Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization
2014-07-25BaiWeiGaoXiangluWuHaotianCaoChengangJiangTao
Bai Wei; Gao Xianglu; Wu Haotian; Cao Chengang; Jiang Tao
(College of Material Science and Chemical Engineering, Tianjin University of Science & Technology, Tianjin 300457)
Preparation of Spherical MgCl2/SiO2/THF-Supported Late-Transition Metal Catalysts for Ethylene Polymerization
Bai Wei; Gao Xianglu; Wu Haotian; Cao Chengang; Jiang Tao
(College of Material Science and Chemical Engineering, Tianjin University of Science & Technology, Tianjin 300457)
A facile and user friendly technique to immobilize the late-transition metal complexes on spherical MgCl2/SiO2/THF support has been developed. The spherical MgCl2/SiO2/THF-supported late-transition metal catalysts 2,6-bis-[1-(2,6-dimethylphenylimino)ethyl]pyridine iron (II) dichloride (SC-A) and 1,4-bis(2,6-dimethylphenyl)- acenaphthene diimine nickel (II) dibromide (SC-B) for ethylene polymerization has been prepared by spray-drying technique using tetrahydrofuran suspension containing MgCl2, SiO2and late-transition metal complexes. The catalysts were characterized by BET, XRD, SEM and the polymers were analyzed using GPC, DSC and13C-NMR. The test results show that spray-drying is a very effective method for immobilizing late-transition metal catalysts for ethylene polymerization. Among six kinds of cocatalysts for olefin polymerization, TMA and TEA were con firmed to be more effective than other compounds for the ethylene polymerization system using the catalyst SC-A. For the case of the catalyst SC-B, DEAC showed the best performance as cocatalysts in ethylene polymerization. The replication of the catalyst morphology was found in the resultant polyethylene.
MgCl2/SiO2/THF support; late-transition metal catalyst; polymerization of ethylene; spray-drying
1 Introduction
Recently, considerable attention has been paid to late-transition metal catalysts for olefin polymerization because of their special characteristics, which can contribute to the synthesis of linear α-olefins and branched polyolefins, even opening up the possibility for preparing functional polyolefin materials via inserting polar comonomers[1–3]. The homogeneous late-transition metal catalysts turned out to be very attractive due to their activity, accessibility, and selectivity for the production of α-olefins and their low sensitivity to the admixtures in a monomer[4]. However, heterogeneous catalysts are the workhorse of many industrial processes, since they have many processing and economic advantages over their soluble counterparts. For most of their practical applications, homogeneous catalysts have to be heterogenized either on inorganic materials, such as silica, MgCl2, Al2O3, molecular sieve, clay[5–16], or on organic materials, such as polystyrene and polypropylene[17], to allow their use as ‘drop-in’ substitutes for hitherto commonly used catalysts in existing industrial production units. Most often, the loss of activity and the leaching of homogenous catalyst which leads to bad polymer morphology and reactor fouling are persistent problems for most of the reported supported catalysts. Improvement in immobilization techniques remains therefore a topic of great importance. The immobilization routes reported in the literature for single site catalyst on various supports can be classified into three main methods. The first one comprises direct impregnation of metal complex on the support, and the support needs to be pretreated by calcination, alkyl aluminums or methylaluminoxane (MAO). The second method involves immobilization of MAO on the support followed by reaction with the metal compounds. A modified version of this method involves the replacement of MAO by common alkyl aluminums. The third method is to immobilize organic ligand on the support followed by the addition of metal salt. The immobilization methods mentioned above are related to the chemistry of the catalytic compounds and usually need tedious preparation and purification steps. Usually, the activity of the supported catalyst is half to a tenth thatof the soluble counterpart. This is ascribed to the diminished diffusion of monomer into the interior pores of the supported catalyst and to the result of fewer active centers that are present in the heterogeneous variant. The active centers may be deactivated when the homogeneous catalyst was supported or cannot be generated from the metalcocatalyst interactions. All above-mentioned methods are short of one or other (like sensitive protocols, support pretreatment, time consumption, etc.) factors to produce commercially viable supported catalysts.
We hereby report a facile protocol for the immobilization of late-transition metal catalysts on MgCl2/SiO2/THF support using the spray-drying technique. The influence of various cocatalysts on the catalytic activity of such supported catalysts in ethylene polymerization and the property of the obtained polyethylene were investigated. Also, the morphology, composition and structure of the supported catalysts and polyethylene products were characterized.
2 Experimental
2.1 Materials
The polymerization-grade ethylene and high-purity nitrogen were obtained from PetroChina’s Daqing Petrochemical Co., Ltd., and were desiccated by passing through the molecular sieve 4A prior to use. Cocatalysts such as trimethylaluminum (TMA), triethylaluminum (TEA), triisobutylaluminum (TIBA), tri-n-hexylaluminum (TNHA) and diethylaluminum chloride (DEAC) were provided by the Daqing Chemical Research Center of PetroChina. A toluene solution of MAO (1.4 mol/L) was purchased from Albemarle. TS610 SiO2(fumed silica, with a diameter of 0.2—0.3 mm and a specific surface area of 125 m2/g) was supplied by the Cabot Corporation and used as received. THF andn-hexane were purchased from the Shanghai Chemical Regent Co., and dried over metallic sodium under refluxing for 24 h. Anhydrous magnesium chloride was obtained from the Beijing Chemical Reagent Co., Ltd. The homogeneous catalysts A and B (Scheme 1) were synthesized according to a previously reported procedure[18].
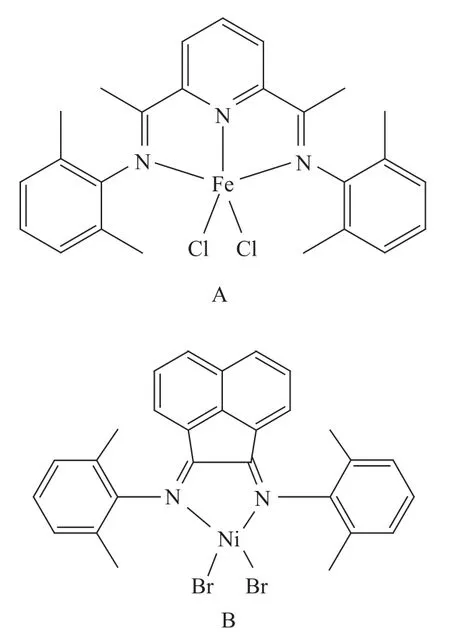
Scheme 1 Structures of homogeneous catalyst A and B
2.2 Synthesis and characterization of catalyst
All operations were carried out under dry nitrogen blanketing using the standard Schlenk techniques. The spherical MgCl2/SiO2/THF-supported late-transition metal catalyst was prepared by spray-drying using the mini-spray dryer B-290 (Buchi, Switzerland). In a flask containing a mixture of THF (130 mL) and anhydrous magnesium chloride (4.8 g) at 65 ℃, 7.5 g of pre-dried cabosil TS-610 (fumed silica) was introduced. Finally, the quantitative complex A or B was added to the slurry. After being stirred for 4 h, the mixture was passed through the Buchi dryer unit to form a spray-dried product composed of substantially uniform and spherical particles.
2.3 Ethylene polymerization
A dried three-necked flask equipped with a stirring bar was flushed with nitrogen gas three times, followed by flushing once with ethylene prior to feeding 50 mL ofn-hexane. The cocatalyst was added via a syringe under stirring. After 2 min, the catalyst was added via the syringe. Polymerization reaction was quenched with acidified ethanol. The polymer was isolated via filtration, washed with ethanol and then dried overnight at 50 ℃ in a vacuum oven.
2.4 Catalyst and polymer characterization
The morphology of the supported catalysts and polyeth-ylene particles was investigated using a Philips environmental scanning electron microscope XL-30ESEMFEG (Philips, The Netherlands, now FEI Co.) equipped with an energy-dispersive X-ray spectrometer (EDX) for determining the distribution of iron or nickel on the catalyst. Elemental analysis was performed with the inductively coupled plasma atomic emission spectroscopy (ICP-AES). The specific surface area and the pore size distribution of the catalyst were measured by means of the N2-BET method using the CE SORPTOMATIC 1990 series instrument. The molecular weight of polymer and its distribution (MWD) were determined by gel permeation chromatography (GPC) on a Waters Alliance GPCV2000 at 150 ℃ with 1,2,4-trichlorobenzene serving as the eluent. Melting points of polymers were measured on a Perkin-Elmer DSC-7 instrument in the standard DSC run mode. The instrument was initially calibrated for the melting point of an indium standard at a heating rate of 10 ℃/min. The polymer sample, weighing about 5 mg, was first equilibrated at 0 ℃, and then heated to 160 ℃ at a heating rate of 10 ℃/min to remove its thermal history. The sample was then cooled again to 0 ℃ at a cooling rate of 10 ℃/min. A second heating cycle was used for collecting the DSC thermo-gram data at a ramping rate of 10 ℃/min. The13C NMR spectrum of the polymer was recorded at 120 ℃ on a Bruker DMX-400 instrument using o-dichlorobenzene-d4 as the solvent.
3 Results and Discussion
3.1 Characterization of the supported catalyst
The analysis data of the supported catalyst in terms of particle size, pores structure and composition are presented in Table 1. The BET results show that the supported catalyst exhibits a lower specific surface area, a higher pore volume and an average pore diameter. The iron content in the catalyst SC-A was 0.24% and the nickel content in the catalyst SC-B was 0.25%.
SEM images of the surface and cross-section of the supported catalyst and polyethylene are shown in Figure 1. It is found out that the supported catalyst particles have good morphology, well particle size distribution and less content of fine powder (Figure 1(a)). We can see from Figure 1(b) that the supported catalyst is comprised of many spherical SiO2particles whose surface was wrapped by MgCl2/THF and the late-transition metal complex. Furthermore, the spatial repartition of iron or nickel, magnesium, chlorine and silica was also investigated on the cross-section by using EDX. It is verified that the different elements were uniformly anchored in the particle, so as to ensure a uniform distribution of the active sites during catalytic polymerization. The polyethylene (PE) grains in Figure 1(c) and 1(d) were also spherical in form and built up from many spherical particles by virtue of duplicating the morphology of the catalyst.

Table 1 Characterization of catalysts SC-A and SC-B
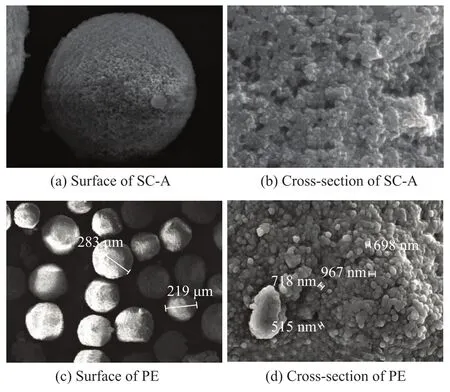
Figure 1 SEM images of the supported catalyst and polyethylene
3.2 Polymerization of ethylene
Although MAO is the best cocatalyst for ethylene polymerization using the homogeneous catalyst A, in our case, it was found out that the role of different cocatalysts was of great interest for the system of supported catalyst A. Theeffect of different cocatalysts on ethylene polymerization catalyzed by the catalyst SC-A was investigated and the results are listed in Table 2.
The catalytic activity of catalyst SC-A with different cocatalysts decreases in the following order: TMA>TEA>TNHA>MAO>TIBA≈DEAC. The iron-catalyzed ethylene polymerization using homogeneous systems typically gives a bimodal polymer molecular weight distribution, and the formation of the low molecular weight fraction is ascribed to the chain transfer to aluminium[19]. A broad molecular weight distribution of PE (Mw/Mn=4.83—7.37), as illustrated in Figure 2(a), is obtained over the catalyst SC-A in all cases. However, there is no formation on the very low molecular weight fraction of PE obtained over the homogeneous catalyst A (Entry 1—3 in Table 2 and Figure 2(a)). In comparison with the homogeneous catalyst A, the catalyst SC-A shows a lower catalytic activity (1.11 vs 5.0×106g PE/mol Fe·h, as evidenced by Entry 1 vs Entry 9 in Table 2). Just as the case with supported metallocene catalyst, the reduction in the catalytic activity is observed under the same condition[20]. This phenomenon can be attributed to the steric effect imposed by the supported system. When the catalyst molecules with large aryl groups are supported on the MgCl2/SiO2/THF channels, the narrow space would limit the incorporation ability of ethylene, which cannot keep in contact with the cationic active centers in the catalyst system easily, leading to reduction in catalytic activity. However, it is worth noting that higher molecular weight and higher melting point of PE could be achieved (Entry 1—8 vs Entry 9 in Table 2). The increase in molecular weight of PE indicates that the MgCl2/SiO2/THF support is very efficient in reducing chain transfer reactions on the catalyst. This can be explained by a larger steric hindrance of the active centers by the channel walls, thus inhibiting the occurrenceof some of the chain transfer reactions, such as the formation of a four-center intermediate, responsible for the b-H elimination. The melting point of the polyethylene obtained over the catalyst SC-A was in the range of around 134.5—135.5 ℃, which was higher than that of PE produced over the homogeneous catalyst A (Tm=132.9 ℃, Entry 1 in Table 2). The elevated melting points of PE could be explained by the increase in molecular weight and crystallinity of the PE obtained over the catalyst SC-A.
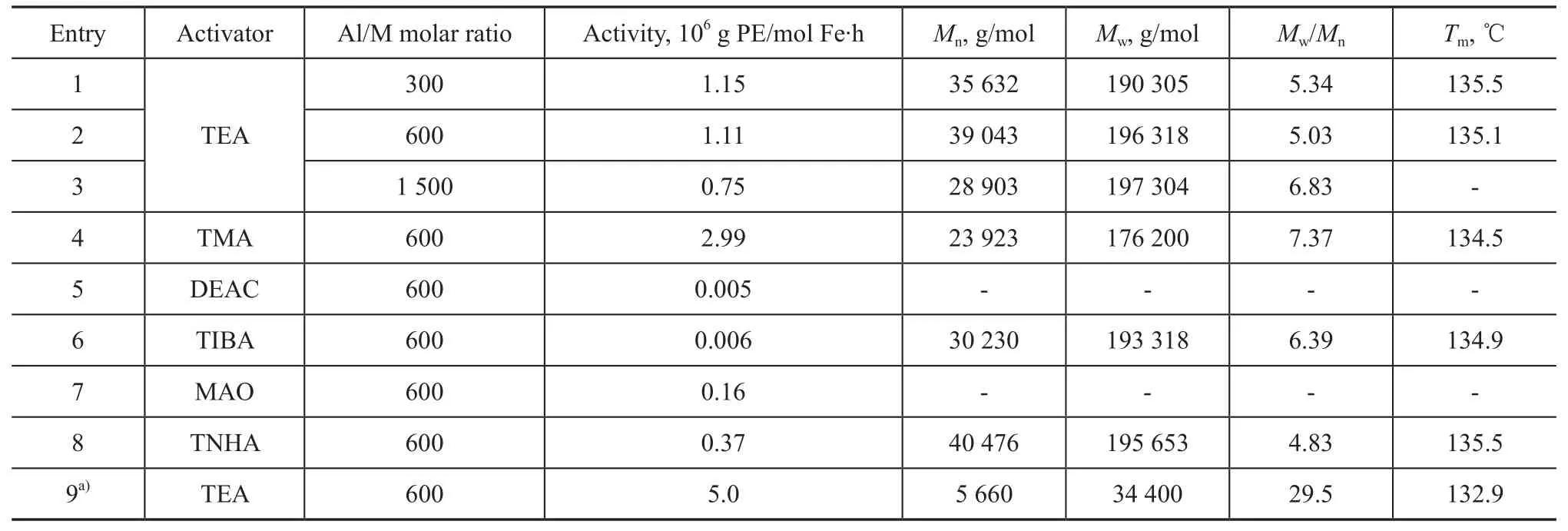
Table 2 Ethylene polymerization catalyzed by supported catalyst A
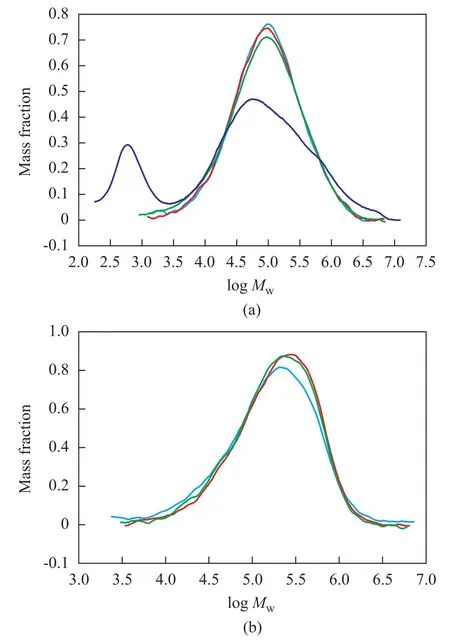
Figure 2 GPC curves of PE obtained over catalysts SC-A (a) and SC-B (b)
The results of ethylene polymerization over the catalyst SC-B are listed in Table 3. DEAC is the best cocatalyst to initiate polymerization of ethylene over the catalyst B. These results are different from the results of polymerization of ethylene over the homogeneous catalyst B[21].
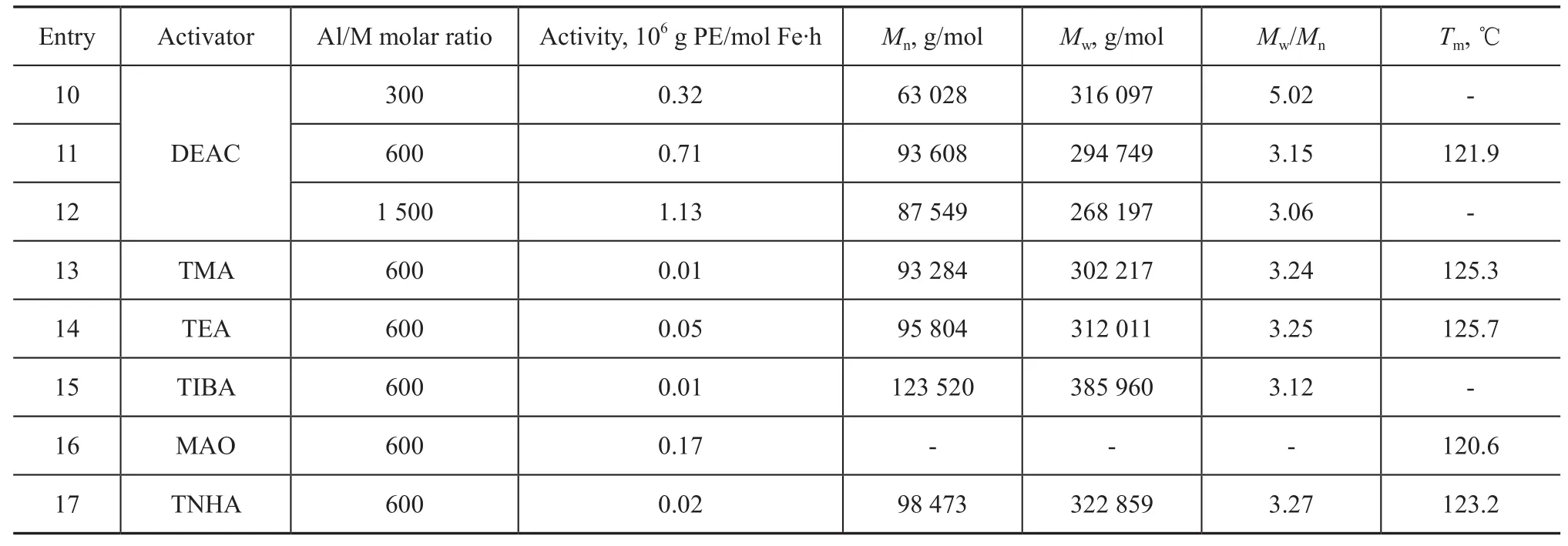
Table 3 Ethylene polymerization catalyzed by supported catalyst B
This better productivity of the catalytic system employing DEAC, in comparison with other generic cocatalysts, can be attributed to the higher acidity of DEAC than other cocatalysts. A similar trend has been observed for the oligomerization of ethylene over nickel complexes in combination with a series of alkylaluminum cocatalysts. This behavior has been assigned to the best combination between the alkylating ability and acidity of the cocatalyst. In accordance with these data, it can be stated that the organochloroaluminate cocatalysts lead to systems more active in nickel catalyzed carbon-carbon bond formation than those obtained with other cocatalysts. It has been reported that the reaction of halocomplexes with MAO in the presence of ethylene or other olefins is presumed to form catalytic cationic active species. This cationic form of organometallic complexes is helpful to polymerization reactions. In our reaction system, DEAC is also presumed to react with the supported nickel α-diimine complex in the presence of ethylene to form similar catalytic cationic active species, while TEA and TIBA probably could reduce the activity of catalyst B excessively and would not form the cationic species.
3.3 Microstructures of polyethylene
The13C NMR spectra of polyethylene products synthesized over catalysts SC-A and SC-B are illustrated in Figure 3. Depending on the catalytic system, it is possible to obtain polymers with different quantities of branches. The polymer in Figure 3(a) has only 1 branch/1 000 C (isolated methyl group). Analysis of the product by13C NMR shows that the PE samples are strictly linear and the end groups are mainly composed of α-olefins[22].
Nickel α-diimine catalyst can produce polyethylene with branch structures according to the mechanism of chain walking without the use of α-olefins comonomers[23]. According to our results, the supported catalyst B shows some effects on the basic modes of chain growth and branching. The DSC results indicate that polyethylene samples exhibit broad melting peaks. It can be suggested that the polyethylene products obtained in our experiment are branched polyethylene. Representative high-temperature13C NMR spectra of polyethylene (Entry 11 in Table 3) prepared over the catalyst SC-B is shown in Figure 3(b). The analysis indicates that the obtained polyethylene isextensively branched mainly with methyl groups. Based on chemical shift calculations performed by the method of Linderman and Adams[24], this sample of polyethylene has 32 branches/1 000 C.
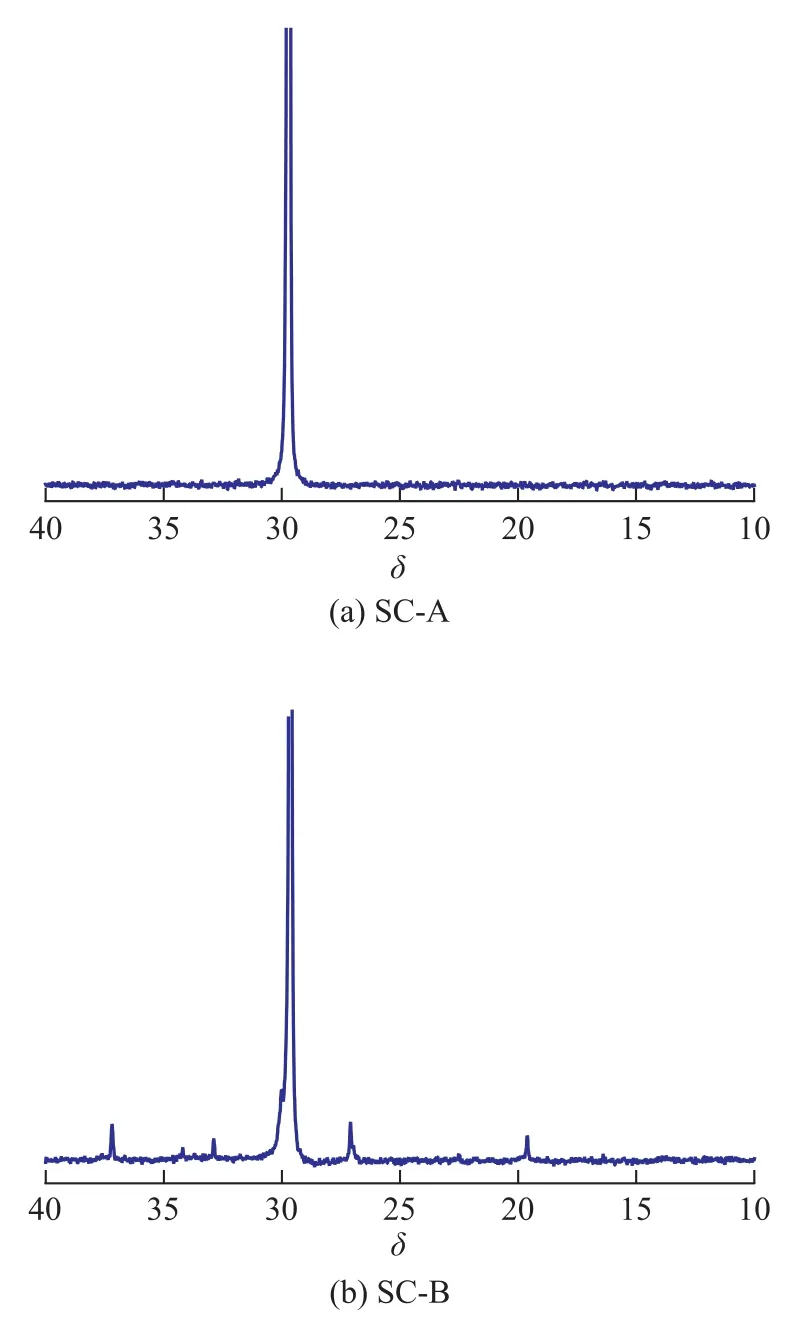
Figure 313C NMR spectra of polyethylene synthesized over catalysts
4 Conclusions
Novel spherical MgCl2/SiO2/THF-supported late-transition metal catalyst systems for ethylene polymerization by spray drying were developed. The particle morphology of the supported catalyst is replicated throughout ethylene polymerization to yield spherical polymers. The molecular weight distribution of PE produced with the immobilized systems was narrower than that obtained over the homogeneous catalytic systems. The pronounced bimodal MWD obtained during homogeneous polymerization was not apparent after catalyst immobilization.
Acknowledgement:This work was supported by the National Natural Science Foundation of China (Grant No.U1162114) and the Science Foundation of Tianjin University of Science & Technology (20090420).
[1] Claudio B, Giuliano G, Lapo L, et al. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands[J]. Coord Chem Rev, 2010, 254(5/6): 431-455
[2] Ittel S D, Johnson, L K. Late-metal catalysts for ethylene homo- and copolymerization[J]. Chem Rev, 2000, 100(4): 1169-1204
[3] Gibson V C, Spitzmesser S K. Advances in non-metallocene olefin polymerization catalysis[J]. Chem Rev, 2003, 103(1): 283-316
[4] Small B L, Brookhart M, Bennet A M A. Highly active iron and cobalt catalysts for the polymerization of ethylene[J]. J Am Chem Soc, 1998, 120(16): 4049-4050
[5] Rossetto E, Caovilla M, Thiele D, et al. Ethylene oligomerization using nickel-α-diimine hybrid xerogels produced by the sol-gel process[J]. Appl Catal A: General, 2013, 454(1): 152-159
[6] Paulino I S, Schuchardt U. Ethylene polymerization using iron catalysts heterogenized in MCM-41[J]. Catal Commun, 2004, 5(1): 5-7.
[7] Ray S, Galgali G, Lele A, et al. In situ polymerization of ethylene with bis(imino)pyridine iron (II) catalysts supported on clay: The synthesis and characterization of polyethylene-clay nanocomposites[J]. J Polym Sci, Part A: Polym Chem, 2005, 43(2): 304-318
我国标准化工作的问题还突出反映在缺乏既具有标准化知识又具有钻井液等专业知识的综合型人才上,虽然最近几年胜利石油管理局加强了对标准制修订人员的技术培训,但是力度还不够。
[8] Kim I I, Han B H, Ha C S, et al. Preparation of silica-supported bis(imino)pyridyl iron (II) and cobalt(II) catalysts for ethylene polymerization[J]. Macromolecules, 2003, 36(18): 6689-6691
[9] Ma Z, Sun W, Zhu N, et al. Preparation of silica-supported late transition metal catalyst and ethylene polymerization[J]. Polymer International, 2002, 51(4): 349-352
[10] Huang R, Koning C E, Chadwick J C. Effects of hydrogen in ethylene polymerization and oligomerization with magnesium chloride-supported bis(imino)pyridyl iron catalysts[J]. J Polym Sci, Part A: Polym Chem, 2007, 45(17): 4054-4061
[11] Zheng Z, Liu J, Li Y. Ethylene polymerization with silicasupported bis(imino)pyridyl iron (II) catalysts[J]. J Catal, 2005, 234(1): 101-110
[12] Jiang H, Lu J, Wang F. Polymerization of ethylene using a nickel α-diimine complex covalently supported on SiO2–MgCl2bisupport[J]. Polym Bull, 2010, 65(8): 767-777
[13] Bahuleyan B K, Oh J M, Chandran D, et al. Highly efficient supported diimine Ni (II) and iminopyridyl Fe (II) catalysts for ethylene polymerizations[J]. Top Catal, 2010, 53(7/10): 500-509
[14] Severn J R, Chadwick J C. MgCl2-based supports for the immobilization and activation of nickel diimine catalysts for polymerization of ethylene[J]. Macromolecules, 2004, 37(17): 6258-6259
[15] Guo C, Jin G, Wang F. Preparation and characterization of SBA-15 supported iron (II)-bisimine pyridine catalyst for ethylene polymerization[J]. J Polym Sci, Part A: Polym Chem, 2004, 42(19): 4830-4837
[16] Huang R, Liu D, Wang S, et al. Preparation of spherical MgCl2supported bis(imino)pyridyl iron (II) precatalyst for ethylene polymerization[J]. J Mol Catal A: Chem, 2005, 233(1/2): 91-97
[17] Liu C, Jin G. Polymer-incorporated iron catalysts for ethylene polymerization—A new approach to immobilize iron olefin catalysts on polystyrene chains[J]. New J Chem, 2002, 26(10): 1485-1489
[18] Britovsek G J P, Bruce M, Gibson V C, et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(imino)pyridyl ligands: Synthesis, structures, and polymerization studies[J]. J Am Chem Soc, 1999, 121(38): 8728-8740
[19] Wang Q, Yang H, Fan Z. Efficient activators for an iron catalyst in the polymerization of ethylene[J]. Rapid Commun, 2002, 23(10/11): 639-642
[20] Lee K S, Oh C G, Yim J H, et al. Characteristics of zirconocene catalysts supported on Al-MCM-41 for ethylene polymerization[J]. J Mol Catal A: Chem, 2000, 159(2): 301-308
[21] Souza C G, Souza R F, Gusma K B. Effect of alkylaluminum cocatalyst on ethylene polymerization with nickel-αdiimine complex[J]. Appl Catal A: General, 2007, 325(1): 87-90
[22] Galland G B, Quijada R, Rolas R, et al. NMR study of branched polyethylenes obtained with combined Fe and Zr catalysts[J]. Macromolecules, 2002, 35(2): 339-345
[23] Johnson L K, Killian C M, Brookhart M. New Pd (II)- and Ni (II)-based catalysts for polymerization of ethylene and alpha-olefins[J]. J Am Chem Soc, 1995, 117(23):6414-6415
[24] Galland G B, Souza R F, Mauler R S, et al.13C NMR determination of the composition of linear low-density polyethylene obtained with [η3-methallyl-nickel-diimine]PF6complex[J]. Macromolecules, 1999, 32(5):1620-1625
Received date: 2014-07-08; Accepted date: 2014-09-06.
Dr. Jiang Tao, E-mail: jiangtao@tust. edu.cn.
猜你喜欢
杂志排行
中国炼油与石油化工的其它文章
- Preparation of Tungsten Film and Its Tribological Properties under Boundary Lubrication Conditions
- Effect of Stirring on Oil-Water Separation in Rare Earth Mixer-Settler
- Spray Characteristics Study of Combined Trapezoid Spray Tray
- Viscoelastic Characteristics of Asphalt Binders at Softening Point Temperature
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Synergetic Effect of Y Zeolite and ZSM-5 Zeolite Ratios on Cracking, Oligomerization and Hydrogen Transfer Reactions
