Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage
2014-05-22MohmmdJoukiSeyedAliMortzviFridehTteiYzdiArshKoochekiNimehKhzei
Mohmmd Jouki Seyed Ali Mortzvi Frideh Ttei Yzdi Arsh Koocheki Nimeh Khzei
a Department of Food Science and Technology, Faculty of Agriculture, Ferdowsi University of Mashhad, P.O.Box 91775-1163, Mashhad, Iran
b Department of Food Science and Technology, Faculty of Agriculture, University of Urmia, Urmia, Iran
Abstract In this study quality changes of rainbow trout fillet wrapped with quince seed mucilage QSM film incorporated with 0–2% (V/V) thyme or oregano essential oil,as natural preservatives,during storage of 18 d at 4°C were investigated.The control and wrapped fillet samples were analyzed for texture,lipid oxidation,and color characteristics.After 6 d of storage,a significantly increasing trend was observed for lipid oxidation in control samples.Peroxidation values (PV) varied for all treatments and remained lower than 8 meq/kg throughout the storage time (18 d).The lowest PV was obtained in fillets wrapped with QSM film containing 2% oregano essential oil.Compared to control samples,fillet samples wrapped with QSM films presented a significant reduction in pH after 12 d (P < 0.05).L* and b* color parameters were increased while parameter a* was decreased linearly for all treatments during the storage time.
Keywords: Edible films; Color; Texture; Lipid oxidation; Rainbow trout fillet
1.Introduction
Fresh fish are perishable foods,which generally spoil faster than other muscle foods [1].This is due to the large amounts of free amino acids and volatile nitrogen bases as well as higher final pH of fish that limit the shelf life of the product [2].Mohan et al.[3]stated that the enzymatic and chemical reactions are usually responsible for the initial loss of freshness,while microbial activity is responsible for subsequent spoilage.Several researches on edible or biodegradable films and edible antimicrobial films based on protein,starch or polysaccharides have been carried out [4–7].
The heightened demands by consumers for better quality and improved freshness of food products have given rise to the development and implementation of edible films.These materials present the possibility of being carriers of different additives,such as antimicrobial,antioxidant,nutraceuticals and flavoring agents [5,6].Among different biodegradable edible films,quince seed mucilage (QSM) films possess hydrophilic characteristics and exhibit good barrier,mechanical and antioxidant properties[8].As was suggested by Schmidt [9],QSM is a complex of a cellulosic fraction with more readily hydrolyzed polysaccharides.Lindberg et al.[10]reported that the major water soluble polysaccharides in the mucilage of the seeds of quince is a partially-O-acetylated (4-O-methyl-D-glucurono)-D-xylan with an exceptionally high proportion of glycuronic acid residues.
In the previous studies theinvitroantimicrobial activity of QSM films containing oregano or thyme essential oil against some pathogenic bacteria (Salmonellatyphimurium,Pseudomonasaeruginosa,EscherichiacoliO157,Listeria monocytogenesandVibriocholera) have been evaluated [5,6].Also the effect of these films on microbiological (aerobic and psychrotrophic count,Pseudomonasspp.,H2S-producing bacteria,lactic acid bacteria,andEnterobacteriaceae),chemical (tert-butanol (TBA),total volatile basic nitrogen (TVB-N),trimethylamine-nitrogen (TMA-N)),and sensory characteristics of rainbow trout fillets has been studied [11].However,some important physico-chemical characteristics of rainbow trout fillet wrapped with QSM films incorporated with oregano or thyme essential oil such as texture,color,pH,peroxide value (PV) have not been studied.The objective of this work is to evaluate the chemical (PV and pH),textural and color characteristics of rainbow trout fillets wrapped with QSM films incorporated with different concentrations of oregano or thyme essential oil and their correlations with studied parameters.
2.Materials and methods
2.1.Preparation of fish fillet
Rainbow trout (Oncorhynchusmykiss) with an average weight of (550 ± 70) g were purchased from a farm in Savojbolagh,Alborz,Iran.Fish were placed in crushed ice and transported to the Food Science Department at University of Tehran within 15 min after catching.They were eviscerated,headed and filleted (approximately 25 cm × 10 cm and weight(165 ± 10) g per fillet) by hand.
2.2.Quince seeds and essential oils
Quince seeds used in this study were purchased from the local market at Tehran in Iran.Oregano and thyme essential oils were purchased from New Directions Aromatics Inc.(Hampshire,UK) and Zardband Company (Tehran,Iran),respectively.Glycerol and Tween 80 (Fluka,Sigma–Aldrich,St.Louis,MO,USA) were used to prepare film-forming dispersions (FFD).
2.3.Film solutions
QSM was prepared according to the method of our previous work [12].Film solution was prepared by slowly dissolving 1%mucilage and glycerol as a plasticizer in 35% (m/m) based on QSM weight under constant stirring (750 r/min) at (45 ± 2)°C for 15 min.Then oregano or thyme essential oil (1,1.5,or 2%,V/V) was dissolved in the solution with agitation using a magnetic stirrer for 1 h at room temperature (25°C).Tween 80 was added as a surfactant at concentrations between 0.1 and 0.2%(m/V).The solution was homogenized (IKA T25-Digital Ultra Turrax,Staufen,Germany) at 12,000 r/min for 5 min to obtain an emulsion [5].Finally,the emulsion was placed into a centrifuge for 3 min at 3800 ×gto remove air bubbles.
2.4.Preparation of antimicrobial–antioxidant films
The film solution (55 mL) was placed on the Teflon coated glass petri dishes (13 cm in diameter).The castings were placed in a fume hood,which was maintained at (37 ± 2)% relative humid and (25 ± 2)°C,for 24 h.The dried QSM films were peeled from the petri-dishes and theirs thickness were measured with a digital micrometer (Mitutoyo No.293-766,Tokyo,Japan)at five random positions on the films.
2.5.Application of films on fish fillets
Fillet samples with average weight of (55 ± 5) g were randomly divided into eight groups:treatment groups consistedof (1) unwrapped samples (control),(2) samples wrapped by QSM film without addition of essential oils (QSMF),(3) samples wrapped by QSM film containing 1% oregano essential oil(QSMF + 1% O),(4) samples wrapped by QSM film containing 1.5% oregano essential oil (QSMF + 1.5% O),(5) samples wrapped by QSM film containing 2% oregano essential oil(QSMF + 2% O),(6) samples wrapped by QSM film containing 1% thyme essential oil (QSMF + 1% T),(7) samples wrapped by QSM film containing 1.5% thyme essential oil (QSMF + 1.5%T),(8) samples wrapped by QSM film containing 2% thyme essential oil (QSMF + 2% T).Wrapped fillets were subsequently labeled and stored at 4°C.The sliced rainbow trout fillets were sampled for examination at storage days of 0,3,6,9,12,15 and 18.On each sampling occasion,fish slices from every batch were subjected to physico-chemical analyses.

Table 1 Chemical composition and their relative abundance of oregano and thyme essential oils.a
2.6.Chemical composition of rainbow trout fillets
The moisture content and crude ash of the fish were determined in an oven at 103°C and 550°C,respectively,until the weight became constant [13].Lipid content was determined by Bligh and Dyer’s [14]method.The total crude protein content was calculated from the nitrogen content determined by Kjeldahl’s method [15].Analyses were conducted in triplicate,and the moisture,protein,lipid and ash contents of the fillets averaged 68.23%,22.54%,3.89% and 3.69%,respectively.
2.7.Chemical composition of essential oils
For the quantification ofindividual components,the essential oils were analyzed using a Hewlett-Packard 6890 series gas chromatograph (Perkin Elmer (PE) Auto System XL,Waltham,MD,USA),connected to a mass spectrometric detector (Hewlett-Packard,model 5973,Agilent Technologies,Wilmington,DE,USA).A capillary column DB-5MS(60 m × 0.320 mm,1 μm) was used for the separation ofindividual components of the essential oil.The chemical composition of essential oils was determined according to the method described by Karabagias et al.[16].Identification of compounds was achieved by comparing the mass spectra of the recorded chromatographic peaks with the Wiley 275 MS database.The major components in oregano and thyme essential oils are listed in Table 1.It can be seen that they are greatly different in their chemical compositions.Thymol (46.42%),p-cymene (22.31%)and carvacrol (12.42%) were the representative components of thyme essential oil.In the case of oregano essential oil,carvacrol(81.85%) was the major component.
2.8.pH measurements
10 g of each sample was homogenized in100 mL of distilled water and the mixture was filtered.The pH of filtrate was measured using a pH meter (HI 221,Hanna Instruments,Woonsocket,RI).
2.9.Estimation of peroxide value
The peroxide content was determined in the total lipid extracts according to Egan et al.[17].Results were expressed in meq oxygen/kg lipid.
2.10.Texture analysis
Texture analyses were performed at room temperature using a Testometric Machine M350-10CT (Testometric Co.Ltd.,Rochdale,Lancashire,England) equipped with a 500 N load cell.Hardness was calculated as described by Liu et al.[18].Trout fillets were cut into small cubes (2.0 cm × 2.0 cm × 2.0 cm) and kept in refrigerator prior to texture analysis.Each sample was compressed using a flat-ended aluminum cylindrical plunger(50 mm diameter) at a constant test speed of 1 mm/s until it reached 60% deformation.Compression of 60% was chosen to ensure a breaking point in almost all samples [19].The compression force was perpendicular to the muscle fiber orientation.The maximum force during compression was recorded as hardness.Analysis was carried out to determine the effect of wrapping treatments and storage time of 0,3,6,9,12,15 and 18 d on the texture of rainbow trout fillets.
2.11.Color measurements
A portable colorimeter (Minolta CR360 Series,Minolta Camera Co.Ltd.,Osaka,Japan) with the following settings was used to measure the meat color in the CIE LAB space (lightness,L*; redness,a*; yellowness,b*) [20].Color measurements were carried out directly on fresh and wrapped/chilled stored fillets.Total color difference (ΔE) was calculated using following equation [21]:
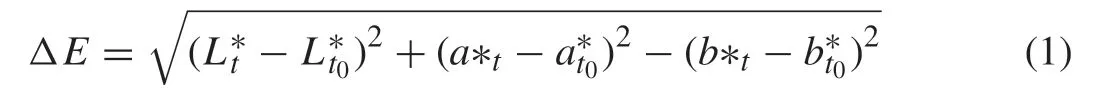
wheretrefers to timetandt0refers to time 0.A mean value of 6 measurements was reported for each color attribute.
2.12.Statistical analysis
A minimum of three observations were collected unless specified otherwise.Microsoft Windows Excel 2007 and SPSS software (Version18.0,SPSS Inc.,Armonk,NY,USA) were used to analyze the data.Data were initially evaluated by analysis of variance (ANOVA),and then a Duncan’s multiple range test was employed to evaluate significant (P< 0.05) differences.

Fig.1.Changes in pH values of unwrapped (control) and wrapped rainbow trout fillets during storage at 4°C.
3.Results and discussion
3.1.Chemical composition rainbow trout fillets
The mean (±SD) compositional contents of moisture,protein,lipid,and ash (g/100 g fish muscle) in the rainbow trout fillet are presented in Table 2.Moisture,protein,lipid and ash contents of the rainbow trout meat averaged 68.23%,22.54%,3.89%and 3.69%,respectively.The results are in good agreement with those reported by Ojagh et al.[7].The present values are favorably comparable with the published reports in different studies[7,22,23](presented in Table 2).As shown in Table 1,rainbow trout fish studied in the present study had lower lipid content(3.89%) and higher protein content (22.54%) when compared to the reported findings by Celik et al.[22],however Gokoglu et al.[23]reported lower lipid content (1.35%) and lower protein content (19.80%).Results in Table 2 showed some differences especially for the lipid content.As González-Fandos et al.[24]stated,such variations in the chemical composition of fish is related to the nutrition,age and size of the fish,living area (quality of water,its pH and temperature),sexual variation,catching season (spawning cycles),as well as other environmental conditions.
3.2.pH measurement
Changes in pH of refrigerated rainbow trout fillets during storage at 4°C are shown in Fig.1.The initial pH of the rainbow trout fillets was 6.34 which was in agreement with that of Arashisara et al.[25]and Gimenez et al.[26].As shown in Fig.1,the pH of rainbow trout fillets decreased until day 3 and increased afterwards.The primary decrease in pH during postmortem is related to the glycogenolysis that occurs after death in fish [27].The reduction of pH in the samples wrapped with QSM films could be related to migrations from the coating itself,which possessed an acid pH value (5.7),to fish fillet.A slight drop in pH was also observed in the samples wrapped with QSM films enriched with essential oils at the beginning of the storage period (Fig.1).This phenomenon could also be contributed to the extending of the preservation of fish by inhibiting the activity ofinternal enzymes and microbial growth in the presence of essential oils [28].Antimicrobial activity of QSM films incorporated with thyme or oregano essential oil was evaluated in our previous studies [5,6].The QSM films containing thyme or oregano inhibited the growth of 11 strains of microorganisms to a varying degree.The antimicrobial activity of thyme and oregano has been attributed to their essential oils,which contain the terpenes:carvacrol (2-methyl-5-[1-methylethyl]phenol) and thymol (5-methyl-2-[1-methylethyl]phenol),respectively [29].Other researchers reported similar results for gilthead sea bream and Pearl spot [30,31].The pH of rainbow trout fillets increased(P< 0.05) during storage.The increase in pH was postulated to be due to the increase in volatile bases produced,e.g.ammonia and trimethylamine,by either endogenous,bacterial metabolites or microbial enzymes [1].As shown in Table 4 correlation between pH and TVB-N,between pH and TVB-N and between pH and TVC was positive and strong.This pH increase has a pronounced negative effect on the quality of the product during storage,especially in terms of sensorial characteristics such as odor,color,and texture,which are negatively affected [1].The application of the pure QSM films indicated no statistically significant effect (P>0.05) on initial pH values when compared with control fish samples.After 6 d of storage at 4°C,the pH value of the control sample (6.51) was significantly (P < 0.05)higher than that of samples wrapped with QSM films (6.46).

Table 2 Proximate composition of the muscle of rainbow trout (Oncorhynchus mykiss).a

Table 3 Color of rainbow trout fillet samples unwrapped and wrapped with QSM films during storage at 4°C for 18 d.a

Fig.2.Changes in PV of fillet samples during refrigerated storage.
3.3.Peroxide value (PV)
Primary lipid oxidation was evaluated by means of PV.The effect of QSM films on changes of PV of rainbow trout fish lipids is depicted in Fig.2.The initial PV (meq peroxide/kg fish sample) in the analyzed trout fillet samples ranged from 1.22 to 1.31.No significant difference (P>0.05) was observed at the first day of storage among different samples.PV significantly increased (P< 0.05) in all treatments throughout the refrigerator storage for all samples,indicating that lipid deterioration continued under the storage temperature conditions (Fig.2).As expected,a positive and high correlation was shown between PV and TBA (Table 4).The PV of rainbow trout fillets wrapped with QSM film (QSMF) was significantly lower than that of unwrapped fillets (control),indicating that the QSM films were effective in reducing lipid oxidation (Fig.2).The QSM film layer on the fish surface was resistant to oxygen diffusion,thus could retard lipid oxidation.We reported that the films based on QSM had a good oxygen barrier and antioxidant properties[5].The results of the present study indicated that QSM-based films enriched with oregano or thyme essential oils were effective in reducing PV in rainbow trout fillets stored at (4 ± 1)°C.The results also showed that the highest level of oregano or thyme essential oils (2%,V/V) had the most obvious effect in slowing down the primary peroxidation process,compared with others (P< 0.05).As we previously reported,oregano and thyme essential oils showed good antioxidant activity when they were incorporated in the QSM film [5,6].On day 18 of storage,PVs of samples wrapped with QSM film incorporated with 2% oregano essential oil (QSM + 2% O) were 43% lower than that of the control sample (Fig.2).This could be attributed to high amounts of carvacrol (81.85%),the major component of oregano essential oil.We also reported that the antioxidant activity of QSM films containing oregano essential oil was higher than that of QSM films incorporated with thyme essential oil [5,6].Meanwhile,PVs of all fish samples were lower than 10 meq/kg of oil until day 18 of storage.In addition,in present study the fillets were stored in a dark refrigerator and were protected from light.These conditions most likely contributed to the inhibition of lipid oxidation even at low essential oil concentrations (1%).Furthermore,PVs of wrapped fish samples increased over time,but at a slower rate compared to the control samples.After 9 days of storage,PVs of unwrapped samples decreased,perhaps due to the breakdown of hydroperoxides or their interaction with muscle proteins [32].Storage time had a significant effect on the PV of each rainbow trout fillets,nonetheless PVs in all samples were well below the proposed acceptable level of 10–20 meq peroxide/kg fish fat [33].These results are in agreement with those of Ojagh et al.[7],who reported that chitosan coating enriched with essential oil was effective in retarding the production of primary lipid oxidation products in refrigerated rainbow trout samples.
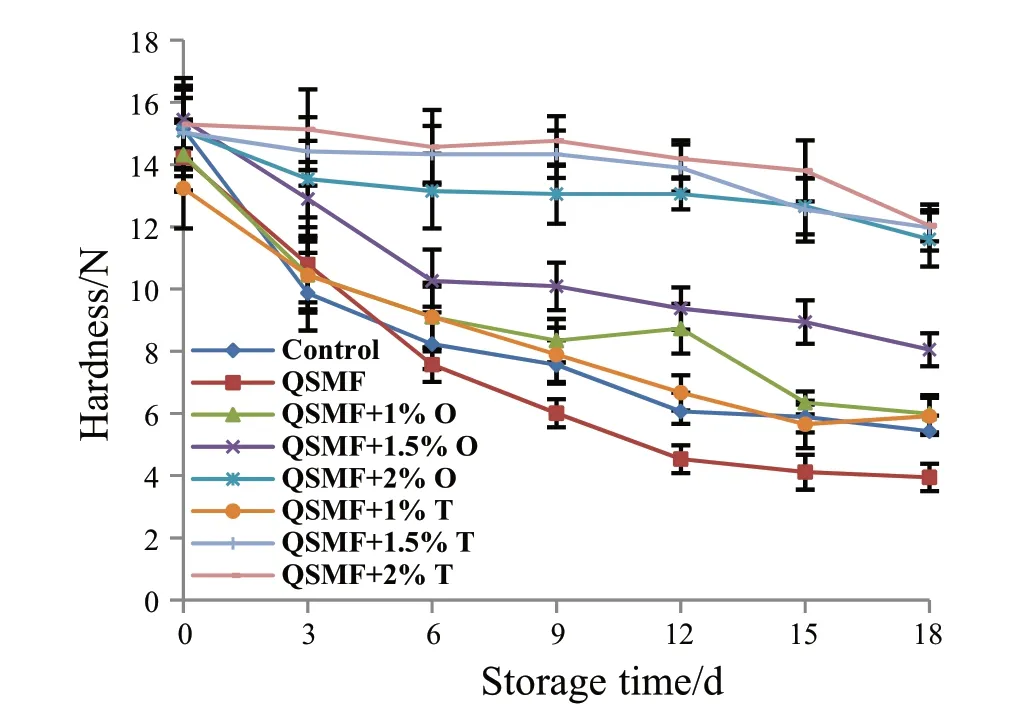
Fig.3.Changes in texture of rainbow trout fillets during storage at 4°C.
3.4.Changes in hardness
The texture of fish is considered as an important quality attribute for fish products’ palatability [31].Thus,texture measurement is a valuable tool to assess the effect of preservation methods on the quality of meat [34].The results of hardness measurements are summarized in Fig.3.For the control samples,hardness decreased during the storage period,showing that fillet became softer.Vaz-Pires et al.[35]also reported two-phase quality loss in fillets of fresh scad (Trachurustrachurus) and rainbow trout (O.mykiss) during refrigerated storage.According to Huss[36],the first phase in texture quality loss is mainly due to the enzymic autolysis (normally first six days in marine fish) and the second phase is due to microbial action.These microorganisms make food organoleptically unacceptable for consumption due to the changes in color,odor and texture [37].As shown in Table 4 correlations between microbiological attribute and texture and between TVB-N and texture were generally strong.Therefore,inhibition of these microorganisms increases the potential shelf life of the flesh.Post mortem changes of fish texture are mainly caused by modifications of myofibrillar proteins,due to both proteases action and variation of physical and chemical conditions [38].We previously reported that the QSM films containing thyme or oregano could inhibit the growth of 11 strains of microorganisms containingE.coli,Yersiniaenterocolitica,Staphylococcusaureus,Bacilluscereus,P.aeruginosa,Lactobacillusplantarum,S.typhimurium,E.coliO157,L.monocytogenes,V.choleraandShewanellaputrefaciens[5,6].In regard to the changes of texture during storage,hardness of fillet wrapped with QSMF + 2% T did not change after 15 d of storage.This may be attributed to QSMF + T functional properties,e.g.antioxidant,antimicrobial and oxygen barrier.The QSMFwrapped samples had the lowest values of hardness (P< 0.05).This could be attributed to the ability of QSM films to uptake huge amounts of water.Chamanara et al.[28]reported similar observations for rainbow trout coated with chitosan.They reported that the texture parameters could be changed by enzymatic and chemical reactions that lead to softening,changes in elasticity,or development of toughness under certain conditions.Poor texture indicated that the shelf life of these fillets was limited by chemical reactions and microbial activity.
3.5.Color analysis
Color values of rainbow trout fillets wrapped with and without QSF films during storage at 4°C for 18 d are shown in Table 3.TheL* values,which refer to lightness,generally increased with storage time in all samples.During storage,the appearance of rainbow trout fillets became whiter and less gray.According to Jung et al.[39]the changes in color of meat and fish during storage could be associated with both enzymatic and non-enzymatic reactions,resulting in degradation of myofibrillar proteins and disorganization of the myofibrils.Similar results for meager and seabass fillets were found by Genc¸ et al.[40]and Chéret et al.[38],reportingL* values increase with increasing storage time.They reported that the storage of sea bass fillets led to an increase inL* values.They also mentioned that the fish fillet color was linked with heme-based pigment,physical structure of muscle and the amount of unbound water which influenced light scattering.Conversely,Ahmad et al.[41]reported that sea bass slices were darker compared to fresh ones.
A decrease in redness,measured using thea* value,was obvious in rainbow trout fillets (Table 3).Wrapping the fillet with QSM films (with or without essential oils) resulted in retention of the red color compared to unwrapped samples(Table 3),as indicated by a slower reduce ina* values in wrapped QSMF samples.As shown in Table 3,the redness of the rainbow trout fillets wrapped with QSM films for 18 d was close to values obtained from unwrapped fillets for 12 d.It was reported that loss of red color occured in parallel with thiobarbituric acid reactive substances (TBARS) development during cold storage of washed cod muscle [42].In the current study,positive and high correlations were shown betweena*value and TBA,betweena* value and TVB-N and betweena*value and TMA-N (Table 3).These data suggest that antioxidant activity of the QSM films retarded oxidation of rainbow trout fillets.According to the previous study [5],QSM films showed radical scavenging activity of 18.39% on DPPH free radical.Films incorporated with thyme essential oil exhibited a higher level of radical scavenging activity with values of 30.11%,37.29% and 43.14% for 1%,1.5% and 2% of thyme essential oil-containing films,respectively.We also reported that QSM films containing oregano essential oils exhibited a higher level of radical scavenging activity with values of 45.09%,56.65%and 61.03% for the films containing 1%,1.5% and 2% of oregano essential oil,respectively [6].

Fig.4.Effect of QSM films on changes of color of rainbow trout fillets after 18 d of storage at 4°C.
Theb* values increased in all samples during the storage time.Fish fillet wrapped with QSM films demonstrated a lowerb* value than fish unwrapped (control) (Table 3).This was presumed to be due to the oxidation of pigment with high content of oxygen [43].Lipid oxidation products,especially aldehydes,could serve as the source of carbonyl compounds for nonenzymatic browning reaction,namely Maillard reaction [44].Wetterskog and Undeland [42]illustrated that the increasing ofb* values along with the reducing ofa* values could be the result of polymerized Schiff bases.Thus,QSM films and essential oils could retard the color changes of rainbow trout fillets to some extent during the storage.Samples wrapped with QSMF + T showed the lowest changes inb* values,probably due to the antioxidant and antimicrobial properties of thyme essential oil [5].Theb* values of QSMF + 2% O wrapped samples were higher than those of other treatments and control samples,which might be due to the color of oregano.We previously reported thatb* values of QSMF + 2% O was 10.03 whileb*values for QSM films and QSMF + 2% T was 3.93 and 8.05,respectively [6].The changes inΔEvalues of fillets are presented in Table 3.HigherΔEresults indicate a greater relative change in color compared to the fish’s original color.After 6 d of chilled storage,ΔEwas 0.76 for control samples and 2.78 for wrapped with QSM film (Table 3).ΔEof control samples was higher than that of other treatments at the end of storage time (18 d).The effect of QSM films on changes of color of rainbow trout fillets after 18 d of storage at 4°C is depicted in Fig.4.According to our results,the quality of QSMF + 2%T wrapped samples were considered more acceptable.Bonilla et al.[45]showed that the color changed as a result of storage conditions and packaging techniques was seemingly influenced by microbiological and chemical loss of quality.

Table 4 Correlation among microbiological,chemical and sensory attributes of rainbow trout fillets.
4.Conclusion
The results of this study showed that QSM films containing oregano or thyme essential oil enhanced the quality of rainbow trout fillets by preventing color and texture changes and lipid oxidation.QSM films containing oregano or thyme essential oil also showed antioxidant effect,since PV was lower than that of control samples at the end of the storage period.Moreover,the protective effect of QSMF + 2% O against lipid oxidation was more pronounced than other films; however,the QSMF + O wrapping made the fish fillet yellow.The results of the present study indicated that the QSMF + 2% T wrapping could maintain the physical-chemical quality of rainbow trout fillet better than that of the control.This film reduced the changes of color,texture and lipid oxidation more efficiently than other treatments,which may be attributed to QSMF + T functional properties,e.g.antioxidant,antimicrobial and oxygen barrier.
Acknowledgement
We gratefully acknowledge the Department of Food Science and Technology,Ferdowsi University of Mashhad for financial support of this work.
杂志排行
食品科学与人类健康(英文)的其它文章
- Effect of casein glycomacropeptide on subunit p65 of nuclear transcription factor-κB in lipopolysaccharide-stimulated human colorectal tumor HT-29 cells
- In vitro antioxidant,antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss.fruit pulp
- Purification,initial characterization and immune activities of polysaccharides from the fungus,Polyporus umbellatus
- Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits
- About the Beijing Academy of Food Sciences
- What could probiotic do for us?
