Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits
2014-05-22
Bioresource Technology Lab, Department of Environmental Sciences, Bharathiar University, Coimbatore 641046, Tamilnadu, India
Abstract The composition of Zizyphus mauritiana mucilage (ZMM) and several properties related to its nutritional quality were investigated.The ZMM with good water holding capacity (25.21 g/g),swelling capacity (19.34 mL/g),glucose dialysis retardation index and in vitro amylolysis kinetics indicate that it may have the capability in controlling the diabetes.Structural characteristics of ZMM were analyzed using the Fourier transform infrared (FT-IR) spectroscopy.In addition,the ZMM has the strong antioxidant potency against ABTS (16,587.32 mmol trolox equiv./g),DPPH(5.27 g mucilage/g DPPH),hydroxyl (76.13%) and superoxide (85.12%) radicals due to the presence of polyphenols (25.54 mg GAE/g mucilage).Besides,the ZMM also inhibited the acetylcholine esterase (86.89%),tyrosinase (47.01%),α-amylase (70.13%) and α-glucosidase (87.14%)enzymes.These properties make the crude mucilaginous fraction from Z.mauritiana fruit an excellent candidate of potential nutraceutical and functional food.
Keywords: Zizyphus mauritiana mucilage; Functional characteristics; Diabetes; Fourier transform infrared (FT-IR); Functional food
1.Introduction
High fiber foods start to gain much attention in the industrial world,due to its importance in human health.Finding new and cheap sources of dietary fiber is extensively needed in developing countries to maintain populational health.The dietary fiber (DF) exists as the soluble or insoluble form; most of the plant foods have the combination of both.The DF endeavors a beneficial role in human health,mainly due to its following attributed qualities.Food rich in DF is unchanged/indigested in the small intestine and it entraps/absorbs the organic molecules(e.g.glucose),which play a potential role in controlling the diabetes.After being absorbed in the large intestine,it is fermented and provides carbohydrates to the colonic bacteria and produces short fatty acid that helps to maintain the colon health[1].Besides,plant-derived DF are also enriched in phytoconstituents which can be considered as dietary antioxidants.Thus,the consumption of diet enriched with DF helps to maintain or reduce the incidence of common disorders such as diabetes,obesity and colon cancer.Most fruits,vegetables and whole grains are good sources of DF.Fruits have relatively more soluble DF(mucilage),i.e.a higher ratio soluble/low insoluble fiber [2].Mucilage is a high molecular weight polyuronides consisting of sugar and uronic acid units.It is partially soluble in water and can form highly viscous solution.It exhibits hampering effect on the diffusion of glucose,and helps to postpone the absorption and digestion of carbohydrates which results in lowered postprandial blood glucose [3].
Zizyphusmauritiana(Indian jujuba) of Rhamanceae family is an indigenous fruit tree of India and grows under varying conditions of climate all over India even at elevations up to 1000 m.Z.mauritianafruits are edible and contain significant levels ofimportant nutrients,including vitamins,minerals,fiber and protein.Interestingly,the content of nutrients inZ.mauritianawas found to be higher than that of some other fruits like mango,guava,orange and strawberry [4].In addition,phytonutrientenrichedZ.mauritianafruits also exhibited antioxidant activity[5].Despite above mentioned facts,the cheap but nutritiveZ.mauritianastill remains underutilized in Indian diet mainly because the consumers have no scientific information about its health promoting effects.The isolated polysaccharides ofZizyphusjujuba(Chinese jujuba) has been characterized,showing antioxidant,hepatoprotective and anti-inflammatory activity[6–8].Most of the information in literatures has been restricted to Chinese jujuba while Indian jujuba requires further research.
Keeping this in mind,the present study isolated the crude mucilage fromZ.mauritianafruit (ZMM) and evaluated its antioxidant activity and functional properties in association withinvitroanti-diabetic effects.Detailed information about the structural and functional characteristics helps us in better understanding its physiological effects and health benefits.
2.Materials and methods
2.1.Chemicals and reagents
All the chemicals used in this study were of analytical grade.2',2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS),butylated hydroxyanisole (BHA),2,2-diphenyl-1-picryl hydrazyl (DPPH),α-amylase,α-glucosidase,tyrosinase,acetylcholinesterase,p-nitrophenyl-α-D-glucopyranoside,L-DOPA,acetylthiocholine iodide andp-nitrophenyl-β-Dglucuronide were purchased from Sigma–Aldrich (St.Louis,MO,USA).All other chemicals were obtained from HiMedia Laboratories (Mumbai,India).Water was treated by arium 67316 reverse osmosis (Sartorius Stedim Biotech GmbH,Germany).All spectrophotometric measurements were done using UV-Vis 100 (Cyberlab,USA).
2.2.Mucilage extraction
The ripen fruits ofZ.mauritianawere purchased from local markets of Coimbatore,Tamilnadu in January 2010.The fruits were thoroughly washed with water to remove dirt and debris.The fresh peel and pulp were separated from seed and mixed with water at a ratio of 1:10.The mixtures were blended using the laboratory blender (Remi Anupam Mixie Ltd,Mumbai,India)and was centrifuged with 3000 ×gfor 30 min.The supernatant was collected and the absolute ethanol was added to the supernatant at the ratio 1:2 to precipitate the mucilage.Then the crude mucilage obtained were collected,dried in the oven at 40°C and ground to fine powder using laboratory blender followed by ball mill MM400 (Retsch,Germany).The powdered crude mucilage was stored in room temperature for further analysis.
2.3.Functional properties
2.3.1.Swelling capacity
0.5 g mucilage powder was precisely weighed into a calibrated cylinder,the volume of solid was recorded (V1) and 10 mL distilled water was added.After mixing,the mixture was left to stand at room temperature for 18 h.The bed volume was recorded (V2).Swelling capacity (SC) was expressed as milliliter per gram of mucilage (mL/g) [9]and calculated as follows:
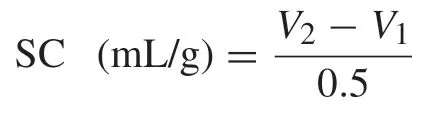
2.3.2.Waterandoilholdingcapacity
Ten milliliters distilled water or refined sunflower oil was added to 0.5 g of three series (triplicate) of mucilage powder in centrifuge tubes.The mixture was stirred carefully and left to stand at room temperature for 60 min.After centrifugation at 3000 ×gfor 15 min,the supernatant was carefully discarded,and the residue was weighed (m).Water holding capacity (WHC)and oil holding capacity (OHC) were expressed as the amount of water or oil retained per gram of mucilage powder (g/g) [9].The WHC and OHC were calculated as follows:

2.3.3.Emulsifyingproperties
The emulsifying activity and stability were determined in triplicates by the methods of Neto et al.[10].Five milliliters of mucilage dispersion in distilled water (10 mg/mL) was homogenized (1 min) with 5 mL refined sunflower oil.The emulsions were centrifuged (1100 ×g,5 min) and the height of the emulsified layer as well as the height of total contents in the tube was determined.The emulsifying activity was calculated as follows:

Emulsion stability (ES) was determined by heating the emulsion (80°C,30 min) before centrifuging (1100 ×g,5 min) and calculated as follows:

The effect of concentration on emulsifying activity and stability of mucilage was studied by preparing 6% (m/V) solution before conducting experiments described above.
2.3.4.Determinationofglucoseabsorptioncapacity
Glucose adsorption capacity was determined according to Ou et al.[3]with slight modifications.A sample of 1 g was mixed with 100 mL glucose solution (concentrations from 10 to 200 mmol/L) and incubated at 37°C for 6 h.When the adsorption reached equilibrium,the sample was centrifuged at 4000 ×gfor 20 min.The glucose content in the supernatant was then determined using glucose assay kit (Sigma–Aldrich,St.Louis,MO,USA).The adsorbed glucose was calculated as the amount of glucose retained by the sample (mmol/g of fiber).
2.3.5.Determinationofglucosedialysisretardationindex(GDRI)
On the basis of the methods of Ou et al.[3]with slight modifications,a mucilage sample (0.5 g),xanthan gum and guar gum(0.5 g) was mixed with 25 mL glucose solution (50 mmol/L),and the mixture solution was dialyzed against 100 mL distilled water at 37°C using a dialysis membrane with a cut off molecular weight of 12,000 Da.After 30,60,90 and 120 min,the glucose content in the dialysate was measured using the glucose assay kit to determine the GDRI as a function of time.A control test was carried out without the addition of fiber.The GDRI was calculated with the following equation:

2.3.6.Effectofthesamplesonamylolysiskineticsinvitro
Forty grams of potato starch were added to 900 mL of 0.05 mol/L phosphate buffer (pH 6.5).The solution,after stirring at 65°C for 30 min,was made up to a final volume of 1000 mL to give a 4% (m/V) starch solution.A starch-α-amylase DF system comprising the above starch solution (25 mL),α-amylase(0.4%),and test samples (1%) were dialyzed in a dialysis bag against 200 mL distilled water at 37°C (pH 7.0) in a shaker water bath.The glucose content in the dialysate was determined at 20,30,60,120 and 180 min,respectively.A control test was carried out without the addition of the sample [3].
2.3.7.Scanningelectronmicroscopy(SEM)
A powder samples of ZMM was loaded on a conducting double-sided carbon tape,sputter coated with gold palladium and scanned using ICON analytical,FEI QUANTA 200.
2.3.8.Fouriertransforminfraredspectroscopy(FTIR)
The FTIR spectrum was used to analyze the chemical structure of the polysaccharide.Fourier transform infrared data was obtained using the Bruker Tensor 27 FTIR spectrophotometer.The spectrum was recorded in the attenuated total reflectance(ATR) mode in the range of 600–4000 cm-1.
2.4.Phytochemical analysis and antioxidant activity
2.4.1.Totalphenoliccontent
The total phenolics content in the crude mucilage was determined according to the method described by Siddhuraju and Becker [11].Known aliquots of the sample solution were taken in a test tube and made up to a volume of 1 mL with distilled water.It was mixed sequentially with 0.5 mL Folin–Ciocalteu(1:1 diluted with water) reagent and 2.5 mL sodium carbonate solution (20%,m/V).The mixture was vortexed and incubated in the dark for 40 min and the absorbance was recorded at 725 nm against a reagent blank.The analysis was performed in triplicates,and the results were expressed as gallic equivalents.
2.4.2.FreeradicalscavengingactivityonDPPH
The radical scavenging activity of extracts was measured using DPPH radical by the method of Sanchez-Moreno et al.[12]with slight modifications.The sample solution of 0.1 mL was mixed with 3.9 mL of DPPH•(0.025 g/L) and incubated in the dark for 30 min.Absorbance was measured at 515 nm and the result was expressed as g extract/g DPPH.
2.4.3.FreeradicalscavengingactivityonABTS•+
The ABTS cationic radical (ABTS•+) decolorization assay was done by the method of Re et al.[13].ABTS•+was generated by adding 2.45 mmol/L potassium persulphate (final concentration) to 7 mmol/L ABTS and incubated in the dark at room temperature for 12–16 h.The stock solution of ABTS•+was diluted with ethanol to give an absorbance of 0.70 ± 0.02 at 734 nm (working solution).10 μL sample was mixed with 1.0 mL ABTS•+working solution and incubated at 30°C for 30 min and the absorbance was measured at 734 nm.The results were expressed as mmol trolox equiv./g extract.
2.4.4.Hydroxylradicalscavengingactivity
Hydroxyl radical (OH•) scavenging activity of extracts was measured using the ascorbic acid-iron-EDTA assay [14].200 μg sample was mixed with 1 mL iron–EDTA solution (0.13% ferrous ammonium sulphate in 0.26% EDTA),0.5 mL of 0.018%EDTA and 1 mL of 0.85% dimethyl sulfoxide (DMSO) solution(in 0.1 mol/L phosphate buffered saline,pH 7.4).The reaction was initiated by the addition of 0.5 mL of 0.22% ascorbic acid and incubated at 80–90°C in water bath for 15 min.After incubation,the reaction was terminated by the addition of 1 mL ice cold trichloroacetic acid (TCA; 17.5m/V).3 mL Nash’s reagent(containing 75 g ammonium acetate,3 mL glacial acetic acid and 2 mL acetyl acetone per liter) was added to the above mixture.And the mixture was allowed to stand at room temperature for 15 min for color development.Absorbance was recorded at 412 nm.The hydroxyl radical scavenging activity (HRSA) was calculated using the following formula:

2.4.5.Superoxideanionradicalscavengingactivity
The superoxide anion radical (O2•-) scavenging capacity of extracts was determined by the method of Martinez et al.[15].Each 3 mL reaction mixture consisting of 50 mmol/L phosphate buffer (pH 7.8),13 mmol/L methionine,2 μmol/L riboflavin,100 μmol/L EDTA,75 μmol/L nitroblue tetrazolium chloride(NBT) and 1 mL sample solution (150 μg/mL) is kept for 10 min under illumination with a 20 W fluorescent lamp.The production of blue formazan was monitored and recorded at 560 nm.The degree of superoxide radical scavenging activity was calculated as follows:
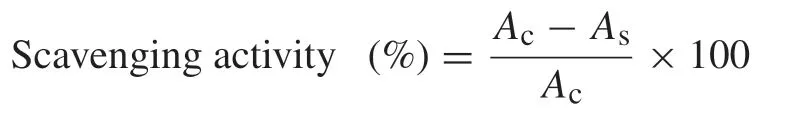
2.5.Analysis of various enzyme inhibitory activities in vitro
2.5.1.α-Amylaseinhibitionactivity
The sample solutions were mixed with 100 μL of 0.02 mol/L sodium phosphate buffer (pH 6.9) and 100 μL ofα-amylase solution (4.5 units/mL/min) and pre-incubated at 25°C for 10 min.Then,100 μL of 1% starch solution was added and incubated at 25°C for 30 min and the reaction was stopped by the addition of 1.0 mL dinitrosalicylic acid.The test tubes were then incubated in a boiling-water bath for 5 min and then cooled to room temperature.The reaction mixture was then diluted 10-fold times with distilled water,and the absorbance was measured at 540 nm[16].The readings were compared to the control (the extract was replaced by sodium phosphate buffer) andα-amylase inhibition activity (%) was calculated as:

2.5.2.α-Glucosidaseinhibitionactivity
The sample solutions were mixed with 100 μL of 0.1 mol/L phosphate buffer (pH 6.9) and 100 μLα-glucosidase solution(1 unit/mL/min) and incubated at 25°C for 5 min.After the pre-incubation,100 μL ofp-nitrophenyl-α-D-glucopyranoside(5 mmol/L) solution was added,and the reaction mixture was incubated at 25°C for 10 min.After the incubation,the absorbance was recorded at 405 nm andα-glucosidase inhibition was calculated as [16]:

where A0 is the absorbance of the control at 540 nm and A1 is the absorbance of the tested sample at 540 nm.
where A0 is the absorbance of the control and A1 is the absorbance of the tested sample.
2.5.3.Tyrosinaseinhibitoryactivity
Tyrosinase inhibitory activity was determined according to the modified method of Chang et al.[17].20 μL tyrosinase(1000 U/mL) was mixed with 100 μL sample solution (dissolved in DMSO) and 1.9 mL of 50 mmol/L phosphate buffer (pH 6.8).The reaction mixture was incubated at 25°C for 5 min.Then,880 μL L-DOPA as a substrate was added to start the reaction.The increase in absorbance was recorded at 475 nm.Kojic acid was used as a positive control.The tyrosinase inhibitory activity was calculated as:

where A0 is the absorbance of the control and A1 is the absorbance of the tested sample.
2.5.4.Acetylcholinesteraseinhibitoryactivity
Acetylcholinesterase (AChE) inhibitory activity was determined according to the modified method of Ellman et al.[18].A 300 μL of 100 mmol/L sodium phosphate buffer (pH 8.0),10 μL sample solution and 40 μL AChE (5.32 × 10-3U) solution were mixed and incubated at 25°C for 15 min and added to 20 μL of 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) (0.5 mmol/L).Then the reaction was initiated by adding 20 μL acetylthiocholine iodide (0.71 mmol/L).The absorbance of the reaction mixture was read at 412 nm.Eserine was used as a reference compound.The inhibition of AChE was calculated by using[(E-S)/E]× 100,whereEis the activity of enzyme without the test sample andSis the activity of enzyme with the test sample.
2.6.Statistical analysis
The data were subjected to one-way analysis of variance(ANOVA),and the significance was determined by Duncan’s multiple-range test (P< 0.05) using SPSS 13.Values expressed are means of triplicate determinations ± standard deviation(n= 3).Pearson’s correlation test was conducted to determine the linear correlations among variables.
3.Results and discussion
3.1.Functional properties of Z.mauritiana mucilage
Hydration properties of mucilage refer to its ability to retain water within the matrix.Fiber with strong hydration properties will increase stool weight and its viscosity properties impedes absorption of macronutrients resulting in increased insulin sensitivity,increased satiety and decreased energy intake.The WHC of crude ZMM was found to be 25.21 g/g (Table 1).The WHC of crude ZMM is higher than those reported in DF of some fruits(e.g.apple,pear,banana,lime peel and bambangan peel) [19,21]which is in the range of 6.3–12.8 g/g.But in comparison with pitaya peels (54.20 g/g) [22]WHC of ZMM is found to be lower.The ability to increase in bulk after absorbing water is one of the important functional properties of fibers.The SC of crude ZMM (19.34 mL/g) is similar to that of coconut (18.3 mL/g)and mango fiber (18.7 mL/g),but it is lower than that of pitaya(35.95 mL/g) and carrot (60.2 mL/g) DF [19,22].It has been suggested that WHC and SC might possibly be affected by the amount of hydrophobic polysaccharide like soluble DF,which is contained within the cell wall of plants [23].Therefore,the high amount of soluble DF in crude ZMM possessed WHC and SC through associating with absorption of large amounts of water.The high WHC of soluble fiber has an effect on their viscosity;the viscous fiber in the intestinal content reduces the absorption of glucose in the gut which helps to reduce the postprandial blood-glucose level.
DF is able to retain oil thus able to absorb/bind bile acids and increase their excretion which is associated with plasma cholesterol reduction.The OHC of crude ZMM (12.53 g/g,Table 1)is much higher than that of other reported DF such as orange,mango,pitaya fiber,which is in range of 1.27–4.65 g/g [21,24].OHC depends on the surface properties of fiber,thickness and the hydrophobic nature of the fiber particle.The exact nature of the interaction between the bile acids and dietary fiber is still unclear to date.It may be due to the hydrophobic nature of DF,which form intraluminal micelle and reduces cholesterol content in plasma,leading to up regulation of LDL receptors and increased clearance of LDL cholesterol [25].
EC is a molecule’s ability to act as an agent that facilitates solubilization or dispersion of two immiscible liquids and ES is the ability to maintain an emulsion and its resistance to rupture.EC and ES of crude ZMM were found to be 54.12% and 42.14%,respectively (Table 1).Mucilage has effective EC via interfacial absorption and the subsequent formulation of condensed films with high-tensile strength that resist coalescence of droplets.Furthermore,it stabilizes oil/water emulsions byforming a strong multimolecular film around each oil globule and thus retards the coalescence by the presence of a hydrophilic barrier between the oil and water phases [26].The EC of fiber is very indicative in health potential benefits.Since it absorbs biliar acids,absorption of these acids in the small intestine and excretion in the feces helps to reduce blood cholesterol levels [27].Therefore,intake ofZ.mauritianafruit enriched in fiber might be helpful to reduce hypercholesterolemic effects (Table 1).

Table 1 Functional properties of ZMM.
3.1.1.Glucoseabsorptioncapacity
The effect of dietary fiber in the absorption of the glucose at different concentrations (10–200 mmol/L) was investigated.This value predicts the behavior of fiber on absorbing the glucose in the gastrointestinal tract.Table 2 reveals that crude ZMM,xanthan and guar gum could bind glucose(0.15–0.97 to 15.34–19.80 mmol/g) at different concentrations(10–200 mmol/L) effectively,and the amount of glucose bound to these dietary fibers were concentration dependent.The absorption of trace amount of the glucose (10 mmol/L) attributes to the fact that crude ZMM could keep the glucose in the intestinal lumen even at low concentrations.In general,the glucose absorption capacity of crude ZMM was found to be lower than that of xanthan and guar gum.
3.1.2.Effectonglucosediffusionandglucosedialysis retardationindex(GDRI)
The effect of various samples on the movement of glucose across the dialysis membrane is presented in Table 3.The diffusion of glucose was time-dependent,and higher amounts of glucose were found in the dialysate with the increasing of time from 30 to 120 min.The glucose content in the dialysate of crude ZMM,xanthan gum and guar gum were elevated from 165.0 to 329.11 μmol,153.0 to 215.54 μmol,and 160.3 to 224.43 μmol respectively,as the time increased from 30 to 120 min.Compared with the control (190.05–412.11 μmol),all the samples significantly reduced the content of diffused glucose.Based on the retardation of glucose diffusion in the dialysis membrane,GDRI for all the samples was obtained (Table 4).GDRI is a usefulinvitroindex to predict the effect of fiber on the delay of glucose absorption in the gastrointestinal tract [28].The GDRI of crude ZMM at 30 min was 37.07%; as the time increased from 60 to 120 min,GDRI generally was diminished,but the retarding effect of crude ZMM in all the time duration was found to be lower compared with that of the xanthan and guar gum.The GDRI value of crude ZMM at 60 min in this study was lower than those reported for mango peel,apple pectin and bambangan peel fiber [19].The mechanism of action in polysaccharides for glucose reduction is mainly due to the formation of viscous gel in aqueous solution by soluble fiber.Viscosity reduces the accessibility of glucose to the absorptive epithelium in the small intestine,thereby blunting postprandial glucose level.This phenomenon reduces the diffusion rate of glucose and may have a potential benefit to control postprandial blood glucose.
3.1.3.Effectofdietaryfiberondiffusionofglucoseina starch-α-amylase-dietaryfibersystem
In humans,starch and its derivatives are digested in several stages.In the mouth,in contact with salivaα-amylase,the starch polymeric chains are cleaved into shorter oligosaccharides.Upon entering the gut,the partially digested material is further hydrolyzed by human pancreaticα-amylase.The main resulting products,maltose and branched dextrins,are converted into glucose by the brush border maltose-glucoamylase and sucrase-isomaltase and then enter the blood stream.Thus plasma glucose level increases about 10 min after a meal containing carbohydrates.We have examined the effect of crude ZMM on the activity ofα-amylase to hydrolyze starch by measuring the amount of glucose in dialysate (Table 4).The diffused glucose content in the dialysate of crude ZMM,xanthan and guar gum were elevated from 110.01 to 378.65 μmol,129.12 to 441.64 μmol and 101.16 to 431.39 μmol respectively,as the time increased from 20 to 180 min.Compared with the control(333.16–866.23 μmol),all the samples significantly reduced the content of diffused glucose.The crude ZMM has reduced the glucose diffusion.However in comparison with the xanthan and guar gum,the crude ZMM showed lower glucose diffusion at all time intervals.The retardation of glucose diffusion is also due to the inhibition ofα-amylase,thereby limiting the release of glucose from the starch.The inhibition ofα-amylase activity might be attributed to several factors such as fiber concentration,the presence ofinhibitors in fibers,encapsulation of starch and enzyme by the fibers present in the sample.These may reduce the accessibility of starch to the enzyme and direct adsorption of the enzyme on fibers,leading to decreased amylase activity [3].Inhibitors of carbohydrate hydrolyzing enzymes delay carbohydrate digestion and prolong overall carbohydrate digestion time,causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise.These observations emphasize that inhibition ofα-amylase is one of the probable mechanisms through which the samples exert its hypoglycemic effect.Further studies are needed to investigate whether the DF consist inhibitors ofα-amylase or simply act as a barrier between enzyme and starch.
3.1.4.FT-IRstudyandSEMexaminationofZMM
FT-IR spectroscopy has been shown to be a useful tool in monitoring structural characteristics.The FT-IR spectrum of crude ZMM is shown in Fig.1.The FT-IR spectra of ZMM showed broad O-H stretching at 3365 cm-1.A short peak observed at 2918 cm-1corresponds to antisymmetric-CH2.The sample exhibited the characteristic’s IR absorption of polysaccharides at 1706 cm-1,which corresponds to carbonyl groups(C=O).The aromatic C=C stretching appeared at 1583 cm-1and C-O stretching of phenolic OH and aryl methyl esters appeared at 1218 cm-1.Combination of C-O stretching and OH deformation appeared at 1010 cm-1,which is a fingerprint region for all the fiber samples.Based on this information,we proposed that the high WHC and OHC of mucilage may be due to the presence of hydroxyl groups.

Table 2 Glucose-adsorption capacity of ZMM,xanthan gum and guar gum at different glucose concentrations.

Table 3 Effect of ZMM,guar gum and xanthan gum on glucose diffusion and GDRI.

Table 4 Effect of mucilage on diffusion of glucose in a starch-α-amylase-DF system.

Fig.1.FT-IR spectrum of ZMM.
The morphological characteristic of crude ZMM was visualized by SEM and shown in Fig.2.It showed more irregular and uneven surfaces with large number of cracks.The available surface and the regiochemistry of the surface layer might play a role in some physicochemical properties (adsorption or binding of some molecules) accounting for some physiological effects DF [29].
3.2.Total phenolic content and antioxidant activity of ZMM
Phenolic compounds are major contributors of the antioxidant capacity of fruits and vegetables.Polyphenols associated with polysaccharides behave as DF constituents.Bioactive compounds (polyphenols) in the polysaccharides have been shown to be protective against various diseases like atherosclerosis,diabetes and neurodegenerative disorders mostly through their antioxidant and free radical scavenging capacities.The total phenolic content of crude ZMM was 25.54 mg/g (Table 5),which was higher than that of bambangan peels (98.3 mg/g)[19],Hayden mango peels (70 mg/g) [30]and Indian mango peel (96.2 mg/g) [20],but similar to that of unripe bambangan peels (24 mg/g) [31].The phenolic compounds in the crude ZMM possessed potential antioxidant activity (Table 2).The value of DPPH and ABTS free radical was expressed as IC50g DPPH/g mucilage and mmol trolox equiv./g mucilage and both the assays were compared with BHA.A lower IC50value reflects a higher scavenging activity.In scavenging the DPPH•and ABTS•+,BHA (0.15 g DPPH/g extract; 654,356.06 mmol trolox equiv./g) exhibited higher radical scavenging activity than the crude ZMM (5.27 g DPPH/g extract; 16,587.32 mmol trolox equiv./g mucilage).Thus,the results indicated that ZMM could also effectively react with DPPH•and ABTS•+to convert them to more stable products by donating the proton and terminating the radical chain reaction.
The superoxide and hydroxyl radical are the powerful oxidizing agents that can react with biological membranes and induce tissue damages.The scavenging capability of these radicals by crude ZMM is directly related to its antioxidant activity(Table 2).The superoxide and hydroxyl radical scavenging ability of crude ZMM was found to be 85.12% and 76.13%,which is higher than that of the previously reportedPhysalisalkekengifruit polysaccharides (17.1%; 21.6%) [32].Compared with the positive control catechin,catechin exhibited higher radical scavenging activity than the crude ZMM.The phenolic compounds in the crude ZMM possessed antioxidant capability toward scavenging DPPH,hydroxyl and super oxide radical; hence,the present study points out crude ZMM as an important antioxidant DF source.

Fig.2.Scanning electron micrograph of ZMM.

Table 5 Total phenolics,DPPH radical,ABTS cation radical,superoxide and hydroxyl radical scavenging activity of ZMM.

Fig.3.Effect of α-amylase,α-glucosidase,acetylcholine esterase and tyrosinase enzyme inhibitory activity by ZMM.Note:ZMM; ACR,acarbose; ESR,eserine;KOJ,kojic acid.
3.3.Effect of ZMM on inhibition of various enzymes in vitro
Earlier reports have revealed that oxidative injury plays a main function in pathogenesis of many diseases,including diabetes,Alzheimer’s disease and skin pigmentation.Besides,oxidation may also change the nutritional quality of food.The crude ZMM,with the presence of polyphenols,inhibits the enzymes responsible for above-mentioned facts.The inhibitory activity ofα-amylase,α-glucosidase,acetylcholine esterase and tyrosinase enzymes is shown in Fig.3.Inhibition of enzymes involved in the hydrolysis of carbohydrates such asαamylase andα-glucosidase have been exploited as a therapeutic approach in controlling postprandial hyperglycemia.Pancreaticα-amylase is involved in the breakdown of starch into disaccharides and oligosaccharides before intestinalα-glucosidase catalyzes the breakdown of disaccharides to liberate glucose,which is later absorbed from the blood circulation.Inhibition of these enzymes would slow down the breakdown of starch in the gastro-intestinal tract,thus reducing postprandial hyperglycemia [33].Theα-amylase andα-glucosidase inhibitory activity of crude ZMM was compared with the control acarbose.The inhibitory effect of crude ZMM onα-amylase andα-glucosidase was 70.13% and 87.14%,respectively,at the concentration of 250 μg/mL.The inhibitory activity of acarbose is high inα-amylase activity,but low forα-glucosidase in comparison with the crude ZMM.Our results were in agreement with those of previous studies,which have demonstrated that plantbased phenolic phytochemicals have lowerα-amylase inhibitory action but stronger inhibitory potential againstα-glucosidase[34],and such natural enzyme inhibitors is likely to offer an attractive therapeutic approach to the treatment of postprandial hyperglycemia,due to lower abdominal side effects arising from excessive inhibition of pancreaticα-amylase by synthetic drugs(acarbose,miglitol and metformin),which results in the abnormal bacterial fermentation of undigested carbohydrates in the colon.Therefore,these results indicate that crude ZMM rich in phenolics have the potential in contributing to the management of type II diabetes.
Tyrosinase,a well-known polyphenol oxidase,widely existing in plants and humans,is responsible for the biosynthesis of melanin.Tyrosinase (binuclear copper containing enzyme),converts the tyrosine to DOPA quinine through monophenolase and diphenolase,which polymerizes to form melanin.Melanin determines the actual color of skin,and it protects skin from UV radiation.The reactive quinine mixes with the polyphenolic subtracts in the presence of oxygen through nonenzymatic coupling and spontaneously evolves to form an enzymatic browning in foods and hyper pigmentation in humans.Enzymatic browning affects the color,flavor and nutritional quality of food and hyperpigmentation affects the skin health of humans.Therefore,the development of natural tyrosinase inhibitor is an important alternative approach in maintaining the food quality and skin health.The inhibitory activity of crude ZMM compared with kojic acid (positive control) was evaluated.Although the tyrosinase inhibitory activity of crude ZMM (47.01%) is lower than that of the kojic acid (90.87%),it is still able to increases the anti-tyrosinase activity due to its antioxidant power.Tyrosinase inhibitors usually chelate with copper within the tyrosinase active site thereby obstructing the substrate–enzyme interaction,or prevent oxidation via an electrochemical process [35].However,the presence of the hydroxyl group in crude ZMM may form hydrogen bond with the active site of enzyme and inhibit the tyrosinase activity.Therefore,crude ZMM may be applied in medicinal,cosmetic and food industry to maintain the skin health and food quality.
The ACh is one of the major compounds by which nerve impulses are transmitted from a neuron to neuron or involuntary muscle.The function of ACh in the nerve impulse transmission is terminated by AChE at the cholinergic synapses by rapid hydrolysis of ACh to choline and acetate.This results in a strategy of treating Alzheimer’s disease,senile dementia,ataxia,myasthenia gravis and Parkinson’s disease [36].Few synthetic medicines (e.g.acrine,donepezil) and the natural product-based rivastigmine are used in the treatment of cognitive dysfunction and memory loss associated with Alzheimer’s disease.These compounds have been reported to have their adverse effects,including gastrointestinal disturbances and problems associated with bioavailability [37],which necessitates the interest in finding better AChE inhibitors from natural resources.The interaction of crude ZMM and eserine (positive control) with AChE was evaluated.The results reveal that crude ZMM inhibited the AChE (57.01%),but it was lower compared with eserine(86.89%).It could be postulated that AChE inhibition activity of crude ZMM might be due to phenolic compounds.
4.Conclusion
The crude mucilage isolated from theZ.mauritianafruit acts as a nutraceutical product through various physiological effects.The role of crude ZMM in controlling the postprandial serum glucose level may be illustrated by three mechanisms.Firstly,ZMM with good WHC and SC,increases the viscosity of small intestine content and hinders the diffusion of glucose.Secondly,glucose binds with the mucilage and decreases the amount of available glucose in the small intestine with high GDRI.Thirdly,it retards theα-amylase andα-glucosidase action and postpones the release of glucose due to the presence of fiber associated total polyphenols.In addition,ZMM exhibited strong antioxidant capacity through scavenging DPPH,ABTS,hydroxyl and superoxide radicals.Besides,promising AChE and tyrosinase inhibitory action reveals that mucilage also has a capability to control the Alzheimer’s disease,skin diseases and food spoilage.Therefore,the novel crude mucilaginous fraction isolated fromZ.mauritianafruit is an excellent source toward its development as a potential nutraceutical/functional food in the prevention and control of diabetes,Alzheimer’s disease and skin disease.Furthermore,it is also important to study the detailed structure and conformation of the above-mentioned polysaccharides and their impacts on health effects throughinvivoexperiments.
Acknowledgement
The financial support by DST-PURSE,New Delhi,India is gratefully acknowledged.
杂志排行
食品科学与人类健康(英文)的其它文章
- Effect of casein glycomacropeptide on subunit p65 of nuclear transcription factor-κB in lipopolysaccharide-stimulated human colorectal tumor HT-29 cells
- In vitro antioxidant,antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss.fruit pulp
- Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage
- Purification,initial characterization and immune activities of polysaccharides from the fungus,Polyporus umbellatus
- About the Beijing Academy of Food Sciences
- What could probiotic do for us?
