A New Nickel(Ⅱ)Complex Based on Novel Pyridyl-carboxylate Schiff-Base Ligand:Synthesis and Crystal Structure
2013-09-29WANGYanWANGTaoWANGKuaiBingLIUGuangXiangCHENYouCunSchoolofChemistryandChemicalEngineeringAnhuiKeyLaboratoryofFunctionalCoordinationCompoundsAnqingTeachersCollegeAnqingAnhui460ChinaStateKeyLaboratoryofCoordinationChemistry
WANG YanWANG TaoWANG Kuai-BingLIU Guang-XiangCHEN You-Cun(School of Chemistry and Chemical Engineering,Anhui Key Laboratory of Functional Coordination Compounds,Anqing Teachers College,Anqing,Anhui 460,China)(State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 0093,China)
A New Nickel(Ⅱ)Complex Based on Novel Pyridyl-carboxylate Schiff-Base Ligand:Synthesis and Crystal Structure
WANG Yan*,1,2WANG Tao1WANG Kuai-Bing1LIU Guang-Xiang1CHEN You-Cun1
(1School of Chemistry and Chemical Engineering,Anhui Key Laboratory of Functional Coordination Compounds,Anqing Teachers College,Anqing,Anhui 246011,China)(2State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
A novel nickel(Ⅱ) coordination polymer{[Ni(HL)2(H2O)2]·2H2O}n(H2L=5-((pyridin-3-ylmethyl)amino)isophthalic acid)(1)has been synthesized by the reaction of Ni(NO3)2·6H2O and H2L under hydrothermal condition.The structure of 1 was determined by single-crystal X-ray diffraction.Complex 1 crystallizes in triclinic space groupwith a=0.76479(11)nm,b=0.87049(13)nm,c=1.08113(16)nm,α=85.583(2)°,β=82.614(2)°,γ=81.565(2)°,V=0.70482(18)nm3,Z=1,Dc=1.586 g·cm-3,μ=0.763 mm-1and F(000)=350.The coordination environment of Ni(Ⅱ) is octahedral,and each ligand links two Ni(Ⅱ)atoms using its pyridyl N atom and carboxylate O atom to generate an infinite one-dimensional(1D)chain structure.The 1D chains are further connected by hydrogen bonds to give a three dimensional structure.CCDC:769438.
self-assembly;crystal structure;nickel(Ⅱ)complex;Schiff-base ligand
During the past decade,many chemists have focused their efforts on the synthesis and investigation of highly organized metal-organic frameworks(MOFs)due to their fantastic structural diversities[1-2]as well as potential applications in many areas including gas storage[3],anion exchange[4],catalysis[5]and so on[6-7].Up to now,multidentate N-or O-donor ligands have been widely used and many MOFs have been constructed bythe reactions of N-containing or O-containing ligands with corresponding metal salts,such as 1,3,5-Tris(imidazol-1-yimethyl)benzene[8],1,3,5-benzenetriacetic acid[9],and benzenedicarboxylic acid[10].
Our research is concentrated on the synthesis of coordination polymers based on N,O-bifunctional ligands.Due to the different coordination abilities of N,O atoms to transition or rare-earth metals,the ligands containing both N and O as coordination donors are now often used to synthesize hetero-metallic lanthanidetransition metal complexes[11],which have attracted many attentions due to theirspecialvalue in investigation on the nature of the magnetic exchange interactions between 3d and 4f metal ions in magnetic materials that contain rare earth metals[12].Meanwhile,Schiff-base ligands have also attracted many attentions of chemists in the past few years[13-14]because of the convenience in synthesis,good coordination ability and wide applications of Schiff-base complexes.Herein,we report the hydrothermal synthesis and crystal structure of a new nickel(Ⅱ)complex based on novel pyridylcarboxylate Schiff-base ligand.
1 Experimental
1.1 General
All commerciallyavailablechemicalsareof reagent grade and used as received without further purification.Solvents were purified according to the standard methods.C,H and N analyses were made on Elementar Vario EL-Ⅲ elemental analyzer.Infrared(IR)spectra were recorded on Nicolet AVATAR 360 FTIR spectrophotometer by using KBr discs.1H NMR spectrawererecorded on theBrukerDRX-500 spectrometer.
1.2 Structure determinations
A suitable colorless single crystal with dimensions of 0.30 mm×0.30 mm×0.10 mm was selected for data collection at 296 K,using a Bruker Smart ApexⅡCCD equipped with a Mo Kα radiation (λ=0.071 073 nm).The structures were solved by direct methods with SHELXS-97 program[15]and refined with SHELXL-97[16]by full-matrix least-squares techniques on F2.All nonhydrogen atomswere refined anisotropically and hydrogen atoms isotropically.The hydrogen atoms except for those of water molecules were generated geometrically.The details of the crystal parameters,data collection and refinement for the compounds are summarized in Table 1,and selected bond lengths and angels with their estimated standard deviations of 1 are listed in Table 2.The hydrogen bond lengths and bond angles are listed in Table 3.
CCDC:769438.

Table 1 Crystallographic data for complex 1

Table 2 Selected bond lengths(nm)and bond angles(°)for complex 1
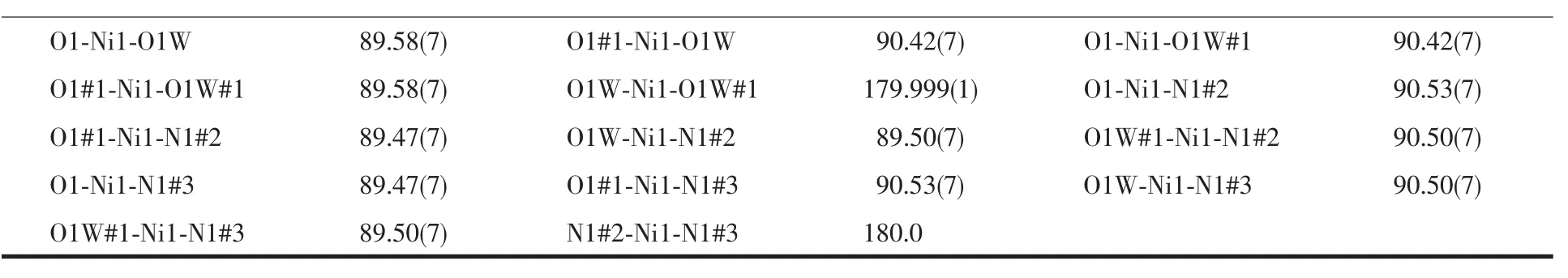
Continued Table 2

Table 3 Hydrogen bond lengths and bond angles for 1
1.3 Synthesis of 5-((pyridin-3-ylmethyl)amino)isophthalic acid(H2L)
The ligand was synthesized according to the previously reported literatures[17].To a dry CH3OH solution(30 mL)of 5-aminoisophthalic acid(50 mmol,9.050 g)was slowly added 3-picolinaldehyde(50 mmol,5.360 g)in 25 mL CH3OH.The resulting yellow solution was stirred over night and then refluxed for 8 h.After been cooled to room temperature,excess NaBH4was added.The pH value of the solution was adjusted to 5.5 with HCl and the pale yellow precipitate was collected,washed with water and recrystallized in ethanol/H2O solution.The ligand was obtained in 75%yield.FTIR (KBr pellet,cm-1):1677s,1672s,1566m,1 525s,1 469s,1 437m,1 421m,1 315s,1 288s,1 174s,835m,777m,763m.1H NMR (500 MHz,d6-DMSO,ppm):δ:8.52(s,1H),8.44(d,1H),7.90(t,2H),7.58(s,2H),7.38(t,1H),4.39(s,2H).
1.4 Synthesis of Ni(HL)2(H2O)2·2H2O(1)
Complex 1 was synthesized by hydrothermal method.To a suspension of H2L (0.027 g,0.1 mmol)in 10 mL water was added NaOH (0.008 g,0.2 mmol),Ni(NO3)2·6H2O (0.029 g,0.1 mmol),and 2 mL C2H5OH.After being stirred for 30 min,the resulting solution was sealed into a bomb equipped with a Teflon liner and heated at 120℃for 72 h.After slow cooling of the reaction mixture to room temperature,green crystals of 1 suitable for X-ray analysis were obtained in 55%
yield.Anal.Calcd.for C28H30N4Ni1O12(%):C 49.95,H 4.50,N 8.32;found(%):C 50.01,H 4.56,N 8.33.
2 Results and discussion
2.1 Structure description
Complex 1 was prepared by the reaction of Ni(NO3)·6H2O with the sodium salt of H2L ligand in C2H5OH/H2O system and crystallized in triclinic system,space groupThe coordination environment around Ni(Ⅱ)centers are depicted in Fig.1.In complex 1,the Ni(Ⅱ)ions are six coordinated,where the equatorial plane is completed by two coordinated water molecules and two carboxylic O atoms from two different ligands with Ni-O distance of 0.202 31(15)and 0.209 89(17)nm,respectively.The apical positions are occupied by two N atoms from another two different ligands with Ni-N distance of 0.2104(2)nm,and the N-Ni-O,O-Ni-O and N-Ni-N bond angles vary from 89.47(7)°to 180°.Thus,the coordination geometry of the six-coordinated Ni(Ⅱ)center can be regarded as a slightly distorted octahedral in N2O4set.It is interesting that although double base has been used,there are still half carboxylate groups are not deprotonated,which just do not participate in the coordination to the metal centers.On the other hand,the deprotonated carboxylate groups coordinate to Ni(Ⅱ) with μ1-η1∶η0coordination mode.
In complex 1,each ligand coordinates to two Ni(Ⅱ)ions with its pyridyl and deprotonated carboxylategroups,and each metal ions links two ligand to form a 22-membered macrocycle,where the Ni…Ni distances are 1.081 nm.The two benzene rings in one cycle are parallel to each other with centroid…centroid distance of 0.412 nm,indicating the existence of rather weak π…πinteractionbetweenthetwoaromaticrings(Fig.2)[18].The dihedral angle of benzene ring and pyridyl ring of the ligand is 73.5°.This macrocycle spread alongcaxis to form a one-dimensional chain structure in 1.

Fig.1 Coordination environment around Ni(Ⅱ)center in complex 1

Fig.2 2D network structure of complex 1 linked by C-H…O hydrogen bonds viewing along theaaxis,where the uncoordinated water molecules were omitted for clarity
The 1D chains are further connected by hydrogen bonds to give a three-dimensional(3D)structure[19-20].The hydrogen bonding data of 1 are summarized in Table 2.First,the C11…O1W#1 distance of 0.335 3(3)nm and C11-H11-O1W#1 angle of 143°indicate the formation of C11-H11…O1W#1 hydrogen bond between the neighboring 1D chains,which link the 1D chains to produce a two-dimensional(2D)network(Fig.2).Furthermore,such 2D networks are linked by another O2W-H2WA…O3#1,N2-H2…O2#2,O2WH2WB…O2#3,O1W-H1WB…O2W#1,O4-H4A…O3#4 hydrogen bonds (Table 2)to generate 3D framework.The results reveal that the hydrogen bonds play important role in stabilizing the whole structure of complex 1.The investigations on reactions of ligand H2L with other metal salts are still in progress.
[1]Ganguly R,Sreenivasulu B,Vittal J J.Coord.Chem.Rev.,2008,252:1027-1050
[2]Batten S R,Robson R.Angew.Chem.,Int.Ed.,1998,37:1460-1494
[3]Suh M P,Cheon Y E,Lee E Y.Coord.Chem.Rev.,2006,252:1007-1026
[4]Xu G C,Ding Y J,Okamura T A,et al.CrystEngComm,2008,10:1052-1062
[5]Hasegawa S,Horike S,Matsuda R,et al.J.Am.Chem.Soc.,2007,129:2607-2614
[6]Su Z,Cai K,Fan J,et al.CrystEngComm.,2010,12:100-108
[7]Chen M S,Chen S S,Okamura T A,et al.J.Coord.Chem.,2009,62:2421-2428
[8]Xu G C,Ding Y J,Okamura T A,et al.Cryst.Growth Des.,2009,9:395-403
[9]LIU Guang-Xiang(刘光祥),CHU Qian(储钱),KAWAGUCHI Hiroyuki(川口博之),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2007,28(7):1203-1207
[10]Su Z,Fan J,Okamura T A,et al.Cryst.Growth Des.,2010,10:1911-1922
[11]Gu X J,Xue D F.Cryst.Growth&Des.,2007,7:1726-1732
[12]Liang Y C,Cao R,Su W P,et al.Angew.Chem.Int.Ed.,2000,39:3304-3307
[13]Bazzicalupi C,Bencini A,Bianchi A,et al.Coord.Chem.Rev.,2008,252:1052-1068
[14]Ganguly R,Sreenivasulu B,Vittal J J.Coord.Chem.Rev.,2008,252:1027-1050
[15]Sheldrick G M.SHELXS-97,Program for Crystal Structure Determination,University of Göttingen,Germany,1997.
[16]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[17]Huang Y T,Ouyang X M,Okamura T A,et al.Chinese J.Inorg.Chem.,2005,21:1479-1482
[18]LIU Shu-Wen(刘书文),WU Xiu-Mei(吴秀梅),LIU Qing-Xiang(柳清湘),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(4):1444-1449
[19]WANG Yan(王彦),WANG Xiao-Feng(王晓锋),OKAMURA Taka-aki(岡村高明),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22(8):1487-1490
[20]ZHANG Shu-Guang(张曙光),FENG Yun-Long(冯云龙),WEN Yi-Hang(温一航).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(4):581-585
新型吡啶基羧酸席夫碱配体镍配合物的合成和晶体结构
王 彦*,1,2王 涛1汪快兵1刘光祥1陈友存1
(1安庆师范学院化学化工学院,安徽省功能配合物重点实验室,安庆 246011)(2南京大学配位化学国家重点实验室,南京 210093)
通过新合成的吡啶基羧酸席夫碱配体(5-(吡啶基-3-亚甲基氨基)间苯二甲酸H2L),和镍盐反应制备了一个新的镍配合物{[Ni(HL)2(H2O)2]·2H2O}n(1),利用元素分析及X-射线单晶衍射对其进行了表征。结构分析结果表明标题化合物属于三斜晶系,P1 空间群,晶胞参数为 a=0.764 79(11)nm,b=0.870 49(13)nm,c=1.081 13(16)nm,α=85.583(2)°,β=82.614(2)°,γ=81.565(2)°,V=0.70482(18)nm3,Z=1,Dc=1.586 g·cm-3,μ=0.763 mm-1,F(000)=350。在配合物的结构中,Ni(Ⅱ)的配位构型为畸变的八面体。配合物中每个配体通过羧酸氧和吡啶氮原子和金属配位形成一维链状结构,并通过体系中丰富的氢键作用链接成为三维框架并加以稳定。
自组装;晶体结构;镍配合物;席夫碱
O614.24+2
:A
:1001-4861(2011)01-0193-04
2010-07-05。收修改稿日期:2010-08-15。
国家自然科学基金资助项目(No.20901004,20971004,20771006)。
*通讯联系人。E-mail:njwangy@126.com
猜你喜欢
杂志排行
无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties
