Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
2014-07-25LiDengShenBenxianSunHui
Li Deng; Shen Benxian; Sun Hui
(Petroleum Processing Research Center, East China University of Science and Technology, Shanghai 200237)
Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
Li Deng; Shen Benxian; Sun Hui
(Petroleum Processing Research Center, East China University of Science and Technology, Shanghai 200237)
1-Decene was oligomerized over the supported AlCl3/γ-Al2O3catalyst in a fixed-bed reactor. The effects of temperature and LHSV on oligomerization of 1-decene were investigated and the synthetic PAO was characterized with GC technique. Furthermore, the life of immobilized catalyst was tested and the mechanism of catalyst deactivation was discussed. The results showed that with an increasing temperature, the PAO yield increased and the kinematic viscosity of oil decreased. The GC results indicated that the synthesized PAO was a mixture consisting of dimers, trimers, tetramers and pentamers. The results of chloride content measurements and BET tests showed that catalyst deactivation could be mainly attributed to the loss of active components.
poly-alpha-olefins, 1-decene, AlCl3/γ-Al2O3catalyst, oligomerization, lubricant base stocks
1 Introduction
Poly-α-olefins (PAOs) are oligomers of linear α-olefins (primarily C8—C12), which are used as base stocks for synthetic lubricants in automotive and industrial fields. Compared with conventional mineral lubricants, PAO-based synthetic lubricants possess excellent performance including wider operating range, higher viscosity index, lower pour point, lower volatility, lower water solubility, and better thermal, oxidative and hydrolytic stability[1-3]. AlCl3is one of the important Friedel-Crafts catalysts widely used in the petroleum refining, chemical, and pharmaceutical industries. Unfortunately, its applicability is seriously restricted on account of its drawbacks: corrosiveness, difficulty in separating the used catalyst from products, and production of a large amount of polluting wastes. A promising improvement of traditional AlCl3catalyst is the immobilization of AlCl3on supports. Immobilization of AlCl3on organic or inorganic supports was achieved and the immobilized materials were used as catalysts in previous studies[4-6]. Early work indicated that the catalysts were prepared by the reaction of anhydrous AlCl3with suitable support originated either from the vapor or solution phase[7-9]. The catalyst has been applied in the Friedel-Crafts alkylation[10-11], gas-phase isomerization and cracking reactions[12], polymerization of aliphatic monomers[13]and polymerization of styrene[14]. But the research on the polymerization of olefins to synthesize lubricants, the catalyst life and the causes leading to its deactivation is less referred to.
In this study, the supported AlCl3/γ-Al2O3catalyst was used for the polymerization of 1-decene in a fixed-bed reactor to synthesize lubricant base stocks. The structural property of synthetic PAO was characterized by GC. In addition, the catalyst life was investigated and the mechanism for deactivation of AlCl3/γ-Al2O3immobilized catalyst was discussed.
2 Experimental
2.1 Materials and reagents
1-Decene had a purity of >95% (manufactured by the Dowpol Chemical International Corporation, China). Anhydrous aluminum trichloride and γ-Al2O3all had a purity of >95% (manufactured by the Sinopharm Chemical Reagent Co., Ltd., China).
2.2 Catalyst preparation
The catalyst preparation method was essentially similar to that reported by Melchor[9]. In brief, 40 g of pretreated γ-Al2O3with a grain size of 10—20 mesh were put into astainless steel autoclave, and a crucible with 12 g of AlCl3was introduced into the autoclave. Then the autoclave was purged by a high-purity nitrogen stream for no less than 10 min. After that, the temperature was increased to 250 ℃and was maintained at that temperature for 6 h. At last, the autoclave was again purged with nitrogen at 250 ℃ until no white precipitates were generated with the exhaust gas being introduced into the AgNO3solution in order to remove the AlCl3adsorbed on the surface of γ-Al2O3support.
2.3 Oligomerization
Oligomerization of 1-decene was carried out in a stainless steel fixed-bed reactor with an inner diameter of 15 mm. The bottom of the reactor was filled with silica sand with a grain size of 10—20 mesh in order to serve as flow distributor in the preheating section. 60 mL of catalyst were added into the reactor after the reactor was thoroughly purged with dried nitrogen. Once the reaction temperature reached the desired value, 1-decene was introduced continuously into the reactor through its bottom by a plunger metering pump. The liquid hourly space velocity (LHSV) varied from 0.25 h-1to 2 h-1. The oligomers were further separated by vacuum distillation and the fractions with distillation temperature exceeding 280 ℃ were collected for analysis.
2.4 Characterization and analysis
2.4.1 Characterization
The degree of oligomerization was analyzed by using a GC-14C gas chromatograph (made by the Shimadzu Co., Japan) equipped with a flame ionization detector.
The BET specific surface area and pore diameter distribution of catalysts were determined by an ASAP 2010 microstructure analysis apparatus (made by the Micromeritics Instrument Co., USA). Before measurements, samples were degassed at 300 ℃ for 5 h under vacuum.
2.4.2 Measurement of chlorine content
Both of the fresh and deactivated catalysts (weighing almost 1 g apiece) were soaked in 40 mL of 0.5 mol/L of dilute nitric acid for 8 h. Then the soaked liquid was diluted with water to 250 mL. The mass concentration of chlorine ion was measured by a ZWC-2001 salt content tester (made by the Jiangyan Jierui Instrument & Equipment Co., Ltd., China).
The chlorine content of the synthetic PAO was measured by using a WK-2D microcoulometric analyzer (made by the Jiangfen Electroanalytical Instrument Co., Ltd., China).
2.4.3 Measurement of PAO viscosity
The kinematic viscosity of the synthetic oil samples was measured by a SYD-265C viscosimeter (made by the Shanghai Geological Instrument Factory). The kinematic viscosity of oil samples was tested at 40 ℃ and 100 ℃, with the corresponding viscosity values denoted asv40andv100, respectively.
3 Results and Discussion
3.1 Influence of temperature on oligomerization
Under the test conditions covering a LHSV of 0.5 h-1and an atmospheric pressure, the influence of temperature on oligomer yield is shown in Figure 1. It can be seen from Figure 1 that the temperature had a significant effect on PAO yield and product properties. The yields of oligomer increased with an increasing reaction temperature. It can be explained that the reaction rate of 1-decene oligomerization increased with an increasing reaction temperature. The oligomer yield reached 84.72% at a reaction temperature of 80 ℃, and rose slowly as the temperature increased further.

Figure 1 Effect of reaction temperature on oligomer yield
Figure 2 shows the change of oligomer viscosity with the reaction temperature. It can be seen from Figure 2 that the viscosity of oligomer decreased with an increasing reaction temperature. The kinematic viscosityv100of oligomer was reduced from 22.75 mm2/s at 50 ℃ to 6.35 mm2/s at 110 ℃,and thev40of oligomer was decreased from 177.28 mm2/s at 50 ℃ to 31.3 mm2/s at 110 ℃.

Figure 2 Effect of reaction temperature on kinematic viscosity of PAO
At a lower polymerization temperature, the probability of chain transfer fell off, leading to the formation of higher molecular weight and higher viscosity oligomers. With an increasing oligomerization temperature, it was possible that the chain transfer took place more easily, so that the intermolecular reaction was intensified and the yield increased[15]. Meanwhile, the incidence of side reactions such as dehydrogenation and superimposition reactions increased. As a result, less high polymers and more dimers are formed, and therefore the product viscosity decreased. In order to identify the influence of temperature on the property of PAO products, the oligomers distribution was investigated at different oligomerization temperatures. The distribution of oligomers at reaction temperatures of 60 ℃, 80 ℃ and 100 ℃, respectively, is shown in Figure 3. At 60 ℃, the content of dimer was only 8.8% while the pentamer content reached as high as 39.1%. With an increasing reaction temperature, the mass fractions of dimer and trimer increased gradually while the mass fractions of tetramer and pentamer decreased. At a reaction temperature of 100 ℃, the pentamer content was reduced to 25.5%. The results are also in agreement with the decreasing trend of kinematic viscosity of PAO as shown in Figure 2, indicating that oligomers with high oligomerization degree are more easily generated at lower reaction temperature. This law is consistent with previous literature information[16]. Overall, the optimal reaction temperature was selected as 80 ℃ on account of the high yield and appropriate kinematic viscosity of PAO product.

Figure 3 Effect of reaction temperature on product distribution
3.2 Influence of LHSV on oligomerization
The effect of LHSV on oligomerization of 1-decene was studied at 80 ℃. As LHSV varied from 0.25 h-1to 2 h-1, the changes in yield of PAO product are shown in Figure 4. Figure 4 demonstrates a rapid decrease in PAO yields with an increasing LHSV value. It can be interpreted that the larger the LHSV was, the shorter the contact time of 1-decene monomer with the catalyst would be. As a result, there was less chance for oligomerization and the PAO yield declined. On the other hand, LHSV should not be too low because of the demand for a high throughput. The selected LHSV used in the experiment was 0.5 h-1, at which the PAO yield was greater than 80%.

Figure 4 Effect of LHSV on PAO yield
3.3 Stability and life of catalyst
The stability and life of AlCl3/γ-Al2O3immobilized catalyst were investigated in a continuous fixed-bed reactorunder operating conditions including a reaction temperature of 80 ℃ and a LHSV of 0.5 h-1. The synthetic PAO samples formed at different time-on-stream were collected and their yields and kinematic viscosity values were measured with the results shown in Figure 5. It revealed that in the early period of reaction, the yield of PAO dropped very quickly with time, from 93.63% at a reaction time of 2 h to 53.12% at a reaction duration of 48 h. Then the yield of PAO reached a flat value of almost 50% after 70 h of timeon-stream. Although the yield of PAO decreased rapidly, the kinematic viscositiesv40andv100were almost equal to stable value of 56 mm2/s and 10 mm2/s, respectively, and the catalyst could be recovered to a certain extent.

Figure 5 Yield and kinematic viscosity of PAO vs time-on-stream
3.4 Characterization of AlCl3/γ-Al2O3catalyst
The deactivation of catalyst was analyzed in previous literature information[11,17], such as poisoning by the residual water in the raw materials, and “pore-clogging”effect caused by the stagnation of large product molecules within the pores of the catalyst. In this paper, the cause of catalyst deactivation was also investigated, some new reasons were found and discussed in detail here.
Given that the supporting aluminum content will be affected by the supporter, the content of active components was determined by analyzing the chlorine content, which has been confirmed as an effective method[18]. The results on analysis of chlorine content in the catalyst are listed in Table 1. It can be clearly seen that the PAO yield declined with a decrease in chlorine content. The chlorine content of the fresh catalyst was found to be 15.51% and decreased to 12.45% after 10 h of time-on-stream, then reduced to 7.98% after more than 40 h of time-on-stream.
The chlorine loss rate is faster at the first 30 h than that experienced in the succeeding 40 h. During the oligomerization process, some active components were washed off by raw materials because of the weak binding force between the active components and the supporter surface. During the period covering 50—70 h of operation, the chlorine content reached nearly a stable value of 7.5%, while the reaction, thereupon, also gave rise to a stable PAO yield.

Table 1 Chlorine content of catalyst and yield of oligomervstime-on-stream
Furthermore, the chlorine content in the synthetic PAO was also analyzed with the results shown in Figure 6. During the first 2 h of operation, the average chlorine content in collected PAO product was found to be 2 186.97 mg/L, and the chlorine content decreased to 628.35 mg/L after 15 h of operation. The results are in agreement with the relationship between the chlorine content of the catalyst and the change in PAO yield. The decrease of PAO yield, therefore, can be attributed to the loss of active components from the immobilized catalyst.
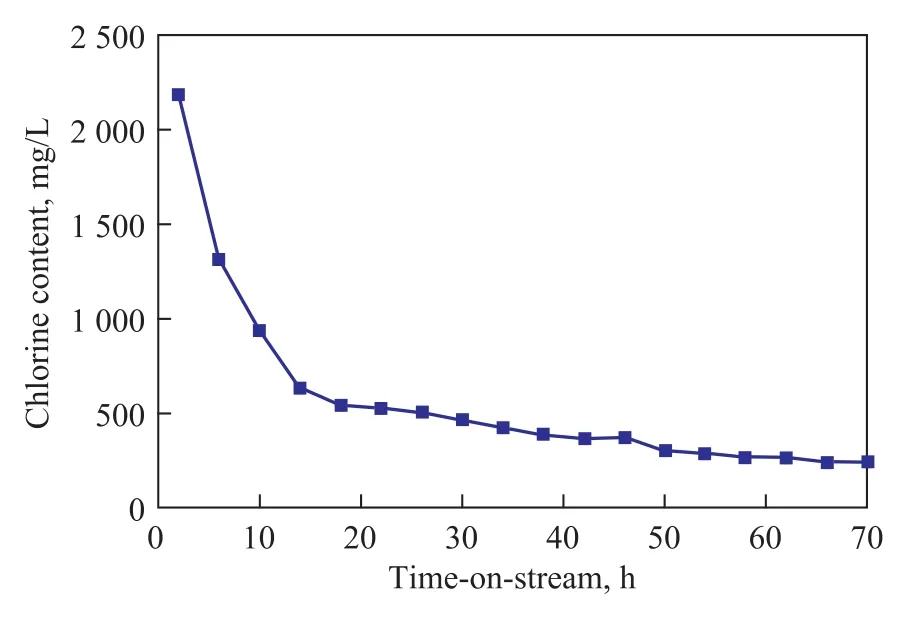
Figure 6 Chlorine content of oligomer vs time-on-stream
In order to identify the roles the coverage of active sites with large molecules plays in the decrease of PAO yield, the pore structures of immobilized catalysts were studiedand compared. Table 2 shows the specific surface areas, pore volumes, and pore size distribution of the support, and the fresh and deactivated catalyst samples. It can be seen that the specific surface area of the support was equal to 281.38 m2/g. After loading of active components, the specific surface area and pore size of catalyst samples decreased, indicating that some small pores were occupied by active components. Compared to the fresh catalyst, the specific surface area of deactivated catalyst showed a slightly larger specific surface area, smaller pore volume, and smaller average pore diameter. These differences between fresh and deactivated catalyst samples are possibly ascribed to the loss of active components which have been loaded on the support.

Table 2 BET of support, fresh and deactivated catalyst
4 Conclusions
The optional conditions for 1-decene oligomerization were specified at a reaction temperature of 80 ℃ and a LHSV of 0.5 h-1. The PAO yield from 1-decene oligomerization was greater than 90% during the first several hours of reaction, and the catalyst life could last for more than sixty hours with the PAO yield exceeding 50%. With an increasing reaction temperature, the PAO yield increased while the kinematic viscosity, and the ratio of tetramers and pentamers in synthesized PAO product all decreased. The chlorine measurement and BET analysis of the catalyst showed that the loss of active components was one of the main reasons leading to deactivation of AlCl3/γ-Al2O3catalyst. The test data obtained from the fixed-bed reactor have indicated that this catalyst system has the potential for industrial application for oligomerization of α-olefins, and the next work phase should be focused on making more stabilized active components and achieving longer life cycle of catalyst.
Acknowledgement:The authors are grateful to the SINOPEC Corporation for the financial support.
[1] Yadav G D, Doshi N S. Development of a green process for poly-α-olefin based lubricants[J]. Green Chemistry, 2002, 4(6): 528-540
[2] Guo F, Li C F, Liu J W, et al. Application of α-ole fins in the field of synthetic lubricant[J]. Chemical Engineer, 2010, 179(8): 29-33
[3] Moore L D, Fels D R, Seay A B, et al. PAO-based synthetic lubricants in industrial applications[J]. Tribology & Lubrication Technology, 2003, 59(1): 23-30
[4] Li Z, Ma X L, Liu J, et al. Silica-supported aluminum chloride: A recyclable and reusable catalyst for one-pot threecomponent Mannich-type reactions[J]. Journal of Molecular Catalysis A: Chemical, 2007, 272: 132-135
[5] Zhao X S, Lu Max G Q, Song C. Immobilization of aluminum chloride on MCM-41 as a new catalyst system for liquid-phase isopropylation of naphthalene[J]. Journal of Molecular Catalysis A: Chemical, 2003, 191(1): 67-74
[6] Wang Z B, Gao B J. Preparation, structure, and catalytic activity of aluminum chloride immobilized on cross-linked polyvinyl alcohol microspheres[J]. Journal of Molecular Catalysis A: Chemical, 2010, 330(1): 35-40
[7] Shang L, Ji M, Cai T, et al. Highly active and stable immobilized aluminium chloride catalyst for alkylation of benzene with 1-decene[J]. Reaction Kinetics and Catalysis Letters, 2005, 87(1): 101-106
[8] Getty E E, Drago R S. Preparation, characterization, and catalytic activity of a new solid acid catalyst system[J]. Inorg Chem, 1990, 29: 1186-1192
[9] Melchor A, Garbowski E, Mathieu M, et al. Chlorinated alumina acidic properties and catalytic activity towardsnbutane isomerization[J]. J Chem. Soc, 1986, 82(6): 1893-1901
[10] Price P M, Clark J H, Macquarrie D J. Modi fied silicas for clean technology[J]. J Chem Soc, Dalton Trans, 2000(2), 101-110
[11] Hu X, Chuah G K, Jaenicke S. Room temperature synthesis of diphenylmethane over MCM-41 supported AlCl3and other Lewis acids[J]. Applied Catalysis A: General, 2001, 217:1-9
[12] Clark J H, Shorrock K, Budarin V, et al. Chemically modified mesoporous solids and their use in the polymerization of hydrocarbon monomers[J]. J Chem Soc, Dalton Trans, 2002(3), 423-427
[13] Sage V, Clark J H, Macquarrie D J. Cationic polymeriza-tion of styrene using mesoporous silica supported aluminum chloride[J]. Journal of Molecular Catalysis A: Chemical, 2003, 198: 349-358
[14] Shubkin R L, Baylerian M S, Maler A R. Olefin oligomer synthetic lubricants: structure and mechanism of formation[J]. Ind Eng Chem Prod Res Dev, 1980, 19(1): 15-19
[15] Huang Q G, Chen L G, Sheng Y P. Synthesis and characterization of oligomer from 1-decene catalyzed by AlCl3/TiCl4/SiO2/Et2AlCl[J]. Journal of Applied Polymer Science, 2006, 101: 584-590
[16] Madgavkar A M, Swift H E. Fixed-bed catalytic process to produce synthetic lubricants from decene-1[J]. Ind Eng Chem Prod Res Dev, 1983, 22(4): 675-680
[17] Cai T X, He M. New approaches to immobilization of aluminum chloride on γ-alumina and its regeneration after deactivation[J]. Catalysis Letters, 2003, 86: 17-23
[18] Lu P F, Jiang L, Mi P K, et al. Structure of immobilized AlCl3catalyst with Al2O3as support [J]. Petrochemical Technology, 2010, 39(6): 616-619 (in Chinese)
Recieved date: 2014-01-13; Accepted date: 2014-05-09.
Professor Shen Benxian, Telephone: +86-21-64252851; E-mail: sbx@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Preparation of Tungsten Film and Its Tribological Properties under Boundary Lubrication Conditions
- Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization
- Spray Characteristics Study of Combined Trapezoid Spray Tray
- Viscoelastic Characteristics of Asphalt Binders at Softening Point Temperature
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
