Integration of animal behaviors under stresses with different time courses
2014-04-06LunZhengXigengZheng
Lun Zheng, Xigeng Zheng
1 Key Laboratory of Mental Health, Chinese Academy of Sciences, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
2 Graduate School, Chinese Academy of Sciences, Beijing, China
Integration of animal behaviors under stresses with different time courses
Lun Zheng1, Xigeng Zheng2
1 Key Laboratory of Mental Health, Chinese Academy of Sciences, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
2 Graduate School, Chinese Academy of Sciences, Beijing, China
We used animal models of “forced swim stress” and “chronic unpredictable stress”, and tried to reveal whether a passive coping style of high fl otation behavior in forced swim stress predicts anhedonia behavior after chronic unpredictable stress, and whether the dopamine system regulates fl oating and anhedonia behaviors. Our results con fi rmed that depression-prone rats use “ fl oating behavior” as a coping strategy in forced swim stress and more readily suffer from anhedonia during chronic unpredictable stress. Intraperitoneal injection or nucleus accumbens microinjection of the dopamine 2/3 receptor subtype agonist ropinirole reduced fl oating behaviors in depression-prone animals, but increased sucrose preference in rats showing anhedonia. These data indicate that fl oating behavior is a defensive mode that is preferred by susceptible individuals under conditions of acute stress. Simultaneously, these animals more readily experienced anhedonia under long-term stress; that is, they were more readily affected by depression. Our results suggest that dopamine 2/3 receptor subtypes in the nucleus accumbens play an important role in fl oating behaviors and anhedonia.
nerve regeneration; brain injury; depression; stress resistance; susceptible to depression; chronic unpredictable stress; forced swim; dopamine; nucleus accumbens; NSFC grant; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 30971057; the Knowledge Innovation Program of the Chinese Academy of Sciences, No. KSCX2-EW-J-8.
Zheng L, Zheng XG. Integration of animal behaviors under stresses with different time courses. Neural Regen Res. 2014;9(15):1464-1473.
Introduction
Depression is a state of low mood, anhedonia and aversion to activity. Patients with depression frequently show a variety of physical symptoms. Their daily lives and social functions are greatly affected, which brings a heavy burden upon patients, family, and the whole community (Palermo-Neto, 1997; Cryan et al., 2001; Yarkov et al., 2003; Papakostas, 2006; Sokoloff et al., 2006; Riddle et al., 2010). Clinical and basic studies have made important progress in developing a treatment for depression, but there are still many problems. For example, the cure rate of depression with medicine is only 50% in the clinic, and almost half of depression patients show no apparent improvement after taking conventional antidepressants (Petersen et al., 2005; Taylor et al., 2006). In patients with effective drug treatment, common antidepressants take effect slowly, and protracted symptoms exist in those undergoing maintenance therapy (Nierenberg and Wright, 1999), with a high relapse rate (Mueller et al., 1999; Solomon et al., 2000).
Clinical studies have con fi rmed that the dopamine system, especially the mesolimbic dopamine system, exerts a vital effect on the pathogenesis of depression (Palermo-Neto, 1997; Cryan et al., 2001; Yarkov et al., 2003; Zhou et al., 2005; Antonijevic, 2006; Bertaina-Anglade et al., 2006; Papakostas, 2006; Sokoloff et al., 2006; Sekine et al., 2007), including decreased dopamine levels or dopamine metabolism (Roy et al., 1985, 1992; Lambert et al., 2000), increased dopamine receptor binding/sensitivity (D’haenen and Bossuyt, 1994; Verbeeck et al., 2001; Klimek et al., 2002) and decreased dopamine transporter activity (Meyer et al., 2001; Neumeister et al., 2001). Among depression patients who committed suicide, the content of the dopamine metabolite dihydroxy-phenyl acetic acid in the nucleus accumbens is remarkably diminished (Bowden et al., 1997). The content of another dopamine metabolite, homovanillic acid, in cerebrospinal fl uid is also noticeably decreased. The content of homovanillic acid in urine is signi fi cantly lower in depressed patients who attempt suicide than in those who do not and healthy controls (Roy et al., 1992). Moreover, homovanillic acid levels in cerebrospinal fluid are negatively associated with the severity of depression (Roy et al., 1985). The above findings indicate that dopamine metabolism is strongly correlated with a patient’s condition. Neuroimaging studies have revealed an increase in dopamine 2/3 receptor binding sites (Yang et al., 2008) in the corpus striatum (Shah et al., 1997) and basal ganglia (D’haenen and Bossuyt, 1994) of depressed patients. Consistent with this phenomenon, a previous study found that the sensitivity of dopamine 2 receptorswas increased in the central nervous system of depressed patients (Verbeeck et al., 2001). Interestingly, this increase in sensitivity of dopamine 2 receptors was associated with taking selective serotonin reuptake inhibitors and tricyclic preparations (Healy and McKeon, 2000). An autopsy study demonstrated an increase in the number of dopamine 2/3 receptor subtype binding sites in the basolateral amygdala and central amygdale, but numbers of dopamine transporter binding sites were obviously reduced in the central amygdala (Klimek et al., 2002). Additionally, single photon emission computerized tomography showed increased dopamine transporter binding activity in the basal ganglia and striatum (Tanda et al., 1994; Laasonen-Balk et al., 1999; Yang et al., 2008). These fi ndings suggested that the dopamine system was strongly associated with the occurrence of depression (Laasonen-Balk et al., 1999; Healy and McKeon, 2000; Lambert et al., 2000; Cryan et al., 2001; Meyer et al., 2001; Neumeister et al., 2001; Klimek et al., 2002; Wall et al., 2003; Millan et al., 2004; Bekris et al., 2005).
Basic studies have also revealed significant pathological changes in the dopamine system of depressed animals under stress (Taylor et al., 1982; Tossman and Ungerstedt, 1986; Simon et al., 1993; Levant, 1997; Steiner et al., 1997; Gendreau et al., 1998; Lawford et al., 2006; Perona et al., 2008; Schneier et al., 2008; van der Wee et al., 2008). After an inescapable uncontrollable electric shock, dopamine 2 receptor density was reduced in the caudate nucleus and core area of the nucleus accumbens of rats experiencing learned helplessness (Winter et al., 2007). In rats with anhedonia under chronic unpredictable stress, the release of dopamine and its metabolites was obviously altered in the prefrontal cortex and corpus striatum. The level of dopamine 2 receptor messenger RNA expression was decreased in the midbrain ventral tegmental area, substantia nigra, core area and shell area of the nucleus accumbens and caudate nucleus (Dziedzicka-Wasylewska et al., 1997; Winter et al., 2007). Additionally, the dopamine 2 receptor binding activity in the nucleus accumbens was apparently reduced (Papp et al., 1994). These changes in the dopamine system could be reversed by slowly injecting antidepressants (Dziedzicka-Wasylewska et al., 1997; Bekris et al., 2005; Yang et al., 2008; Vignisse et al., 2011). Clinical and basic studies have indicated that the dopamine system exerts a crucial effect on the pathological development of depression, but the effects of the dopamine system, especially of dopamine 2/3 receptor subtypes, on the treatment of depression, have not been examined in a comparative study using animal models.
Basic studies addressing stress-induced depression in experimental animals could investigate the relationship between stress and depression (D’haenen and Bossuyt, 1994; Papp et al., 1994; Tanda et al., 1994; Willner et al., 1994). The forced swim test and chronic unpredictable stress have been extensively used to prepare animal models of depression (Strekalova et al., 2008; Bolkunov et al., 2009; Tian et al., 2011; Varga et al., 2011; Vignisse et al., 2011; Vollenweider et al., 2011). In conditions of acute stress, animals show increased depression-like behaviors such as fl oating behaviors. Chronic stress causes persistent anhedonia-like behavior (Sun et al., 2004; Cryan and Holmes, 2005; Mathews and MacLeod, 2005; Remy et al., 2005). This “ fl oating behavior”and “anhedonia” is considered to be a model of depression in behavioral neuroscience studies. Studies have veri fi ed that the sucrose preference in high floating animals (high percentage of fl oating behaviors) after a forced swim test is not decreased, but fl oating behavior is more frequently observed in animals showing anhedonia than in control animals after chronic unpredictable stress (Sun et al., 2004; Cryan and Holmes, 2005; Mathews and MacLeod, 2005; Remy et al., 2005). Therefore, the relationship between animals showing floating behaviors under acute stress and animals showing anhedonia under chronic stress remains poorly understood, as does the mechanism underlying the effects of the dopaminergic system on the above two kinds of models of depression.
In this study, we sought to explore the following three problems. (1) Whether there is a predictive relationship between fl oating behavior of animals after forced swim test and anhedonia behavior of animals after chronic unpredictable stress; and if there is a predictive relationship, do animals showing a high percentage of fl oating behavior easily suffer from anhedonia after chronic unpredictable stress? (2) Whether the dopamine 2/3 receptor subtype regulates floating behavior in models after forced swim test? If yes, does the nucleus accumbens play an important role in this regulation? (3) Whether the dopamine 2/3 receptor subtype regulates anhedonia in models after chronic unpredictable stress? If yes, does the nucleus accumbens play an important role in this regulation?
Materials and Methods
Experimental animals
One-hundred and four adult male Wistar rats were purchased from VitalRiver, Beijing, China. Their initial body weight was between 250 and 270 g. All rats were housed at 20-24°C in 15-20% humidity, with a light cycle of 8:00-20:00, in a specific-pathogen-free room. All rats were acclimated to the conditions for 1 week before experiments. During this week, an experimenter regularly caught and touched these rats to exclude non-experimental speci fi c stress. All protocols were approved by the Animal Ethics Committee of the Chinese Academy of Sciences.
Establishment of rat models in a forced swim test
The forced swim test, also called the desperate experiment or Porsolt test, was fi rst reported by Porsolt et al. (1978). Animals could not escape from the bad surroundings, which resulted in behavioral despair. In our experiments, a transparent cylindrical container (50 cm high, 25 cm diameter) was used. During the test, the depth of water was 35 cm, and the water temperature was 25°C. A 60 W frosted yellow light bulb provided lighting. A camera was placed in the front of the glass bucket to record animal’s behaviors in water. A total of 64 rats were randomly assigned to a stress group (n= 34) and a control group (n= 30). The forced swim test was performed over2 days. On the fi rst day, rats in the stress group were placed in the swimming pond for 15 minutes. The rats were then taken out of the swimming pond, dried and housed in cages for 24 hours. The rats in the stress and control groups were placed in the swimming pond for 5 minutes to observe their swimming behavior and floating behavior. Precise data on swimming and fl oating behaviors were analyzed using Ethovision software, which identified swimming and floating behaviors by analyzing the percentage of changes in animal images.
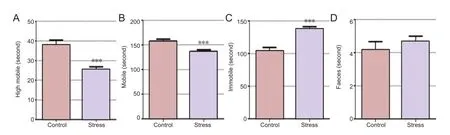
Figure 1 Effects of forced swim stress on fl oating behavior in rats.

Figure 2 Effects of chronic unpredictable stress on sucrose preference and weight in rats.

Figure 3 Predictive effects of acute fl oating behavior on anhedonia in rats.

Figure 5 Individual differences in the percentages of sucrose intake under chronic unpredictable stress.

Figure 4 Effects of a dopamine 2/3 receptor agonist on fl oating behaviors in rats susceptible to depression under acute stress.
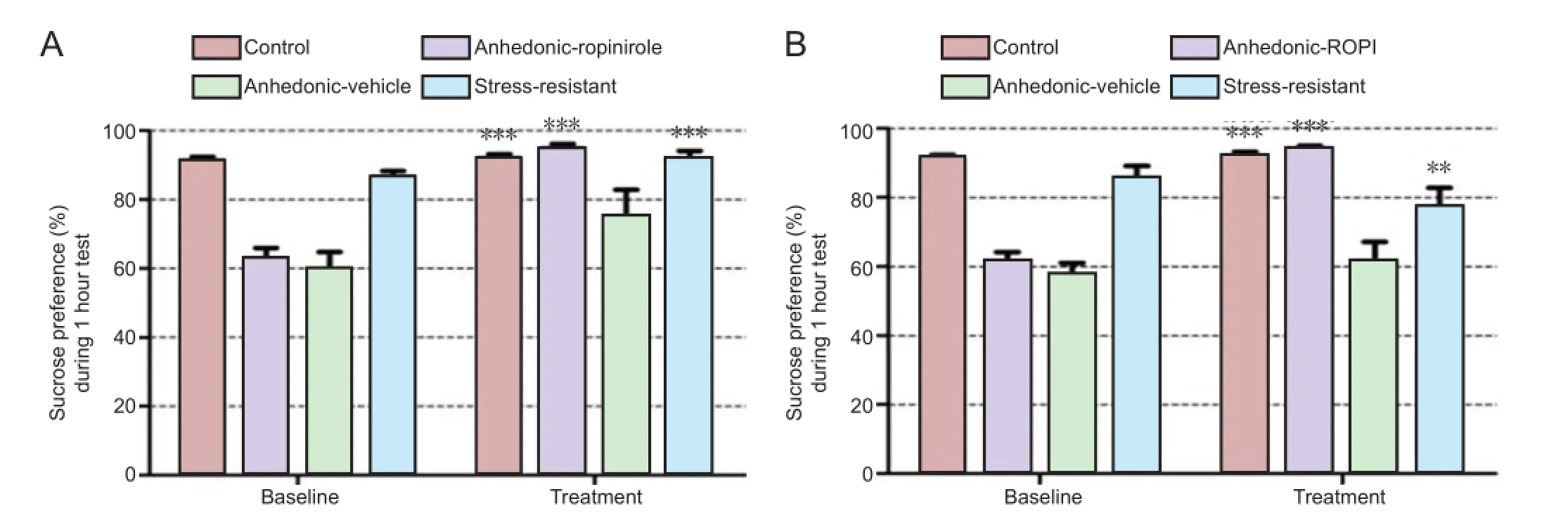
Figure 6 Ropinirole decreased anhedonia in rats susceptible to depression under chronic unpredictable stress.
Establishment of rat models of chronic unpredictable stress
Depression models induced by chronic unpredictable stress were fi rst established by Willner in 1987 (Abdo et al., 2010). The models were established by administering a series of chronic unpredictable mild stresses to simulate various stresses in daily life. The various stresses were given in a pseudo-random method. Stressors included: twice 2-hour restraint stress (Strekalova et al., 2005), twice 30-minute low-temperature stress (0-4°C), three times 8-hour high-temperature stress (32 ± 1°C), three times 12-hour crowded living, twice 12-hour wet floor, three times 18-hour food deprivation, twice 12-hour water deprivation, twice 1-hour empty bottle stress, three times 12-hour strong light exposure, once 5-minute cold water swimming (4°C), four times cage tilt at 45°, and three times strobe light stress (Katz et al., 1981; Valverde et al., 1997).
A total of 40 rats received tests of sucrose preference, were subjected to the elevated plus maze and were weighed. They were divided into a stress group (n= 30) and a control group (n= 10). No significant difference in the above indices was observed between the stress and control groups. Chronic unpredictable stress was performed for 4 weeks. Rats’weights were measured every day, and sucrose preference was measured every week. After stress, the sucrose preferences and weights of animals were measured again. The volumes of sweet water and water consumed within 1 hour and 12 hours were calculated. Sucrose preference (%) was calculated as the volume of sweet water/(the volume of sweet water + the volume of water) × 100%.
Stereotaxic localization of rat brain
The rats were intraperitoneally anesthetized with sodium pentobarbital (55 mg/kg), and intraperitoneally injected with atropine (0.05 mg/kg) to avoid respiratory distress (Agustin Zapata and Chefer, 2009). Rat skulls were fi xed with a stereotaxic apparatus (Woruide, Shenzhen, Guangdong Province, China). In accordance with a stereotaxic atlas (Paxinos and Watson, 1997), the precise sites of the nucleus accumbens injections were + 1.7 mm posterior to the anterior fontanelle, and ± 1 mm lateral to the left and right.
Screening of anhedonia and stress-resistant animals under chronic unpredictable stress and drug intervention
In accordance with sucrose preference at 4 weeks of chronic unpredictable stress, 16 anhedonia rats and 10 stress-resistant rats were selected. Anhedonia rats were further assigned to an administration group (n= 8) and a control group (n= 8). In the ropinirole experiment, the rats were intraperitoneally injected with the dopamine 2/3 receptor subtype agonist ropinirole (1 mg/kg, 0.65 mg/kg), once a day, for 7 consecutive days. On the 7thday, sucrose preference was tested. In the administration test in the nucleus accumbens, all rats received intubation, and were allowed to recover for 6 days after surgery. Sucrose preference was then measured. Ropinirole (1.625 μg/μL) was also injected into the nucleus accumbens of anhedonia rats 30 minutes before the test, while an equal volume of physiological saline was injected in control rats.
Screening of fl oating susceptibility and stress-resistant animals in the forced swim stress and with drug intervention
Desipramine is a tricyclic antidepressant. Its main mechanism of action is inhibiting the reuptake of norepinephrine, but the effects of desipramine on reuptake of serotonin are weak. We observed the effects of desipramine on fl oating behavior during 15-minute pre-processing (forced swimming), selected the most sensitive time for drug treatment, and fi nally identifi ed the depression index, which could be used as a standard to select rats with a high or low percentage of fl oating behavior in the subsequent tests. Thus, 16 rats with a high percentage of fl oating behavior and 8 rats with a low fl oating percentage were selected. The 16 rats with high percentage of floating behavior were assigned to a ropinirole administration group (n= 8) and a control group (n= 8), and subjected to testing.
Statistical analysis
All data were expressed as mean ± SEM, and were analyzed using GraphPad prism 4.0 and SPSS 17.0 software (SPSS, Chicago, IL, USA). Multivariate analysis of variance was applied when experimental data contained two or three factors (twoor three-way analysis of variance. Multiple comparisons of the differences in intergroup data were performed using Duncan’s method or Bonferroni test. Data were compared between groups using the two samplet-test. A value ofP< 0.05 was considered statistically signi fi cant.
Results
Forced swim stress apparently increases fl oating behavior of rats in the stress group
在以往的研究中,吉卜林“长期以来被视为英国在印度的殖民统治的辩护人,背负着‘帝国主义作家’的恶名”。譬如,评论家艾德蒙·威尔逊就批评基姆“讲自己所爱的人送入英国侵略者之手”,“利用自己对当地的了解来防止和压制当地人对英国的反抗”而基姆——吉卜林的代言者——之所以为英国情报机构工作,完全可以是为了挽救已经满是伤痕的印度于另一场侵略战争之中。这也就是为什么,英国的情报机构有印度人的忠诚参与。我们不能因为吉卜林是英国人,就臆测他的一切行为都是以英国的殖民统治为出发点的(当然无法忽视的是他身上仍旧带有时代的局限性)。
Fifteen-minute pre-processing before the forced swim test resulted in significant model effects, with significantly diminished high-intensity exercise and movement time in the forced swim test in the stress group, and increased fl oating time (Figure 1A-C,P< 0.001). No signi fi cant di ff erence in fecal excretion was observed between the stress group and control group (Figure 1D), which indicated that the increase in fl oating behavior was not induced by non-speci fi c emotional changes in the stress group.
Chronic unpredictable stress obviously decreases sucrose preference in rats in the stress group
Under the initial state, no signi fi cant di ff erence in weight was detected between the stress and control groups (337 gvs. 338 g,P= 0.8676). It is clearly observed that the increase in weight in the stress group was significantly slower than that in the control group, and the weights of rats in the stress group even diminished. Repeated-measures analysis of variance revealed signi fi cant di ff erences in weight between the stress and control groups from day 2 of stress (F(1,37)= 88.54,P< 0.001;Figure 2B).
Acute fl oating behavior is predictive of anhedonia in rats
A dopamine 2/3 receptor agonist reduces fl oating behaviors in depression-susceptible rats under acute stress conditions
As shown inFigure 4, no significant difference in floating behaviors was detected between ropinirole-treated (intraperitoneal injection of ropinirole 0.65 mg/kg per day) and non-model rats showing a high percentage of floating behaviors (P> 0.05). Significant differences in floating time were observed between the high-percentage fl oating behavior saline control group (HI-vehicle) and the saline control group (vehicle) (P< 0.001). Signi fi cant di ff erences in fl oating time were also observed between the ropinirole- and saline-treated rats showing a high percentage of fl oating behaviors (P<0.05). Signi fi cant di ff erences in fl oating time were detectable between the high-percentage fl oating behavior saline control group (HI-vehicle) and the low-percentage fl oating behavior group (low-immobile) (P< 0.001).ese fi ndings suggested that intraperitoneal injection of a dopamine 2/3 receptor agonist decreased fl oating behaviors (Figure 4B).
Noticeable “individual difference” in rats under chronic unpredictable stress
Chronic unpredictable stress can be used to induce depression models of strong and persistent stress. The reduction in sucrose preference did not appear in all rats in the stress group.e sucrose preference level began to decrease from 1 week aer stress in some rats, and was maintained until anhedonia appeared. By contrast, the sucrose preference level was high during the test, which was called stress resistance (Figure 5).
During chronic unpredictable stress, the sucrose preference level gradually diminished with time in rats with anhedonia. Conversely, the sucrose preference level remained at a high level in rats with stress resistance. Repeated measures analysis of variance results demonstrated a signi fi cant main effect among the anhedonia, stress-resistance and control groups (F(2,26)= 27.715,P< 0.001), a signi fi cant main e ff ect of time (F(4,104)= 4.23,P< 0.001), and a significant interaction of “group” × “time” (F(8,104)= 4.938,P< 0.001). The Bonferroni test indicated that, from 2 weeks, significant di ff erences were observed between the anhedonia group and the stress resistance group (P< 0.05 orP< 0.001). Simultaneously, signi fi cant di ff erences were also apparent between the anhedonia group and control group from 2 weeks (P<0.001 orP< 0.001).ese results indicated that, because of innate di ff erences in susceptibility, sucrose preference levels were quite di ff erent among groups.
A dopamine 2/3 receptor agonist diminishes anhedonia in rats susceptible to depression under conditions of chronic unpredictable stress
Intraperitoneal injection of ropinirole for 1 week signi fi cantly increased sucrose preference in rats with anhedonia (P< 0.001,Figure 6A). Nucleus accumbens microinjection of ropinirole significantly increased the sucrose preference level in rats with anhedonia (P< 0.001;Figure 6B).
Discussion
The forced swim test is characterized by a short stress time, easy to identify behavioral output, and sensitivity to antidepressants, and has been extensively used to induce animal models of depression (Porsolt et al., 1977). If forcing rats to swim in a limited space, the rats will finally stop trying to escape, a show of fl oating behavior. The 15-minute pre-processing before forced swim test is an inevitable stress. During the test, their behavior will alter after struggling for a time. That is, their behavior changes from a positive status (vio-lent struggle) to a negative status (keeping the head above the surface), which is associated with rats’ recognition about their own state. The first time the rats entered the pool, they attempted to get out of this predicament. After failure, behavioral inhibition appeared. After re-entering the same environment (they cannot escape from the pool), the sooner they understood this situation, the earlier the passive avoidance behavior occurred: rats showed a short struggling time, early appearance of immobile status, and a long duration of immobility. Many investigators believe that this typical stable fl oating behavior re fl ects a desperate state in rats. Moreover, multiple effective depression treatment reduced floating behavior. This model-induced depression-like behavior can be relieved by effective “non-drug treatments”, including electroconvulsive therapy, REM sleep deprivation and rich environmental exposure (Porsolt et al., 1978).
In the present study, 15-minute pre-processing obviously enhanced floating behavior in the stress group (24 hours later). Some investigators believe that floating behavior possibly benefits survival and is an adaptive behavior. In a long-term forced swim test, animals with more fl oating behaviors could fl oat in the water, and did not sink (Nishimura et al., 1988); these animals could better cope with negative stress. Nevertheless, many researchers believe that 15-minute pre-processing would cause a negative perception of the environment by animals, believing they cannot escape from the negative stress no matter how to struggle (Tian et al., 2011). A previous study verified that defecation frequency increased in rats during two forced swim tests (Armario et al., 1988). Defecation re fl ects the emotional reactions of animals. A clinical study on depression con fi rmed that negative perception was strongly associated with depression (Mathews and MacLeod, 2005). However, sucrose preference levels did not decrease in rats with a high percentage of floating behaviors, but their sucrose intake could be increased because of the large consumption of physical energy. These fi ndings indicated that animals with a high percentage of floating behavior screened in the 15-minute forced swimming did not display anhedonia, but their depression was temporary, so it was called state depression. Therefore, their floating behavior was elevated remarkably during the forced swim test. Animals sensitive to antidepressants possibly experience negative perception most readily when facing acute, inescapable, uncontrollable stress. These animals probably suffered from anhedonia under repeated stresses.
Models of chronic unpredictable stress are very typical and commonly used animal models of depression. A series of depression-like behaviors in rats are possibly induced by giving long-period unpredictable stresses and simulating human uncertain stress events during daily life. The main change in behaviors is the decrease in sucrose preference. Animals generally prefer sweet water, but this preference in depressed rats becomes weak, with the presence of anhedonia. Experimental results veri fi ed that, after chronic unpredictable stress, the sucrose preference level was noticeably lower in the stress group compared with the control group at 2 weeks, and the decreased sucrose preference persisted until the end of the stress. A previous study veri fi ed that chronic unpredictable stress decreased the response of animals to reward, including reducing the approach of mice to food in a new environment (Tannenbaum et al., 2002), and decreasing the nose touch response to predictable sugar reward (Phillips and Barr, 1997). The above results suggest that animals present with anhedonia under conditions of chronic unpredictable stress. Anhedonia is a core symptom of depression. This kind of depression is persistent, difficult to recover from, and can lead to other kinds of depression,e.g., constitutional depression. Some studies have shown that chronic unpredictable stress can increase fl oating behaviors in rats. What is the relationship between fl oating behavior and anhedonia?
Our experimental results demonstrated that susceptible animals using fl oating behavior as a coping strategy during a forced swim test easily suffer from anhedonia following chronic unpredictable stress. This study established a connection in terms of behavioral indicators between acute stress models and chronic stress models, and found that state depression animals with floating behavior under the acute stress are more readily affected by anhedonia during chronic stress. These data indicate that fl oating behavior is a defensive mode that susceptible individuals tend to use under conditions of acute stress. However, this negative coping strategy probably causes anhedonia, because these animals could not effectively cope with the subsequent chronic stress.
Considering the key effect of the dopaminergic system in stress-related mental disorders, the present study further investigated the effects of the dopamine system on fl oating behaviors and anhedonia in model animals subjected to the forced swim stress and chronic unpredictable stress. After screening animals with high-floating level and anhedonia, this study demonstrated that 1-week intraperitoneal injection of ropinirole effectively reduced floating behaviors during the forced swim test, and reversed the reduction in sucrose preference level in animals showing anhedonia. Simultaneously, we also explored the effect of the nucleus accumbens-which has crucial effects on mood and reward-part of the dopamine system, on depressive behaviors. The results from this study suggest that, after screening animals with a high percentage of fl oating behaviors and anhedonia, microinjection of ropinirole into the nucleus accumbens before the forced swim test and sucrose preference test could effectively diminish fl oating behaviors under acute forced swim stress, and reversed the decrease in sucrose preference level in animals showing anhedonia. The above results suggest that the dopamine system exerts a crucial antidepressant effect on state depression and constitutional depression induced by acute stress and chronic stress, for which dopamine 2/3 receptor subtypes and the nucleus accumbens are important.
Numerous studies have demonstrated that, in a learned helplessness model, chronic injection of the dopamine receptor agonist quinpirole, the dopamine 1 receptor subtype agonist SKF38393, the dopamine 2 receptor subtype agonist quinpirole, or the dopamine 2/3 receptor subtype agonists ropinirole or S32504 could reverse helpless behaviors in-duced by uncontrollable shock (Takamori et al., 2001; Millan et al., 2004; Bertaina-Anglade et al., 2006). In chronic unpredictable stress models, the dopamine 2 receptor subtype agonists pramipexole and quinpirole increased the reduction in the amount of sugar consumed and sucrose preference level in the stress group (Muscat et al., 1992; Willner et al., 1994). However, the above antidepressant effect disappeared after withdrawal of quinpirole, but re-administration of quinpirole in a subsequent sucrose test could normalize sucrose preferences (Muscat et al., 1992). In a previous study, injection of a dopamine 2/3 receptor subtype agonist, twice a day, could restore sucrose preferences in rats with depression induced by withdrawal of metamphetamine (D’Aquila et al., 1994), suggesting that dopamine 2/3 receptor subtypes could be a key target in the treatment of withdrawal-induced depression. Our findings show that 1-week administration of ropinirole diminishes fl oating behaviors during a forced swim test, reverses sucrose preference levels in rats with anhedonia under chronic unpredictable stress, and further con fi rmed that dopamine 2/3 receptor subtypes are signi ficant in the treatment of depression.
A clinical study found that the content of the dopamine metabolite dihydroxy acid was significantly reduced in the nucleus accumbens of depression patients who committed suicide (Bowden et al., 1997). Long-term use of most antidepressants and repeated electroconvulsive therapy would obviously upregulate dopamine receptor messenger ribonucleic acid receptor expression in the nucleus accumbens shell (Meyer et al., 2001). The studies mentioned above veri fi ed that dopamine 2/3 receptor subtypes in the nucleus accumbens had important effects on the pathogenesis of depression. Our results con fi rmed that nucleus accumbens microinjection of a dopamine 2/3 receptor subtype agonist could effectively diminish floating behaviors under acute forced swim stress, and reverse sucrose preference levels in animals showing anhedonia under chronic unpredictable stress, which indicates that dopamine 2/3 receptor subtypes in the nucleus accumbens have important signi fi cance and clinical application prospects for the treatment of depression.
In this study, we explored the relationship between depression and the dopamine system from the perspective of individual differences, and, in parallel, compared acute stress and chronic stress. This approach is very rare in the field, with most studies using models of forced swim stress or chronic stress (Bekris et al., 2005; Gronli et al., 2005; Vignisse et al., 2011). We established a connection in terms of behavioral indicators between acute stress models and chronic stress models, and found that state depression animals showing floating behaviors under acute stress are more readily susceptible to constitutional depression (anhedonia) during chronic stress. This connection has not previously been shown. Additionally, we focused on individual differences in different animals under the same stress condition in terms of aspects of their behavior. We screened animals based on floating behaviors under acute stress after pre-processing with antidepressants and anhedonia under chronic stress. Animals showing real depression in the stress group were selected for subsequent drug treatment in this study. We did not neglect individual differences like most similar studies (Steimer and Driscoll, 2003; Wall et al., 2003; Yeritsyan et al., 2003). While behavioral research was conducted, this study further employed behavioral pharmacology by injecting drugs into the abdominal cavity and nucleus accumbens, investigated the regulatory effects of dopamine 2/3 receptor subtypes on depression in susceptible animals under acute and chronic stresses, and found that dopamine 2/3 receptor subtypes mediated the changes in the above behaviors. Our findings are significant for the integration of animal behaviors under stresses with different time courses and for further identifying the effect of the dopamine system on the onset and treatment of depression.
We primarily showed that acute floating behavior as a passive coping strategy is a main reason for anhedonia under conditions of long-term stress. Nevertheless, other indices and effects of coping strategies on behaviors after long-term stress still need to be investigated. Further studies will investigate the effects of the dopamine system on different stages of depression from the aspects of transmitter release, receptor changes and morphological changes using microdialysis, polymerase chain reaction and immunohistochemistry.
Author contributions:Zheng L wrote the manuscript. All authors implemented and designed the study, and approved the final version of the paper.
Con fl icts of interest:None declared.
Abdo AA, Ackermann M, Agudo I (2010) Fermi Large Area Telescope and multi-wavelength observations of the flaring activity of PKS 1510-089 between 2008 September and 2009 June. Astrophys J 721:1425-1447.
Agustin Zapata, Chefer VI (2009) Microdialysis in Rodents. Curr Protoc Neurosci. Chapter 7: Unit7.2
Antonijevic IA (2006) Depressive disorders--is it time to endorse di ff erent pathophysiologies? Psychoneuroendocrinology 31:1-15.
Armario A, Gavaldà A, Martí O (1988) Forced swimming test in rats: e ff ect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility and defecation rate. Eur J Pharmacol 158:207-212.
Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z (2005) Behavioural and neurochemical e ff ects induced by chronic mild stress applied to two di ff erent rat strains. Behav Brain Res 161:45-59.
Bertaina-Anglade V, La Rochelle CD, Scheller DKA (2006) Antidepressant properties of rotigotine in experimental models of depression. Eur J Pharmacol 548:106-114.
Bolkunov A, Redkozubova O, Malatynska E, van Miegem V, Vankin G, Strekalova T, Bachurin S (2009) Aging-related anhedonia in C 57 mice and e ff ects of dimebone. J Neurochem 110:50.
Bowden C, Cheetham SC, Lowther S, Katona CLE, Crompton MR, Horton RW (1997) Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res 769:135-140.
Cryan JF, Dalvi A, Jin SH, Hirsch BR, Lucki I,omas SA (2001) Use of dopamine-beta-hydroxylase-de fi cient mice to determine the role of norepinephrine in the mechanism of action of antidepressant drugs. J Pharmacol Exper 298:651-657.
D’Aquila PS, Brain P, Willner P (1994) E ff ects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol behav 56:861-867.
D’haenen HA, Bossuyt A (1994) Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiat 35:128-132.
Dziedzicka-Wasylewska M, Willner P, Papp M (1997) Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol 8:607.
Gendreau PL, Petitto JM, Gariepy JL, Lewis MH (1998) D2-like dopamine receptor mediation of social-emotional reactivity in a mouse model of anxiety: strain and experience e ff ects. Neuropsychopharmacology 18:210-221.
Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R (2005) E ff ects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 84:571-577.
Healy E, McKeon P (2000) Dopaminergic sensitivity and prediction of antidepressant response. J Psychopharmacol 14:152.
Katz RJ, Roth KA, Carroll BJ (1981) Acute and chronic stress e ff ects on open fi eld activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 5:247-251.
Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA (2002) Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiat 52:740-748.
Laasonen-Balk T, Kuikka J, Viinam ki H, Husso-Saastamoinen M, Lehtonen J, Tiihonen J (1999) Striatal dopamine transporter density in major depression. Psychopharmacology 144:282-285.
Lambert G, Johansson M, Agren H, Friberg P (2000) Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 57:787.
Lawford BR, Young R, Noble EP, Kann B, Ritchie T (2006) The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry 21:180-185.
Mathews A, MacLeod C (2005) Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 1:167-195.
Meyer JH, Kr¨¹ger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Mini fi e C, Houle S, Hussey D, Kennedy SH (2001) Lower dopamine transporter binding potential in striatum during depression. Neuroreport 12:4121.
Millan MJ, Brocco M, Papp M, Serres F, La Rochelle CD, Sharp T, Peglion JL, Dekeyne A (2004) S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors: III. Actions in models of potential antidepressive and anxiolytic activity in comparison with ropinirole. J Pharmacol Exper 309:936.
Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, Warshaw M, Maser JD (1999) Recurrence aer recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry 156:1000-1006.
Muscat R, Papp M, Willner P (1992) Antidepressant-like effects of dopamine agonists in an animal model of depression. Biol Psychiat 31:937-946.
Neumeister A, Willeit M, Praschak-Rieder N, Asenbaum S, Stastny J, Hilger E, Pirker W, Konstantinidis A, Kasper S (2001) Dopamine transporter availability in symptomatic depressed patients with seasonal affective disorder and healthy controls. Psychol Medicine 31:1467-1473.
Nierenberg AA, Wright EC (1999) Evolution of remission as the new standard in the treatment of depression. J Clin Psychiat 60:7-11.
Nishimura H, Tsuda A, Oguchi M, Ida Y, Tanaka M (1988) Is immobility of rats in the forced swim test “behavioral despair?”. Physiol behav 42:93-95.
Palermo-Neto J (1997) Dopaminergic systems: dopamine receptors. Psychiatr Clin North Am 20:705-721.
Papakostas GI (2006) Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 16:391-402.
Papp M, Klimek V, Willner P (1994) Parallel changes in dopamine D 2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology 115:441-446.
Perona MTG, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR (2008) Animal models of depression in dopamine, serotonin and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol 19: 566.
Petersen T, Papakostas GI, Posternak MA, Kant A, Guyker WM, Iosifescu DV, Yeung AS, Nierenberg AA, Fava M (2005) Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharm 25:336-341.
Phillips A, Barr AM (1997) E ff ects of chronic mild stress on motivation for sucrose: mixed messages. Psychopharmacology 134:361-362.
Porsolt RD, Lepichon M, Jalfre M (1977) Depression - new animal-model sensitive to antidepressant treatments. Nature 266:730-732.
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379-391.
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128:1314.
Riddle DL, Wade JB, Jiranek WA (2010) Major depression, generalized anxiety disorder, and panic disorder in patients scheduled for knee arthroplasty. J Arthroplasty 25:581-588.
Roy A, Karoum F, Pollack S (1992) Marked reduction in indexes of dopamine metabolism among patients with depression who attempt suicide. Arch Gen Psychiatry 49:447.
Roy A, Pickar D, Linnoila M, Potter WZ (1985) Plasma norepinephrine level in affective disorders: relationship to melancholia. Arch Gen Psychiatry 42:1181.
Schneier FR, Martinez D, Abi-Dargham A, Zea-Ponce Y, Simpson HB, Liebowitz MR, Laruelle M (2008) Striatal dopamine D2 receptor availability in OCD with and without comorbid social anxiety disorder: preliminary fi ndings. Depress Anxiety 25:1-7.
Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR (2007) Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse 61:72-77.
Shah P, Ogilvie A, Goodwin G, Ebmeier K (1997) Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 27:1247-1256.
Simon P, Panissaud C, Constentin J (1993) Anxiogenic-like e ff ects induced by stimulation of dopamine receptors. Pharmacol Biochem Behav 45:685-690.
Sokolo ff P, Diaz J, Foll B, Guillin O, Leriche L, Bezard E, Gross C (2006)e dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets 5:25-43.
Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea T, Coryell W, Warshaw M, Turvey C, Maser JD, Endicott J (2000) Multiple recurrences of major depressive disorder. Am J Psychiatry 157:229-233.
Steimer T, Driscoll P (2003) Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress 6:87-100.
Steiner H, Fuchs S, Accili D (1997) D3 dopamine receptor-deficient mouse: evidence for reduced anxiety. Physiol Behav 63:137-141.
Strekalova T, Spanagel R, Dolgov O, Bartsch D (2005) Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol 16:171.
Strekalova T, van Miegem V, Redkozubova O, Dolgov O, Larde G, Beznosko B, Vankin G, Bachurin S (2008) Sucrose test method: facts, artifacts and application in anhedonia models with young and old C57BL/6 mice. Int J Neuropsychoph 11:128-128.
Sun HX, Yang YP, Shi SX, Lu Z, Zhang MY (2004)e control study of the major depression disorder comorbid with anxiety disorder in psychiatric patient. Int J Psychol 39:454-454.
Takamori K, Yoshida S, Okuyama S (2001) Repeated treatment with imipramine, fl uvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sci 69:1919-1926.
Tanda G, Carboni E, Frau R, Dichiara G (1994) Increase of extracellular dopamine in the prefrontal cortex - a trait of drugs with antidepressant potential. Psychopharmacology 115:285-288.
Tannenbaum B, Tannenbaum G, Sudom K, Anisman H (2002) Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: implications for allostatic load. Brain Res 953:82-92.
Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM (2006) Psychomotor slowing as a predictor of fl uoxetine nonresponse in depressed outpatients. Am J Psychiatry 163:73.
Taylor DP, Riblet LA, Stanton HC, Eison AS, Eison MS, Temple DL (1982) Dopamine and antianxiety activity. Pharmacol Biochem Behav 17:25-35.
Tian M, Mao RR, Wang LP, Zhou QX, Cao J, Xu L (2011) Interaction between behavioral despair and addictive behaviors in rats. Physiol Behav 102:7-12.
Tossman U, Ungerstedt U (1986) Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand 128:9-14.
van der Wee NJ, van Veen JF, Stevens H, van Vliet IM, van Rijk PP, Westenberg HG (2008) Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-{beta}-(4-iodophenyl)-Tropane SPECT. J Nucl Med 49:757-763.
Valverde O, Smadja C, Roques BP, Maldonado R (1997)e attenuation of morphine-conditioned place preference following chronic mild stress is reversed by a CCKB receptor antagonist. Psychopharmacology (Berl) 131:79-85.
Varga J, Domokos A, Barna I, Jankord R, Bagdy G, Zelena D (2011) Lack of vasopressin does not prevent the behavioural and endocrine changes induced by chronic unpredictable stress. Brain Res Bull 84:45-52.
Verbeeck WJC, Berk M, Paiker J, Jersky B (2001) The prolactin response to sulpiride in major depression: the role of the D2 receptor in depression. Eur Neuropsychopharm 11:215-220.
Vignisse J, Steinbusch HWM, Bolkunov A, Nunes J, Santos AI, Grandfils C, Bachurin S, Strekalova T (2011) Dimebon enhances hippocampus-dependent learning in both appetitive and inhibitory memory tasks in mice. Prog Neuro-Psychoph 35:510-522.
Vollenweider I, Smith KS, Keist R, Rudolph U (2011) Antidepressant-like properties of alpha2-containing GABA(A) receptors. Behav Brain Res 217:77-80.
Wall PM, Blanchard RJ, Yang M, Blanchard DC (2003) Infralimbic D2 receptor in fl uences on anxiety-like behavior and active memory/attention in CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry 27:395-410.
Willner P, Lappas S, Cheeta S, Muscat R (1994) Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology 115:454-462.
Winter C, von Rumohr A, Mundt A, Petrus D, Klein J, Lee T, Morgenstern R, Kupsch A, Juckel G (2007) Lesions of dopaminergic neurons in the substantia nigra pars compacta and in the ventral tegmental area enhance depressive-like behavior in rats. Behav Brain Res 184:133-141.
Yang YK, Yeh TL, Yao WJ, Lee IH, Chen PS, Chiu NT, Lu RB (2008) Greater availability of dopamine transporters in patients with major depression--A dual-isotope SPECT study. Psychiatry Res 162:230-235.
Yarkov AV, Hanger D, Reploge M, Joyce JN (2003) Behavioral e ff ects of dopamine agonists and antagonists in MPTP-lesioned D3 receptor knockout mice. Pharmacol Biochem Behav 76:551-562.
Yeritsyan N, Navasardyan G, Strekalova T, Avetisyan L (2003) Hypokinesia in rats leads to de fi cits in complex instrumental learning. Behav Pharmacol 14:S35-35.
Zhou FM, Liang Y, Salas R, Zhang LF, De Biasi M, Dani JA (2005) Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron 46:65-74.
Copyedited by McGowan D, Yajima W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
Lun Zheng, Key Laboratory of Mental Health, Chinese Academy of Sciences, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China, zhengluntj@163.com.
10.4103/1673-5374.139464
http://www.nrronline.org/
Accepted: 2014-06-19
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Growth factor- and cytokine-stimulated endothelial progenitor cells in post-ischemic cerebral neovascularization
- The role of DJ-1 in the oxidative stress cell death cascade after stroke
- Perspectives on the neural connectivity of the fornix in the human brain
- Optimal therapeutic dose and time window of picroside II in cerebral ischemic injury
- Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus
- Pretreatment with Danhong injection protects the brain against ischemia-reperfusion injury
