcimagne R, Berbigier P, Bonnefond JM, Ceschia E,D’hou P, Henault C, Laville P, Martin C, Pinare`s-Patino C. 2007. The role of grazing management for The net biome productivity and Greenhouse gas Budget (CO2, N2O and CH4) of semi-natural Grassland. Agriculture,Ecosystems and Environment, 121: 47−58.
Allison LE. 1965. Organic carbon. In: Black, C. A., Evans, D. D., White, J. L.,Ensminger, L. E., Clark, F. E. (Eds.), Methods of Soil Analysis, Part 2,Chemical and Microbiological Properties. Madison: American Society of Agronomy, p. 1367.
Aradottir A, Ssavarsdottir L, Kristian H, Jonsson P, Gudbergson G. 2000.Carbon accumulation in vegetation and solids by reclamation of degraded areas. Icelandic Agricultural Sciences, 13:99−113.
Arevalo CBM, Bhatti JS, Chang SX, Sidders D. 2009. Ecosystem carbon stocks and distribution under different land-uses in north central Alberta,Canada. Forest Ecology and Management, 257(8): 345–357.
Birdsey R, Heath I, Williams D. 2000. Estimation of Carbon Budget Model of the United State Forest Sector. In: Advances in Terrestrial Ecosystem Carbon Inventory, Measurements and Monitoring Conference in Raleigh,North Carolina, October 3-5, 2000, 51-59.
Blake GR, Hartge KH. 1986. Bulk density. In: Klute, A. (Ed.), Methods of Soil Analysis. Part I. Physical and Mineralogical Methods. Soil Sci Soc Am Pub, 9: 1363–376.
Bouyoucos GJ. 1962. Hydrometer method improved for making particle size analysis of soils. Argon J, 56: 464–465.
Bremner JM, Mulvaney CS. 1982. Nitrogen-total. In: Page, A.L., Miller, R.H.,Keeney, R.R. (Eds.), Methods of Soil Analysis, Part 2. Second ed. Madison,WI: American Society of Agronomy, pp.595–624.
Cannell R, Dewar RC. 1995. The carbon sink provided by plantation forests and their products in Britain. Forestry, 68(1): 35–48
Cannell R. 2003. Carbon sequestration and biomass energy offset: theoretical,potential and Achievable capacities globally, in Europe and UK. Biomass and Bioenergy, 24: 97–116.
Ceulemans R, Janssens IA, Jach ME. 1999. Effects of CO2 Enrichment on Trees and Forests: Lessons to be learned in View of Future Ecosystem Studies. Annals of Botany, 84: 577–590.
Davis MR, Condron LM. 2002. Impact of grassland afforestation on soil carbon in New Zealand: a Review of paired-site studies. Australian Journal of Soil Research, 40: 675–690.
Dewar RC, Cannell MGR. 1992.Carbon sequestration in the trees, soil and wood products of Managed forests. Trees physiology, 8: 239–258.
Dinakaran J, Krishnayya NSR. 2008. Variations in type of vegetal cover and heterogeneity of soil Organic carbon in affecting sink capacity of tropical soils. Current Science, 94(9): 1144–1150.
Grunzweig JM, Lin T, Rotenberg E, Schwartz A,Yakir D. 2003. Carbon Sequestration in Arid-land Forest. Global Change Biology, 9: 791–799.
Hernandez R, Koohafkan P, Antoine J. 2004. Assessing Carbon Stocks and modeling win-win Scenarios of carbon sequestration through land-use changes. Food and Ariculure Organization, p.166.
Honda Y, Yamamoto H, Kajiwara K. 2000. Biomass Information in Central Asia. Chiba University: Center for Environmental Remote Sensing, p.1–33
Hoover GM, Birdsey RA, Heat LS, Stout SL. 2000. How to estimate Carbon sequestration on small Forest Tracts. Journal of Forestry, 98: 13–19.
House JI, Colin Prentice C, Le Quere C. 2002. Maximum impacts of future reforestation or deforestation On atmospheric CO2. Global Change Biology,8: 1047–1052.
Hu YL, Zeng DH, Fan ZP, Chen GS, Zhao Q, Pepper D. 2008. Changes in ecosystem carbon stocks following grassland afforestation of semiarid sandy soil in the southeastern Keerqin Sandy Lands, China. Journal of Arid Environments. 21:72–81.
IPCC. 2000. Land Use, Land Use Change and Forestry. A Special Report,Inter-Governmental Panel on Climate Change. Cambridge, UK: Cambridge University Press, pp.127–180.
IPCC. 2001. Climate Change 2001: The Scientific Basis. Cambridge, UK:Cambridge University Press. p.881.
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH. 2002.Ecosystem carbon loss With woody plant invasion of grasslands. Nature,418: 22–26.
Johnson DW, Todd J, DE, Tolbert VR. 2003. Changes in ecosystem carbon and nitrogen in a Loblolly pine plantation over the first 18 years. Soil Sci Soc Am J, 67: 1594–1601.
Kerckhoffs LHJ, Reid JB. 2007. Carbon sequestration in the standing biomass of orchard crops in New Zealand. Report prepared for Horticulture New Zealand Ltd. New Zealand Institute for Crop & Food Research Ltd, RD2,Hastings, New Zealand.
Laclau P. 2003. Biomass and Carbon Sequestration of Ponderosa Pine Plantations and Native Cypress forests in Northwest Patagonia. Forest Ecology and Management, 180(1-3): 317–333.
Lal R. 2005. Forest soils and carbon sequestration. Forest Ecology and Management, 220: 242–258.
Lemma B, Kleja DB, Olsson M, Nilsson I. 2007. Factors controlling soil organic carbon sequestration under exotic tree plantations: A case study using the CO2Fix model in southwestern Ethiopia. Forest Ecology and Management, 252: 124–131.
Losi CJ, Siccama TG, Juan RC, Morales E. 2003. Analysis of alternative methods for Estimating carbon stock in young tropical plantations. Forest Ecology and Management, 184(1-3): 355–368.
Luciuk GM, Bonneau MA, Boyle DM, Vibery E. 2000. Prairie Farm Rehabilitation Administration. Paper, Carbon Sequestration-Additional Environmental Benefits of Forests in the PFRA, p.33.
MacDicken KG. 1997. A Guide to Monitoring Carbon Storage in Forestry and Agro forestry Projects. Winrock International Institute for Agricultural Development, Forest Carbon Monitoring Program, p.91.
Mendham DS, O'Connell AM, Grove TS. 2003. Change in soil carbon after land clearing or afforestation in highly weathered lateritic and sandy soil ofSouth-Western Australia. Agriculture, Ecosystems and Environment, 95(1):143–156.
Oliver GR, Beets PN, Garrett LG, Pearce SH, Kimberly MO, Ford-Robertson JB, Robertson KA. 2004. Variation in soil carbon in pine plantations and implications for monitoring soil carbon stocks in relation to land-use change and forest site management in New Zealand. Forest Ecology and Management, 203(1-3): 283–295.
Pablo C. Benítez, Ian McCallum, Michael Obersteiner, Yoshiki Yamagata,2003. Global potential for carbon sequestration: Geographical distribution,country risk and policy implications. Ecological Economics, 60: 572–583.
Peng XH, Zhang B, Zhao QG. 2004. A review on relationship between soil organic carbon pools and soil structure Stability. Acta Pedol Sin, (In Chinese), 41: 618–623.
Redondo-Brenes A. 2007. Growth, carbon sequestration and management of native tree plantation in humid regions of Costa Rica. New Forests, 34:253–268.
Redondo-Brenes A, Mantagnini F. 2006. Growth, productivity, aboveground biomass and carbon sequestration of pure and mixed native tree plantation in the Caribbean lowlands of Costa Rica. Forest Ecology and Management,232(1-3): 168–178.
Rossi J, Govaerts A, De Vos B, Verbist B, Vervoort A, Poesen J, Muys B,Deckers J. 2009. Spatial structures of soil organic carbon in tropical forests—a case study of Southeastern Tanzania. Catena, 77: 19–27.
Sandra B. 2000. Forest carbon monitoring Program. Winrock International,U.S.A. (www. winrock.org), p. 91.
Satoo T, Madgwick HAI. 1982. Biomass. In: H. A. I. Madgwick (eds), Forest Biomass. Forestry Sciences, 6: pp 46-89
Scott N, Kelvin A, Tate R, Gilt rap D, Wilde HR, Davis M. 2000. Land-cover effects on soil Carbon storage in New Zealand: A national monitoring system. In: Advances in Terrestrial Ecosystem Carbon Inventory,Measurements, and Monitoring Conference in Raleigh, North Carolina,October 3-5, 2000.
Schuman GE, Janzen H, Herrick JE. 2002. Soil carbon information and potential carbon sequestration by rangelands. Environmental Pollution, 116:391–396.
Smith KA. 1999. After the Kyoto protocol: can soil scientists make a useful contribution?. Soil Use and Management, 15: 71–75.
Steele SJ, Gower ST, Vogel JG, Norman JM. 1997. Root mass, net primary production and Turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiology, 17(8-9): 577–587.
Stevens A, Wesemael Bv. 2008. Soil organic carbon stock in the Belgian Ardennes as affected by afforestation and deforestation from 1868 to 2005.Forest Ecology and Management, 256(8): 1527–1539.
Vallet P, Meredieu C, Seynave I, T Be louard I, Dhote JF. 2009. Species substitution for carbon storage: Sessile oak versus Corsican pine in France as a case study. Forest Ecology and Management, 257: 1314–1323.
Varamesh S, Hosseini SM, Abdi N, Akbarinia M. 2010. Effects of afforestation on soil carbon sequestration in an urban forest of arid zone in Chitgar forest park of Tehran. Nauka za Gorata, 47(3): 75–90.
Varamesh S, Hosseini SM, Abdi N. 2011. Estimating Potential of Urban Forests for Atmospheric Carbon Sequestration. Journal of Environmental Studies, 37: 113–120.
Vedrova EF. 2005. Biochemistry of carbon and nitrogen in the Siberian afforestation experiment. In: Binkley, D., Menyailo, O. (eds.), Tree Species Effects on Soils: Implications for Global Change. Dordrecht: Kluwer Academic, pp. 281–292.
Walker SM, Desanker PV. 2004. The impact of land use on soil carbon in Miombo Woodlands of Malawi. Forest Ecology and Management, 203(1-3): 345–360.
Watson RT. 2000. Land Use, Land-Use Change, and Forestry: A Special Report of the IPCC. Cambridge: Cambridge University Press, p. 377.
Wauters JB, Coudert S, Grallien E, Jonard M, Ponette Q. 2008. Carbon stock in rubber tree plantations in Western Ghana and Mato Grosso (Brazil).Forest Ecology and Management, 255: 2347–2361.
WU QB, Wang XK, Oyang ZY. 2009. Soil organic carbon and its fractions across vegetation types: effects of soil mineral surface area and micro aggregates. Pedosphere, 19(2): 258–264.
杂志排行
Journal of Forestry Research的其它文章
- Effects of harvesting intensities and techniques on re-growth dynamics and quality of Terminalia bellerica fruits in central India
- Long-term vegetation development on a wildfire slope in Innerzwain(Styria, Austria)
- Herbaceous species diversity in relation to fire severity in Zagros oak forests, Iran
- Effects of selective logging on tree diversity and some soil characteristics in a tropical forest in southwest Ghana
- Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery
- Development and evaluation of local communities incentive programs for improving the traditional forest management: A case study of Northern Zagros forests, Iran
