Herbaceous species diversity in relation to fire severity in Zagros oak forests, Iran
2014-10-18MortezaPourrezaSeyedMohsenHosseiniAliAkbarSafariSineganiMohammadMatinizadehSeyedJalilAlavai
Morteza Pourreza • Seyed Mohsen Hosseini • Ali Akbar Safari Sinegani Mohammad Matinizadeh • Seyed Jalil Alavai
Introduction
In many parts of the world, fire as a natural disturbance factor can have major influences on vegetation composition (Agee 1993; Nasi et al. 2002; Herath et al. 2009; Schaffhauser et al.2012). In savannah ecosystems, Mediterranean countries of Europe, and boreal forests, fire is a part of the ecosystem and a common environmental disturbance factor (Weber and Flannigan 1997; Enright et al. 2005; Andersen et al. 2005). Fire severity is an accepted term for describing the ecological effects of a specific fire that describes the degree of change in ecosystem components (DeBano et al. 1998). These changes are generally measured by visual indicators corresponding both soil and vegetation characteristics after fire (Ryan 2002).
Depending on site conditions, fire severity and fire regimes determine forest age structure, species composition and physiognomy, biodiversity (Uys et al. 2004; Safford and Harrison 2004; Zyranova et al. 2007) and change the soil properties and quality (DeBano et al. 1998; Arocena and Opio 2003; Martinez-Salgado et al. 2010). Dominant native species that produce high vegetative cover often have the greatest potential to deter establishment of non-native species at small scales (Huston 2004;Smith et al. 2004; Bremner et al. 1982).
The level of disturbance is also important in altering species diversity (Walpole and Sheldon 1999; Willott et al. 2000; Cleary et al. 2006; Elliott and Vose 2010; Maia et al. 2012). Low fire severity can promote herbaceous abundance and diversity (Hutchinson et al. 2005) and increase availability of nutrients to plants (Gilliam 1988; Certini 2005; Knoepp et al. 2009; Scharenbroch 2012). Moderate levels of disturbance enhance diversity by providing additional ecological niches and preventing competitive exclusion of rarer species (Rosenzweig 1995; Elliott et al.1999; Elliott and Vose 2005, 2010). Elliott and Vose (2010)demonstrated that herbaceous layer cover was either increased or not changed depending on site conditions and no significantchange in species diversity occurred after burning at any site. In contrast, Kuenzi et al. (2008) and Huisinga et al. (2005) reported that high fire severity can promote plant species cover and diversity more than low fire severity. Post-fire increase in plant species richness can also occur as a result of reduced vegetation biomass and cover, and consequently reduced competition for light (Pekin et al. 2012). Varying reports on the impacts of fire on herbaceous species diversity emphasize the need for more information across the full range of vegetation community types to better understand fire as an important ecological factor (Elliott and Vose 2010).
Forest biodiversity encompasses a wide range of trees, shrubs,wild animals, and micro-organisms. It can be considered at genetic, species, population, community, landscape and ecosystem levels (Gillison and Liswanti 2004; Granke et al. 2009). Biodiversity is very complex and generally described by two indices richness and evenness. Richness simply refers to the count of species present in a unit area. Evenness refers to the relative abundance of species (Rousseau and Van Hecke 1999). Various indices have been proposed to measure and quantify species diversity (Scherer et al. 2005). Although the ecological impacts of fire on forest ecosystems have been widely investigated,comparatively little is known about the ecological effect of fire on vegetation and herbaceous diversity in Zagros forests. Zagros forests range from northwest to southeast in Iran and are classified as semi-arid forests (Sagheb-talebi et al. 2004). Oak species are dominant in Zagros forests and more than 90% of them occur in coppice form (ibid.) due to frequently cut. So in these oak coppice forests each tree accounts for several stems from a single stump and occupies the forest floor in patches that vary in area depending on density of sprouts. Increasing numbers of fire reports in these forests during recent decades (Pourreza 2009; Mohammadi et al. 2011) implicate fire as an important factor influencing biodiversity and structural change in these forests. In this study the herbaceous diversity was investigated in the first growing season after fire in Zagros coppice forests. Our main objectives were to (1) document herbaceous composition inside and outside of the microsites of oak sprout clumps and (2) indentify how herbaceous species composition and diversity change in areas subjected to different fire severity.
Materials and methods
Study area
The study area was located in Ravansar, Kermanshah province,Iran with latitude 34° 51' 50" N and longitude 46° 31' 02" E (Fig.1). The climate of the study area is Mediterranean according to Demarton's classification with average annual precipitation about 600 mm. The mean annual temperature is 14.9 °C (August: 29.3°C and January: 1.8 °C). The soil texture is generally sandy clay loam. The soil of the Zagros Mountains in this region is also generally calcareous (Jazirehi and Ebrahimi 2003). Quercus brantii Lindl. is the main tree species with coppice form of sprout clumps that occupy forest area like patches. More than 100 ha of this forest were burned in early September 2010. The forest surface was completely burned but fire severity was different as a result of the heterogeneity and spatial pattern of fuel.In other words, inside the sprout clumps contained higher load of fuel compared to the outside of them (forest floor). We used an unburned area adjacent to the burned area helped to compare herbaceous diversity and soil characteristics in burned and unburned areas. The unburned area (25 ha) was adjacent to the burned area and separated by a valley. Both sites were located along the same hillside with north-west geographical aspect, with the same slope (45%), elevation (1,700−1,820 m above sea level),soil properties and vegetation covers.
Fig.1. The location of study area (●) in Iran’s map
Experimental design and data collection
In both burned and unburned area, 5 parallel transects were established along the hillside slop with 100 m distance from each other (Fig.2). We considered six treatments namely: unburned-inside of sprout clumps (Ni), unburned-outside of sprout clumps (No), inside of sprout clumps that burned with high severity (H), inside of sprout clumps that burned with moderate severity (M), and outside of sprout clumps (forest floor) that burned with low severity (OH and OM). The treatments were selected randomly along transects (Fig.2). Although we expected that treatments OH and OM (low fire severity) would contain the same herbaceous structure we considered them separately with respect to their nearest burned sprout clump (M or H)(Fig.2). The inside of unburned sprout clumps (Ni) is generally covered by litter, sprouts and grasses. So it generally contains a high load of fuel in contrast to the outside of sprout clumps (No)that covered just by grasses (Table1).
We used visual indicator such as litter depth and sprouts consumption, ash, sprout mortality and vegetation re-establishment to classify fire severity (Table1). Vegetation re-establishment is closely dependent upon soil seed bank density and viability that is affected by fire severity. Vegetation re-established occurred with high density in both low severity burning (OH and OM) and moderate severity burning, while in high burning severity we observed low vegetation re-establishment. The different load of fuel inside of sprout clumps resulted in moderate or high severity burning. We assigned moderate and high severity burning treat-ments by considering the fuel consumption and the mortality of sprout clumps and vegetation re-establishment (Table1).

Table1. Treatments and the indicators for treatments classification
Fifteen 1.5 m × 1.5 m quadrats were considered for each treatment to record herbaceous data using the Van der Marel(1979) method (Table2). The surface area of sprout clumps varied from 6 to 10 m2and the quadrats were established in the center of sprout clumps. To compare some physical-chemical properties of soils at sites subjected to different fire severity, 10 composite soil samples were collected from the top 5 cm of soil in areas representing each burning level of severity one year after fire. For soil data, the treatments OH and OM were considered the same and reported as Low fire severity. The soil physical-chemical analyses were determined in triplicate for all characteristics. N was determined by the Kjeldahl method (Bermner and Mulvaney 1982). Total organic C was determined using a wet oxidation technique (Walkley and Black 1934). Soil moisture was checked by removing 5 g (wet weight) soil samples and weighing them before and after oven-drying at 105°C for 24 h.Soil electrical conductivity (EC) and pH were measured in deionised water (1:5 and 1:2.5 soil/water ratio, respectively)(McLean 1982).

Table2. The classifications of species cover percentage
Data analysis
The detrended correspondence analysis (DCA) ordination method was employed to analyze the herbaceous data inside and outside the oak sprout clumps using Canoco for windows version 4.5 (ter Braak & Šmilauer 2002). To summarize species composition in each treatment, the following parameters were calculated: cover (CVR), relative cover (RCVR=individual species cover/∑CVR of all species), frequency (FRQ=% of the total number of quadrats in which a species occurred) (McEwan and Muller 2011). To measure diversity components we simply used the number of species (S) as richness, and Shannon–Weiner diversity index (H'):

where, piis the proportion of individuals of the ith species.
Pielou index (Pielou 1966) was used for measuring the evenness(E):

where, S is the number of species and H’ is Shannon’s index.
Sorenson’s similarity index (SI):

where, c is the number of common species in both treatments, a is the total number of species occurred only in treatment a, and b is the total number of species occurred only in treatment b) wasalso used to compare the similarity of species composition among treatments (Magurran 2004; Eliotte and Vose 2010). The frequency of life form (Raunkiaer 1934) in each treatment was calculated. The normality distribution and variance homogeneity of data were tested by Kolmogorov-Smirnov and Leven’s test respectively. One-way analysis of variance (ANOVA) followed by Tukey’s HSD was used to compare the means of diversity indices, and soil physical-chemical properties. SPSS software was employed to do statistical comparisons.
Results
The detrended corresponding analysis (DCA) of herbaceous cover indicated that apart from the fire effect, herbaceous composition was different inside of the sprout clumps microsites (Ni)compared to outside (No) or on the forest floor (Fig.3). The quadrats of inside and outside the microsites of oak sprout clumps were separated by a second axis. This meant that post-fire herbaceous composition was also specified by microsite conditions.
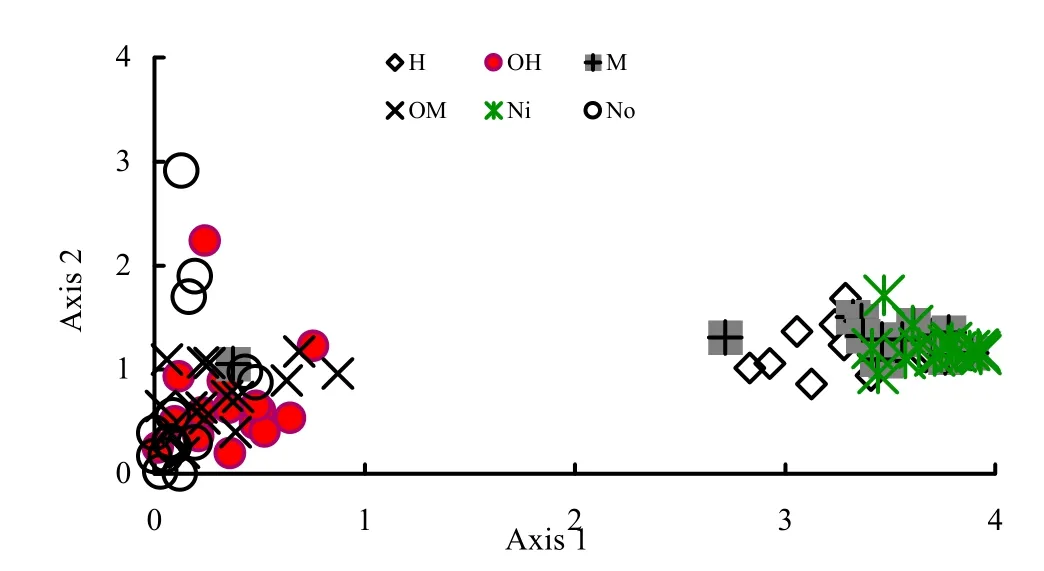
Fig.3. The result of detrended corresponding analysis (DCA) of herbaceous composition with respect to the microsites of inside of sprout clumps (H, M, Ni) at the right side and outside of them (OH, OM, No) at the left side. Ni is unburned sprout clumps; No is outside of Ni; H is sprout clumps burned with high severity; M is sprout clumps burned with moderate severity; OH and OM are outside of H and M.
The summarized species composition by parameters: cover,relative cover, and frequency indicated that Biberstinia multifida had greatest CVR and RCVR, and Galium aparine was the most frequent in the quadrats inside of the burned and unburned sprout clumps (Ni, H, M) (Appendix 1). Brumos tecterom had highest CVR, RCVR and frequency in quadrats outside the burned and unburned sprouts clumps (OH, OM, No), (Appendix 1).
The analysis of variance (ANOVA) for herbaceous species richness, evenness, Shannon-Weiner indices, and the number of individuals showed significant differences between treatments inside of sprout clumps microsites. Tukey’s post hoc test indicated a significant difference of herbaceous richness inside of the sprout clumps (Ni) compared to outside of them (No) (Table3).The outside of sprout clumps had the greater richness than inside of them.
Low fire severity (OM and OH) and moderate fire severity (M)significantly increased herbaceous richness compared to the unburned area (No and Ni, respectively). However richness was not significantly changed in high fire severity compared to Ni.The plant abundance inside and outside of sprout clumps (Ni and No) was similar. Low fire severity (OH and OM) significantly increased plant abundance. The sprout clumps subjected to high fire severity (H) had the fewest individuals. Evenness was only significantly increased after high fire severity.
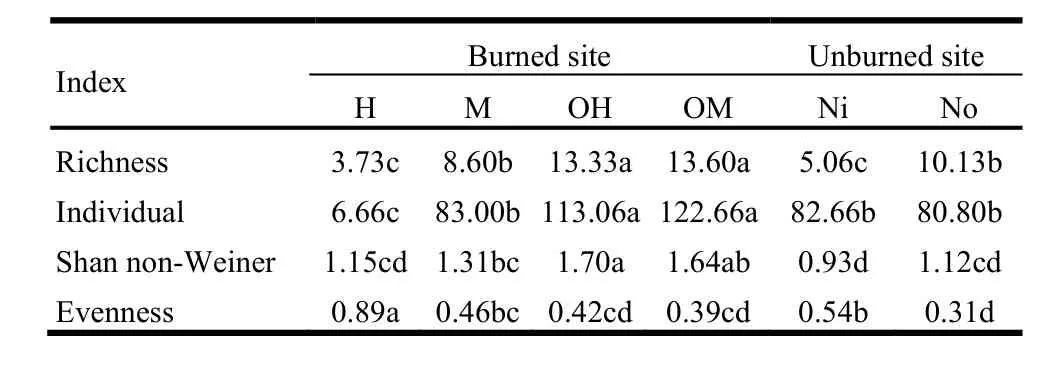
Table3. The result of Tukey’s test for herbaceous individual, richness,evenness, and Shannon-Weiner indices among treatments
There was no significant difference between moderate fire severity (M) and unburned sprout clumps (Ni) in terms of herbaceous abundance. The diversity was not significantly different between unburned inside and unburned outside of sprout clumps(Ni and No). However, diversity increased in areas subjected to both low and moderate fire severity compared to No and Ni,respectively. The evenness index was significantly different between unburned inside and unburned outside of sprout clumps(Ni and No) (Table3).
Comparing the Sorenson’s similarity index between unburned inside and unburned outside of sprout clumps indicated that the value of this similarity was 51% between Ni and No and it was increased to 71% after moderate fire severity (between M and OM). While this value decreased to 41% by high fire severity(between H and OH). Also the results indicated that OH and OM were the most similar treatments (91%) as we expected (Table4).
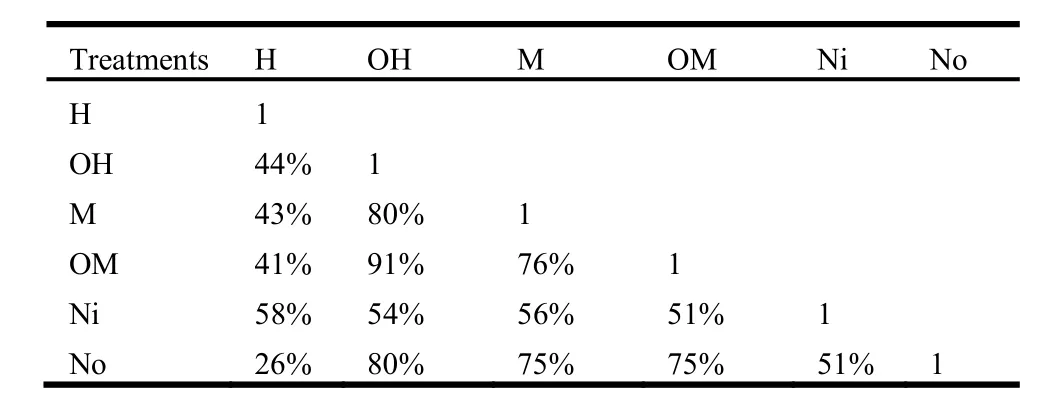
Table4. Sorenson similarity index (%) among treatments
Therophytes were the most frequent life form in both unburned and burned areas (Fig.4). The frequency of therophytes increased after moderate fire severity (M) compared to unburned sprout clumps (Ni) and high fire severity (H).
Soil physical-chemical properties were also changed depending on fire severity. The soil pH did not differ significantly between inside of sprout clumps or Ni (7.64) and outside of sprout clumps or No (7.59), (Table5). Soil pH was significantly higher(p <0.05) in the high fire severity or H (7.94) compared to moderate fire severity or M (7.74) and Ni (7.64). It was not significantly different between low fire severity (7.66) and No (7.59).The soil organic carbon (SOC) of Ni (65.2) was potentially higher than No (27.47) (Table5). SOC was significantly lower (p<0.05) both in M (56.08) and H (35.9) compared to Ni. It was also significantly lower (p <0.05) in H compared to M. SOC was not significantly changed by low fire severity (23.8) compared to No. The variability of the N concentrations among treatments was similar to the C concentrations except that it was not significantly differed between M (5.8) and H (4.8) and also M and Ni(6.14). The electrical conductivity (EC) was significantly different between Ni (360.6) and No (213), however no significant change was observed by the other different burning severity(Table5).
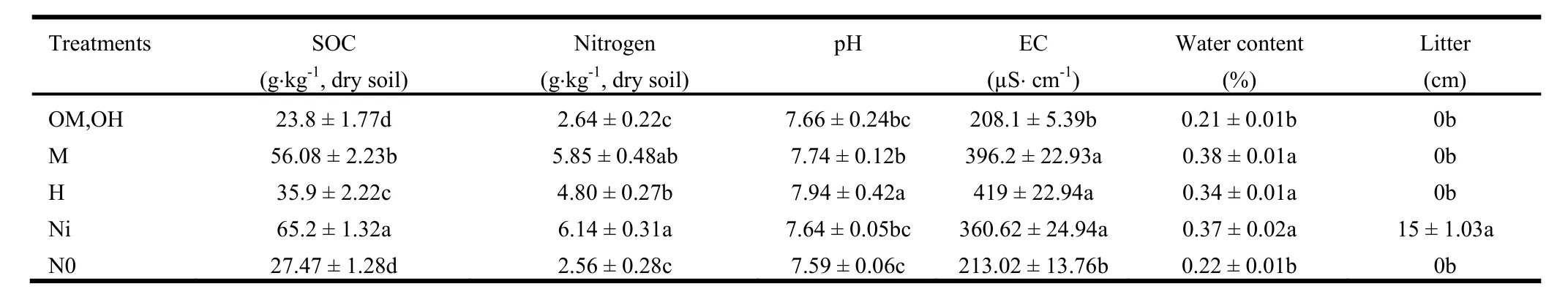
Table5. Physical and chemical variables (mean ± SE) of soil measured in the unburned area (inside and outside of the sprout clumps) and in burned area(with low, moderate and high severity) after one year
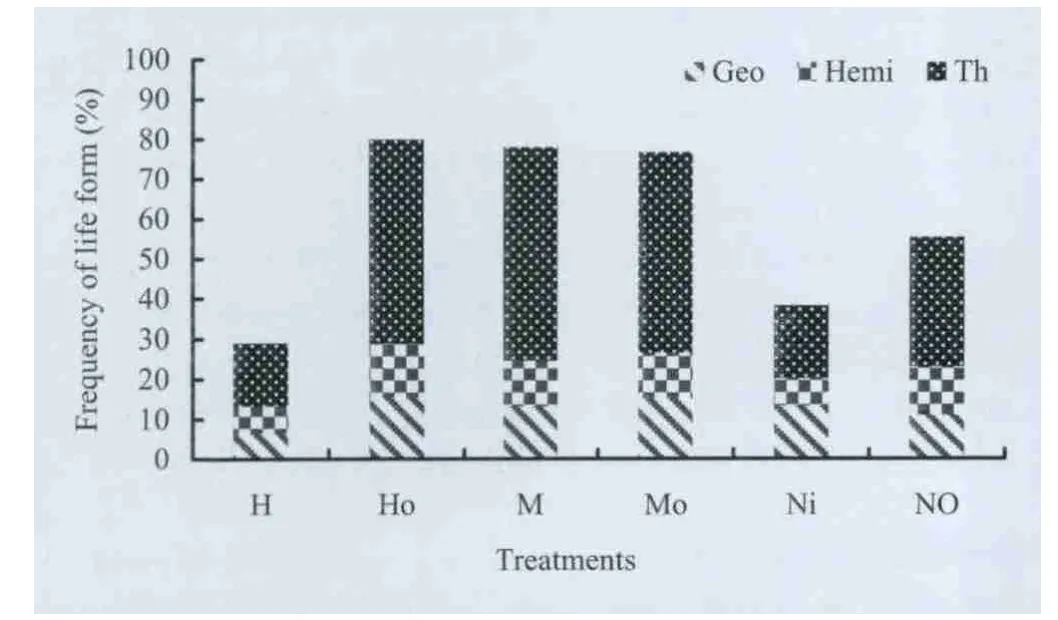
Fig.4. Life form frequency of herbaceous species in different treatments.Therophyte (Th), Hemiphyte (Hemi) and geophyte (Geo), Ni is unburned sprout clumps; No is outside of Ni; H is sprout clumps burned with high severity; M is sprout clumps burned with moderate severity; OH and OM are outside of H and M.
Discussion
In this study we intended to understand the fire influence on herbaceous diversity in relation to the microsites of oak sprout clumps and fire severity. Apart from fire effect the results simply implied a different herbaceous composition between inside and outside of sprout clump microsites (Ni and No). Also, inside of sprout clumps (Ni) soils contained significantly higher SOC, N and water than outside of them (No). It is well known that herbaceous community composition is dependent upon microsite conditions and soil fertility (Small and McCarthy 2005; McEwan and Muller 2011). The different conditions in microsites (e.g.litter, organic matter, light availability and moisture content)probably determine the composition of herbaceous species.Bromus tectorum was the most frequent herbaceous species with highest CVR and RCVR outside of sprout clumps (i.e. on the forest floor). While for the microsites inside of the sprout clumps,Biberstinia multifida had highest CVR and RCVR and Galium aparine was most frequent, probably due to relatively high moisture content.
In this study, burning time coincided with the dormant season and this promoted survival of the soil seed banks and root systems of herbaceous species (Elliott and Vose 2010) although it is highly dependent upon fire severity (Maia et al. 2012). Low and moderate fire severity promoted post-fire species richness and diversity. While high fire severity (H) reduced the herbaceous cover, increased evenness but did not significantly change richness or diversity.
Our results are consistent with some studies and inconsistent with others. Hutchinson et al. (2005) found that low fire severity can promote abundance and diversity of herbaceous cover. While Elliott and Vose (2010) demonstrated that low fire severity had no significant impact on species diversity. Others have reported that moderate levels of disturbance enhance diversity by providing additional ecological niches and preventing competitive exclusion of rare species (Rosenzweig 1995; Elliott et al. 1999;Willott et al. 2000; Cleary et al. 2006).
Microsites subjected to moderate fire severity (M) increased heterogeneity and contained the same species presented outside of the sprout clumps (OH, OM, No). Reportedly, fire can recreate heterogeneity and promote biodiversity (Fule et al. 2004;Cleary et al. 2006; Odion and Sarr 2007). Post-fire plant species richness also tends to increase in response to lower vegetation biomass and cover probably by reducing competition for light(Pekin et al. 2012).
In our study the inside of the sprout clumps contained much higher organic matter than outside and this led to moderate and high fire severity. Burning severity was low on the forest floor or outside of sprout clumps (OM and OH) that was covered by with grasses cover. Fire severity in grasses is generally low because the same amount of heat does not transfer to the soil due to quick move of fire through grasses (Neary et al. 1999). This contrasts with slow-moving fires in areas with moderate and heavy fuels.The post-fire data indicated that low fire severity did not significantly change SOC content but both moderate and high fire severity significantly reduced SOC content. Soil organic C is one of the most important indicators of soil quality (Martinez-Salgado et al. 2010). Total N was changed similar to SOC.Loss of organic C and N depends on fire intensity and combustion of organic matter. The losses of organic C and N occur at temperatures of 220–460°C (DeBano et al. 1998). Soil pH was significantly increased only by high fire severity. Increase in soil pH after fire only occurs once temperatures exceed 450-500 °C as a result of denaturation of organic acids (Certini 2005).
Site conditions and vegetation type are key factors that can affect the impacts of wildfire. For instance Elliott and Vose(2010) considered two levels of fire severity (low and moderate)and concluded that herbaceous diversity was not changed after low severity fires but was changed after moderate severity fire.Huisinga et al. (2005) found that total plant species richness and abundance increased after high fire intensity in Grand Canyon National Park. Similarly Kuenzi et al. (2008) found that high fire severity is more influential in increasing plant species richness and exotic species than low fire severity. In contrast, Scharenbroch (2012) reported that high fire severity had negative impacts on vegetation (presence of exotic species) and soil nutrient cycling while species diversity was increased by low fire severity and no negative impacts were detected. We found that both low and moderate fire severity increased species richness and diversity. Although high fire severity reduced vegetation cover and plant abundance, it did not significantly influence richness and diversity.
Conclusions
Different fire severity can result in different vegetation composition and diversity. On the other hand different ecosystems may respond differently to the fire. This makes the influence of fire on vegetation complicated to predict and impossible to extrapolate from one ecosystem to another. Fire ecologists and managers have used prescribed fire as a tool to control vegetation composition and diversity in fire-prone lands. Our results indicated that the forest managers can take advantages of low and moderate fire severity in Zagros forest (that have never been used) as a tool for forest management. However, further studies are needed to better understand fire effects on species community composition and diversity.
This study was part of the PhD thesis research at Tarbiat Modares University. The authors thank Dr. Nastaran Jalilian, and Dr.Masoomeh Khanhasani from the Department of Botany of the Agricultural and Natural Resources Research Center of Kermanshah province for identifying herbaceous species.
Agee JK. 1993. Fire ecology of the Pacific Northwest. Washington, D.C.:Island Press, p. 493.
Andersen AN, Cook GD, Corbett LK, Douglas MM, Eager RW, Russell-Smith J, Setterfield SA, Williams RJ, Woinarski JCZ. 2005. Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology, 30:155−167.
Arocena JM and Opio C. 2003. Prescribed fire-induced changes in properties of sub-boreal forest soils. Geoderma, 113: 1−16.
Bremner JM, Mulvaney CS. 1982. Nitrogen total. In: A.L. Page, R.H. Miller and D.R. Keeney (eds), Methods of soil analysis. Part 2, chemical and microbiological properties. Madison: American Society of Agronomy, pp.595−624
Certini G. 2005. Effects of fire on properties of forest soils: a review. Oecologia,143: 1–10.
Cleary DFR, Fauvelot C, Genner MJ, Menken S BJ, Mooers AØ. 2006. Parallel responses of species and genetic diversity to El Nino Southern Oscillation-induced environmental destruction. Ecology Letter, 9: 304–310.
DeBano LF, Neary DG, Ffolliott PF. 1998. Fire Effects on Ecosystems. New York: John Wiley & Sons, p. 333.
Elliott JK, Hendrick RL, Major AE, Vose JM, Swank WT. 1999. Vegetation dynamics after a prescribed fire in the southern Appalachians. Forest Ecology and Management, 114: 199–213.
Elliott JK, Vose JM. 2005. Effects of understory prescribed burning on short leaf pine (Pinus echinata Mill.) mixed hardwood forests. Bulletin of the Torrey Botanical Club, 132: 236–251.
Elliott JK, Vose JM. 2010. Short-term effects of prescribed fire on mixed oak forests in the southern Appalachians: vegetation response. Bulletin of the Torrey Botanical Club, 137(1): 49–66.
Enright NJ, Lamont BB, Miller BP. 2005. Anomalies in grass-tree fire history reconstructions forsouth – western Australian vegetation. Austral Ecology,30: 668−673.
Fule PZ, Crouse JE, Cocke AE, Moore MM, Covington WW. 2004. Changes in canopy fuels and potential fire behavior 1880–2040: Grand Canyon,Arizona. Ecological Modeling, 175: 231–248.
Gilliam FS. 1988. Interactions of fire with nutrients in the herbaceous layer of an utrient-poor Coastal Plain forest. Bulletin of the Torrey Botanical Club,115: 265–271.
Gillison AN, Liswanti N. 2004. Assessing biodiversity at landscape level: the importance of environmental context. Agricultural Ecosystem and Environment, 104: 75−86.
Granke O, Kenter B, Kriebitzsch WU, Köhl M, Köhler R, Olschofsky K. 2009.Biodiversity assessment in forests - from genetic diversity to landscape diversity. iForest, 2: 1−3.
Herath DN, Lamont BB, Enright NJ, Miller BP. 2009. Impact of fire on plant-species persistence in post-mine restored and natural shrubland communities in southwestern Australia. Biological Conservation, 142(10):2175–2180.
Huisinga KD, Laughlin DC, Fule PZ, Springer JD, McGlone CM. 2005.Effects of an intense prescribed fire on under story vegetation in a mixed conifer forest. Bulletin of the Torrey Botanical Club, 132: 590–601.
Huston MA. 2004. Management strategies for plant invasions: manipulating productivity, disturbance, and competition. Diversity and Distribution, 10:167–178.
Hutchinson TF, Boerner REJ, Sutherland S, Sutherland EK, Ortt M, Iverson LR. 2005. Prescribed fire effects on the herbaceous layer of mixed-oak forests. Canadian Journal of Forest Research, 35:877–890.
Jazirehi MH, Ebrahimi M. 2003. Silviculture in Zagros. Tehran: University of Tehran Press. p. 560. (in Persian)
Knoepp JD, Elliott KJ, Vose JM, Linton BD. 2009. Effects of prescribed fire in mixed-oak forests of the southern Appalachians: forest floor, soil, and soil solution nitrogen responses. Bulletin of the Torrey Botanical Club, 136:380–391.
Kokaly RF, Rockwell BW, Haire SL, King TVV. 2007. Characterization of postfire surface cover, soils, and burn severity at the Cerro Grande Fire, New Mexico, using hyperspectral and multispectral remote sensing. Remote Sensing of Environment, 106: 305−325.
Kuenzi A, Fule PZ, Sieg CH. 2008. Effects of fire severity and pre-fire stand treatment on plant community recovery after a large wildfire. Forest Ecology and Management, 255: 855–865.
Magurran AE. 2004. Measuring Biological Diversity. Oxford UK: Blackwells,p. 256.
Maia P, Pausas JG, Arcenegui V, Guerrero C, Pérez-Bejarano A,Mataix-Solera J, Varela MET, Fernandes I, Pedrosa ET, Keizer JJ. 2012.Wildfire effects on the soil seed bank of a maritime pine stand - The importance of fire severity. Geoderma, 191: 80−88.
Martinez-Salgado MM, Gutiérrez-Romero V, Jannsens M, Ortega-Blu R.2010. Biological soil quality indicators: a review. In: A. Mendez-Vilas (ed.),Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Spain: Formatex Research Center, pp.319−328
McEwan RW, Muller RN. 2011. Dynamics, diversity, and resource gradient relationships in the herbaceous layer of an old-growth Appalachian forest.Plant Ecology, 212:1179–1191.
McLean EO. 1982. Soil pH and lime requirement, In: A.L. Page, R.H. Miller and D.R. Keeney (eds), Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. Madison, WI : American Society of Agronomy,SSSA, pp. 199–224
Mohammadi F, Shabanian N, Pourhashemi M, Fatehi P. 2011. Risk zone mapping of forest fire using GIS and AHP in a part of Paveh forests. Iranian Journal of Forest and Poplar Research, 18:569–586. (in Persian)
Nasi R, Dennis R, Meijaard E, Applegate G, Moore P. 2002. Forest fire and biological diversity. Unasylva, 209: 36–40.
Neary DG, Klopatek CC, DeBano LF, Ffolliott PF. 1999. Fire effects on belowground sustainability: a review and synthesis. Forest Ecology and Management, 122: 51–71.
Odion DC, Sarr DA. 2007. Managing disturbance regimes to maintain biological diversity in forested ecosystems of the Pacific Northwest. Forest Ecology and Management, 246(1): 57–65.
Pekin BK, Wittkuhn RS, Boer MM. 2012. Response of plant species and life form diversity to variable fire histories and biomass in the Jarrah forest of south-west Australia. Austral Journal of Ecology, 73: 330–338.
Pielou EC. 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13: 131−144.
Pourreza M, Safari H, Khodakarami Y, Mashayekhi Sh. 2009. Preliminary results of post fire resprouting of manna oak (Quercus brantii L.) in the Zagros forests, Kermanshah. Iranian Journal of Forest and Poplar Research, 17: 225−236. (in Persian)
Raunkiaer C. 1934. The life form of plants and statistical plant geography.Oxford: The Clarendon Press, p. 632.
Rosenzweig ML. 1995. Species Diversity in Space and Time. Cambridge:Cambridge University Press, p. 460.
Rousseau R, Van Hecke P. 1999. Measuring biodiversity. Acta Biotheoretica,47: 1−5.
Ryan KC. 2002. Dynamic interactions betweenforest structure and fire behavior in boreal ecosystems. Silva Fennica, 36: 13 –39.
Safford HD, Harrison S. 2004. Fire effects on plant diversity in serpentine VS.sandstone chaparral. Ecology, 85: 539–548.
Sagheb-Talebi K, Sajedi T, Yazdian F. 2004. Forests of Iran. Tehran: Research Institute of Forests & Rangelands, p. 24.
Schaffhauser A, Curt T, Véla E, Tatoni T. 2012. Fire recurrence effects on the abundance of plants grouped by traits in Quercus suber L. woodlands and maquis. Forest Ecology and Management, 282: 157–166.
Scharenbroch BC, Nix B, Jacobs KA, Bowles ML. 2012. Two decades of low-severity prescribed fire increases soil nutrient availability in Midwestern, USA oak (Quercus) forest. Geoderma, 183-184: 80 –91.
Scherer-Lorenzen M, Potvin C, Koricheva J, Schmid B, Hector A, Bornik Z.2005. The design of experimental tree plantations for functional biodiversity research. In: M. Scherer-Lorenzen, Ch. Korner and. E.-D. Schulze (eds),Forest Diversity and Function. Temperate and Boreal Systems. Berlin Heidelberg: Springer-Verlag, pp. 347–376.
Small CJ, McCarthy BC. 2005. Relationship of understory diversity to soil nitrogen, topographic variation, and stand age in an eastern oak forest, USA.Forest Ecology and Management, 217: 229–243.
Smith M.D, Wilcox JC, Kelly T, Knapp AK. 2004. Dominance not richness determines invisibility of tall grass prairie. Oikos, 106: 253–262.
Uys RG, Bond WJ, Everson TM. 2004. The effect of different fire regimes on plant diversity in southern African grasslands. Biological Conservation,118: 489–499.
Van der marel E. 1979. Transformation of cover- abundance value in phytosociology and its effects on community similarity. Journal of Vegetation Science, 39: 97−114.
Walkley A, Black IA. 1934. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Science, 37: 29−37.
Walpole MJ, Sheldon IR. 1999. Sampling butterflies in tropical rainforest: an evaluation of a transect walk method. Biological Conservation, 87: 85–91.
Weber MG, Flannigan MD. 1997. Canadian boreal forest ecosystem structure and function in a changing climate: impact on fire regimes. Environmental Review, 5:145−-166.
Willott SJ, Lim DC, Compton SG, Sutton SL. 2000. Effects of selective logging on the butterflies of a Bornean rainforest. Conservation Biology, 14:1055–1065.
Zyranova OA, Yaborov VT, Tchikhacheva TL, Koike T, Makoto K, Matsuura Y, Satoh F, Zyryanov VI. 2007. The structure and biodiversity after fire disturbance in Larix gmelinii Rupr. forests, Northeastern Asia. EurasianJournal of Forest Research, 10:19−29.
杂志排行
Journal of Forestry Research的其它文章
- Effects of harvesting intensities and techniques on re-growth dynamics and quality of Terminalia bellerica fruits in central India
- Long-term vegetation development on a wildfire slope in Innerzwain(Styria, Austria)
- The impact of land afforestation on carbon stocks surrounding Tehran,Iran
- Effects of selective logging on tree diversity and some soil characteristics in a tropical forest in southwest Ghana
- Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery
- Development and evaluation of local communities incentive programs for improving the traditional forest management: A case study of Northern Zagros forests, Iran
