5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia******☆
2012-01-04BailiuYaLanZhangLiZhangYaliLiLinLi
Bailiu Ya, Lan Zhang Li Zhang Yali Li Lin Li
1 Department of Pharmacology, Xuanwu Hospital of Capital Medical University, Beijing Geriatric Medical Research Center, Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Beijing 100053, China
2 Department of Physiology, Jining Medical University, Jining 272067, Shandong Province, China
5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia******☆
Bailiu Ya1,2, Lan Zhang1, Li Zhang1, Yali Li1, Lin Li1
1Department of Pharmacology, Xuanwu Hospital of Capital Medical University, Beijing Geriatric Medical Research Center, Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Beijing 100053, China
2Department of Physiology, Jining Medical University, Jining 272067, Shandong Province, China
In the present study, we hypothesized that 5-hydroxymethyl-2-furfural could attenuate ischemic brain damage by reducing oxidative injury. Thus, mice were subjected to bilateral common carotid artery occlusion to establish a model of permanent forebrain ischemia. The mice were intraperitoneally injected with 5-hydroxymethyl-2-furfural 30 minutes before ischemia or 5 minutes after ischemia. The survival time of mice injected with 5-hydroxymethyl-2-furfural was longer compared with untreated mice. The mice subjected to ischemia for 30 minutes and reperfusion for 5 minutes were intraperitoneally injected with 5-hydroxymethyl-2-furfural 5 minutes prior to reperfusion, which increased superoxide dismutase content and reduced malondialdehyde content, similar to the effects of Edaravone, a hydroxyl radical scavenger used for the treatment of stroke. These findings indicate that intraperitoneal injection of 5-hydroxymethyl-2-furfural can prolong the survival of mice with permanent forebrain ischemia. This outcome may be mediated by its antioxidative effects.
5-hydroxymethyl-2-furfural; forebrain ischemia; survival time; oxidative stress; superoxide dismutase; malondialdehyde; mouse
Research Highlights
(1) 5-hydroxymethyl-2-furfural pretreatment and treatment prolonged the survival of mice with permanent forebrain ischemia. In addition, these effects were associated with increased brain superoxide dismutase activity and reduced malondialdehyde content.
(2) The ability of 5-hydroxymethyl-2-furfural to prolong survival may be mediated by these antioxidative effects.
Abbreviations
BCAO, bilateral common carotid artery occlusion; MDA, malondialdehyde; SOD, superoxide dismutase
lNTRODUCTlON
5-hydroxymethyl-2-furfural (molecular structure shown in Figure 1) is a five carbon-ring aromatic aldehyde[1].
Controversial results about its toxicity have been published over the last few decades. Some studies found that it has toxicity, mutagenicity and carcinogenicity[2-6], and significant adverse effects on the overall metabolism of human blood cells[4], leading to increased activity of hepatic enzymes, altered serum protein profiles and hepatic fatty degeneration[5], as well as the formation of colonic aberrant crypt foci in rats[6]. However, some evidence shows that 5-hydroxymethyl-2-furfural may bebeneficial to health at an appropriate dose. It is currently in phase I clinical trials for treating sickle cell anemia. At a concentration of 100 μM, 5-hydroxymethyl-2-furfural can inhibit lipid accumulation to influence the differentiation of mouse 3T3-L1 preadipocytes into adipocytesin vitro[7]. 5-hydroxymethyl-2-furfural in the steamed root ofRehmanniae Radixmay be responsible for the clinical efficacy of the plant, which is used for treating anemia, palpitations and other conditions[8].
Recent studies indicate that 5-hydroxymethyl-2-furfural is an anti-oxidant[9]and protects against hypoxiain vitro[10]andin vivo[11].

Figure 1 Structure of 5-hydroxymethyl-2-furfural (A) and Edaravone (B).
Based on these observations, we hypothesized that 5-hydroxymethyl-2-furfural would protect neurons against oxidative stress in ischemic brain injury. To test this hypothesis, we investigated the neuroprotective effect of 5-hydroxymethyl-2-furfural using mice subjected to bilateral common carotid artery occlusion (BCAO; a model of forebrain ischemia) and compared its effects with that of Edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolone-5-one), a hydroxyl radical scavenger commonly being used in the management of forebrain ischemic stroke[12].
RESULTS
Quantitative analysis of experimental animals
In experiment 1, 40 male ICR mice were used. They were equally and randomly assigned to model (normal saline), 4 mg/kg 5-hydroxymethyl-2-furfural, 12 mg/kg 5-hydroxymethyl-2-furfural and Edaravone (10 mg/kg) groups. The treatments were given by intraperitoneal injection 30 minutes before BCAO. All of the mice were included in the final analysis.
In experiment 2, 40 male ICR mice were used. They were equally and randomly assigned to model (normal saline), 4 mg/kg 5-hydroxymethyl-2-furfural, 12 mg/kg 5-hydroxymethyl-2-furfural and Edaravone (10 mg/kg) groups. The treatments were given by intraperitoneal injection 5 minutes after ischemia. One mouse died after anesthesia in the 4 mg/kg 5-hydroxymethyl-2-furfural group. The remaining 39 mice were included in the final analysis. In experiment 3, 50 male ICR mice were used. They were equally and randomly assigned to sham-surgery (normal saline), model (normal saline), 4 mg/kg 5-hydroxymethyl-2-furfural, 12 mg/kg 5-hydroxymethyl-2-furfural and Edaravone (10 mg/kg) groups. The treatments were given by intraperitoneal injection 5 minutes after reperfusion. All of the mice were included in the final analysis.
5-hydroxymethyl-2-furfural prolonged survival of mice with permanent forebrain ischemia
In experiment 1, the Kaplan-Meier survival plots showed that the survival time curves were shifted rightward after 5-hydroxymethyl-2-furfural treatment, indicating that the survival time was longer in the 5-hydroxymethyl-2- furfural groups compared with the model group (Figure 2A). Pretreatment with low and high doses of 5-hydroxymethyl-2-furfural prolonged the survival time by 29% and 33%, respectively, compared with the model group. Edaravone also significantly prolonged survival time (Figure 2B).
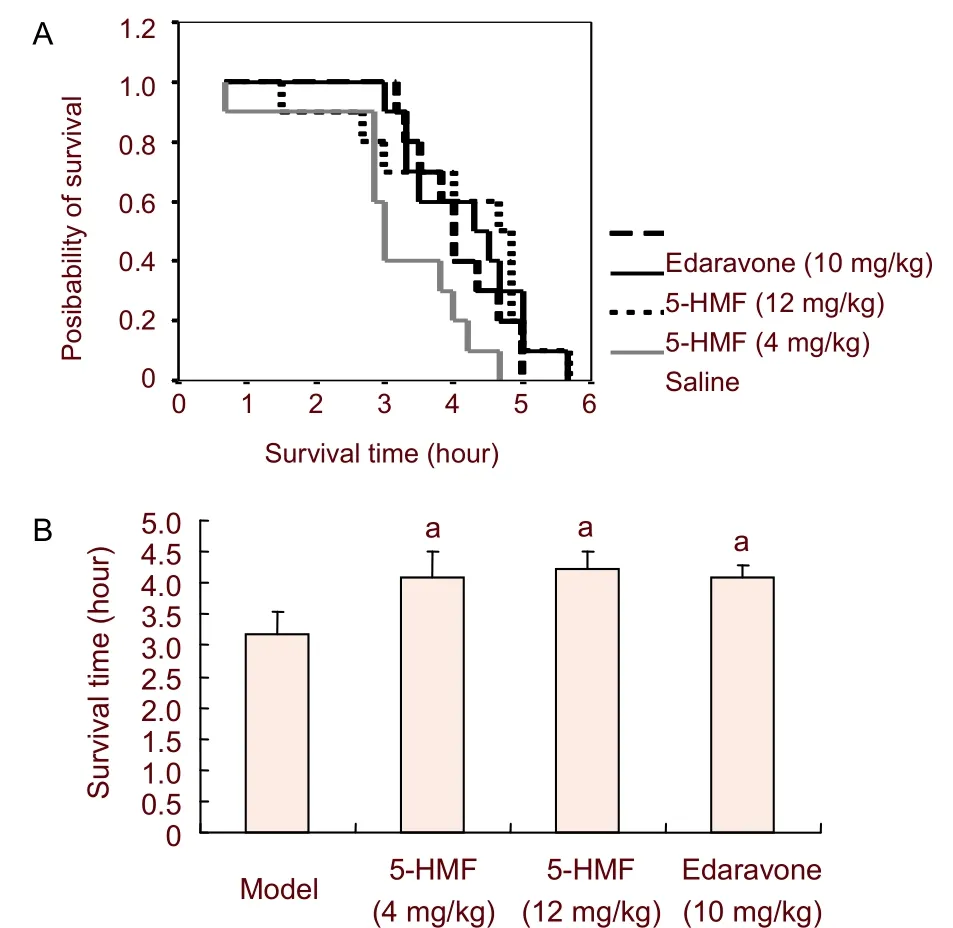
Figure 2 Effect of pretreatment with 5-hydroxymethyl-2-furfural (5-HMF) on the survival time of mice with permanent forebrain ischemia.
In experiment 2, the Kaplan-Meier survival plots showed that the survival time curves were shifted rightward after3A), indicating a significant increase in survival time (31%) compared with the model group. Edaravone exhibited similar effects as 4 mg/kg 5-hydroxymethyl-2-furfural, not affecting survival time (Figure 3B).
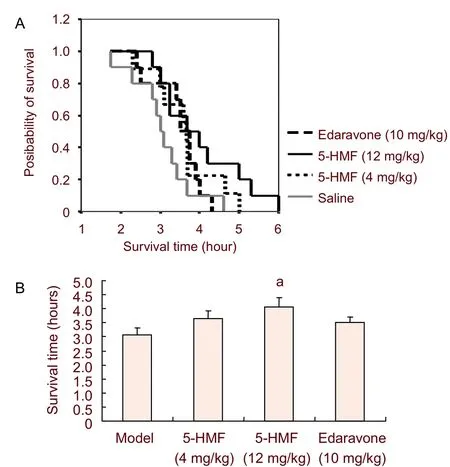
Figure 3 Effect of 5-hydroxymethyl-2-furfural (5-HMF) treatment after ischemia on the survival time in mice with permanent forebrain ischemia.
5-hydroxymethyl-2-furfural reduced malondialdehyde (MDA) content in brain tissues of mice with transient forebrain ischemia
To gain insight into the neuroprotective mechanism underlying the capacity of 5-hydroxymethyl-2-furfural to prolong survival time, we evaluated the ability of the compound to protect against oxidative stress. MDA is a metabolite of lipid peroxidation, which is commonly used to assess the level of oxidative stress in the brain[13]. In experiment 3, transient forebrain ischemia was achieved by performing 30 minutes of BCAO followed by 24 hours of reperfusion. Drugs were given 25 minutes after ischemia (5 minutes before the onset of reperfusion).
There was a significant increase in the concentration of MDA in the brain tissue of the model group compared with the sham-surgery group (P< 0.01). Treatment with 4 and 12 mg/kg 5-hydroxymethyl-2-furfural significantly reduced MDA contents, by approximately 15% and 21%, respectively, compared with the model group (P< 0.01; Figure 4).
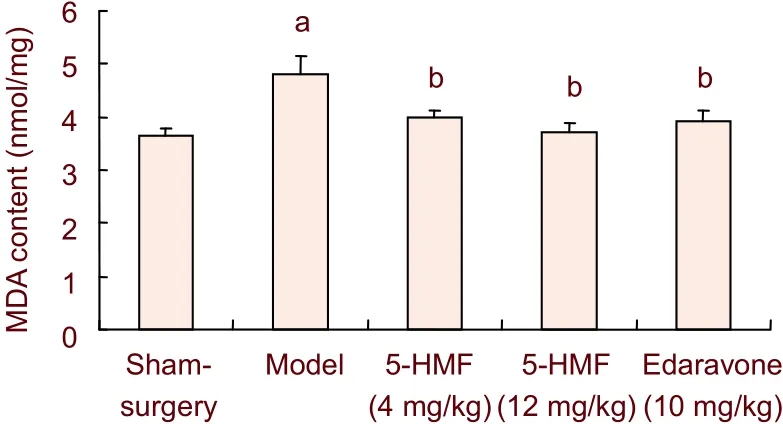
Figure 4 Effect of 5-hydroxymethyl-2-furfural (5-HMF) on malondialdehyde (MDA) content in brain tissue of mice with transient forebrain ischemia.
5-hydroxymethyl-2-furfural increased superoxide dismutase (SOD) activity in brain tissues of mice with transient forebrain ischemia (Figure 5)
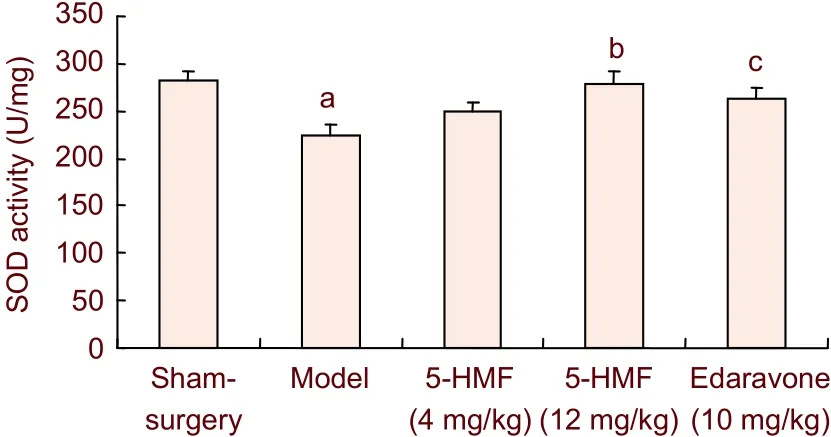
Figure 5 Effects of 5-hydroxymethyl-2-furfural (5-HMF) on superoxide dismutase (SOD) activity in brain tissue in mice with transient forebrain ischemia. Transient forebrain ischemia was induced in mice by 30 minutes of ischemia and 24 hours of reperfusion.
DlSCUSSlON
The BCAO model has been extensively used to study the impact of ischemic brain injury. It simulates the effects produced by the systemic decrease in blood flow, affects the entire brain, and resembles clinical transient forebrain ischemia in a number of pathological aspects[15-16]. In the present study, the BCAO model was used to evaluate the effect of 5-hydroxymethyl-2-furfural on the survival time of mice. We found that intraperitoneal injection of 5-hydroxymethyl-2-furfural 30 minutes before ischemia or 5 minutes after ischemia significantly prolonged the survival time of mice subjected to BCAO. This finding indicates that 5-hydroxymethyl-2-furfural may not only have a preventive effect against ischemic brain injury, but it may also be beneficial for the treatment of acute stroke. Furthermore, we attempted to identify the mechanisms underlying the ability of 5-hydroxymethyl-2-furfural to prolong survival time.
Reactive oxygen species have been shown to contribute to ischemic brain damage. Enhanced generation of reactive oxygen species during brain ischemia may easily overcome anti-oxidant defenses as anti-oxidant enzymes are present in relatively low concentrations in the brain. Reactive oxygen species cause lipid peroxidation, protein oxidation and DNA damage[17-19]. Furthermore, reactive oxygen species can activate diverse downstream signaling pathways, thus regulating the expression of a variety of proinflammatory proteins[20-21]. Such phenomena may become prominent during reperfusion wherein the supply of oxygen and substrates to energy-deprived and metabolically perturbed tissue is restored[22-23]. Antioxidant defenses, including free radical scavengers and antioxidants, are considered the most effective means of attenuating tissue injury induced by ischemia/reperfusion[24-26]. Therefore, the effects of numerous antioxidants on ischemic tissue damage have been evaluated over the last two decades.
To investigate the mechanism underlying the protection mediated by 5-hydroxymethyl-2-furfural against ischemic brain injury, lipid peroxidation and antioxidant defenses in the injured brain tissue of mice were measured in the present study. We found that 30 minutes of BCAO ischemia and 24 hours of reperfusion induced an increase in the content of MDA, a metabolite of lipid peroxidation, and a decrease in the activity of SOD, an antioxidant enzyme, in the brain tissue of mice. Intraperitoneal injection of 5-hydroxymethyl-2-furfural 25 minutes after BCAO ischemia (5 minutes before the onset of reperfusion) inhibited lipid peroxidation, as shown by decreased MDA content, and enhanced antioxidant defenses by increasing the activity of SOD, which scavenges free radicals, such as superoxide anions. These results suggest that the neuroprotective effect of 5-hydroxymethyl-2-furfural may be attributable to its antioxidant action. Further study is required to elucidate whether the effects of 5-hydroxymethyl-2-furfural involve other mechanisms.
In summary, 5-hydroxymethyl-2-furfural prolongs the survival time of mice subjected to forebrain ischemia induced by BCAO. One of the underlying neuroprotective mechanisms may involve the ability of 5-hydroxymethyl-2-furfural to increase SOD activity and decrease free radical levels.
MATERlALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
This experiment was performed at the Animal Laboratory of Xuanwu Hospital of Capital Medical University, China from May to December 2009.
Materials
Animals
以上就是二连浩特口岸志的全部内容。虽然二连口岸现在仍存在许多问题,如口岸基础建设较简单薄弱、口岸依托城市南北经济面貌差异较大、受国际关系影响波动较大等问题有待于进一步提升,但随着国家“一带一路”倡议的逐步落实和中蒙俄经济走廊建设加快推进的不断深入,二连浩特口岸深入推进重点开发开放试验区、中蒙边民互市贸易区建设,区位优势不断凸显,这不仅对本地发展带来机遇,也能够更好地发挥二连口岸作为国家门户和桥梁、窗口的积极作用。
A total of 130 male ICR mice of specific pathogen-free grade, aged 3 months, weighing 25-30 g, were purchased from Beijing Vitalriver Experimental Animal Co., Beijing, China (certificate No. SCXK (Jing) 2007-0001), and were housed under a 12-hour light/dark cycle and specific pathogen-free conditions. During the entire experiment, the mice were allowed free access to food and water. All experimental procedures were conducted in accordance with the Provisions and General Recommendations of Chinese Experimental Animal Administration Legislation.
Drugs
5-hydroxymethyl-2-furfural was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in sterile saline. 5-hydroxymethyl-2-furfural molecular formula is C6H6O3, with a molecular weight of 126.11.
Edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolone-5-one) injection was purchased from Nanjing Simcere Pharmaceutical Co., Ltd. (Nanjing, China; No. 070313, certificate No. H20031342).
Methods
Establishment of forebrain ischemia model
Forebrain ischemia model was produced according to a previously described method[27-28]. Briefly, the animals were fasted overnight from food but allowed free access to water. The mice were anaesthetized with 10% chloral hydrate (0.4 mL/kg, intraperitoneal injection) and allowed to breathe spontaneously. During the surgical procedure, the body temperature of the animal was maintained at 37.0 ± 0.5°C with a heating pad. Permanent forebrain ischemia was induced by BCAO. A midline incision was made in the neck, and the bilateral common carotid arteries (BCAs) were carefully exposed and separated from the surrounding connective tissue and nerve fibers, and encircled with a 4/0 silk thread. Then the BCAs were completely occluded using the silk threads. For transient forebrain ischemia, the BCAs were carefully exposed and encircled loosely with 4/0 silk threads to enable later occlusion with non-traumatic artery clips. The BCAs were then occluded using artery clips for 30 minutes. Following the occlusion, the clips were removed to allow complete reperfusion. The sham-surgery mice were subjected to the same procedures except for occlusion of the BCAs. After surgery, the wound was sutured and the animals were placed under heat lamps to maintain body temperature until they fully recovered from anesthesia.
The mice were returned to their cage and given free access to water and food. All mice were kept warm using an electric heating blanket before they awoke and were able to move spontaneously.
Interventions
The experimental protocols were as follows:Experiment 1 investigated the effect of pretreatment with 5-hydroxymethyl-2-furfural on the survival time of mice after permanent forebrain ischemia. Mice in the 5-hydroxymethyl-2-furfural and Edaravone groups were intraperitoneally injected with 5-hydroxymethyl-2-furfural (4 or 12 mg/kg) and Edaravone (10 mg/kg), respectively, 30 minutes before ischemia. The selection of the dosages of 5-hydroxymethyl-2-furfural and Edaravone was based on a preliminary experiment in the present study and on a previous study[29]. Mice in the model group were injected with 0.9% saline (5 mL/kg). The survival time, defined as the time between the onset of ischemia and respiratory arrest (the last observable inspiratory movement), was determined.
Experiment 2 examined the effect of 5-hydroxymethyl-2-furfural treatment on the survival time of mice after permanent forebrain ischemia. Mice were intraperitoneally injected with 5-hydroxymethyl-2-furfural (4 or 12 mg/kg) or Edaravone (10 mg/kg) 5 minutes after ischemia. Mice in the model group were administered 0.9% normal saline. Survival time was determined as in experiment 1.
Experiment 3 evaluated the effect of 5-hydroxymethyl-2-furfural on oxidative stress after transient forebrain ischemia induced by 30 minutes of BCAO ischemia followed by 24 hours of reperfusion. Mice in the 5-hydroxymethyl-2-furfural groups and Edaravone group were intraperitoneally injected with 5-hydroxymethyl-2-furfural (4 or 12 mg/kg) and Edaravone (10 mg/kg), respectively, 25 minutes after ischemia (5 minutes before the onset of reperfusion). The mice in the model and sham-surgery groups received 0.9% normal saline. The mice were sacrificed 24 hours after ischemia/reperfusion. The brain tissues were harvested and homogenized in phosphate buffer to determine MDA levels and SOD activity.
Determination of brain MDA content and SOD activity
Twenty-four hours after forebrain ischemia/reperfusion, the animals were re-anesthetized. Their brains were removed quickly and stored at -80°C until use. For measurements of MDA level and SOD, the brain tissues were homogenized in 0.1 M sodium phosphate buffer (pH 7.4) at a ratio of 1:10 (w/v). Then the homogenate was centrifuged at 3 000 ×gat 4°C for 10 minutes. Protein concentrations were determined utilizing Lowry’s method[30], with bovine serum albumin as the standard. MDA level and SOD activity were determined following the kit instructions (SOD Detection Kit and MDA Detection Kit were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, MDA content was assessed by measuring levels of thiobarbituric acid reactive substances at 532 nm using a spectrophotometer (WFZ800-3, Beijing Beifen-Ruili Analytical Instrument (Group) Co., Ltd., Beijing, China). The assay for total SOD activity was based on the enzyme’s ability to inhibit the oxidation of oxyamine by O2-produced from the xanthine-xanthine oxidase system. One unit of SOD activity was defined as the capability to reduce the absorbance at 550 nm by 50%. MDA was assayed based on the reaction of MDA with thiobarbituric acid to form a pink chromogen. The results from the thiobarbituric acid reactive substances assay were expressed as MDA equivalents. The measurements were performed according to the detailed procedures provided in the manufacturer’s instructions[31-34].
Statistical analysis
All values were expressed as mean ± SD. The survival time was analyzed with log rank survival analysis of the Kaplan-Meyer survival curves. Statistical significance of differences for SOD activity and MDA levels was calculated using one-way analysis of variance followed bypost hocFisher protected least significant differencet-test. A value ofP< 0.05 was considered statistically significant.
Funding: This project was supported by the National Basic Research Program of China (973 Program), No. 2003CB517104; the National Natural Science Foundation of China, No. 30973513; Beijing Municipal Science and Technology Program, No. D0206001043191; the Natural Science Foundation of Beijing, No.7112061; Beijing Key Foundation of Traditional Chinese Medicine, No. KJTS2011-04; and Beijing Health and Technical Personal of High-Level Plan, No. 2009-3-66.
Author contributions: Bailiu Ya and Lan Zhang designed the study, conducted experiments, analyzed data and wrote manuscript. Li Zhang and Yali Li provided technical support. Lin Li was in charge of funds, authorized this study and revised this manuscript. All authors read and approved the final manuscript. Conflicts of interest: None declared.
Ethical approval: This study received permission from the
Animal Care and Research Committee of Xuanwu Hospital of Capital Medical University, China.
[1] Wu CD. Grape products and oral health. J Nutr. 2009; 139(9):1818S-1823S.
[2] Janzowski C, Glaab V, Samimi E, et al. 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem Toxicol. 2000;38(9):801-809.
[3] Lee YC, Shlyankevich M, Jeong HK, et al. Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylic sulfuric acid ester. Biochem Biophys Res Commun. 1995;209(3):996-1002.
[4] Nässberger L. Influence of 5-hydroxymethylfurfural (5-HMF) on the overall metabolism of human blood cells. Hum Exp Toxicol. 1990;9(4):211-214.
[5] Ulbricht RJ, Northup SJ, Thomas JA. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Fundam Appl Toxicol. 1984;4(5):843-853.
[6] Zhang XM, Chan CC, Stamp D, et al. Initiation and promotion of colonic aberrant crypt foci in rats by 5-hydroxymethyl-2-furaldehyde in thermolyzed sucrose. Carcinogenesis. 1993;14(4):773-775.
[7] Matsuda E, Yoshizawa Y, Yokosawa Y, et al. Effects of Eucommia ulmoides Oliver leaf extract on 3T3-L1 differentiation into adipocytes. J Nat Med. 2006;60(2):26-29.
[8] Lin AS, Qian K, Usami Y, et al. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J Nat Med. 2008;62(2):164-167.
[9] Li YX, Li Y, Qian ZJ, et al. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. J Microbiol Biotechnol. 2009;19(11):1319-1327.
[10] Li MM, Wu LY, Zhao T, et al. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2011; 16(3):267-273.
[11] Li MM, Wu LY, Zhao T, et al. The protective role of 5-hydroxymethyl-2-furfural (5-HMF) against acute hypobaric hypoxia. Cell Stress Chaperones. 2011;16(5):529-537.
[12] Zhang P, Li W, Li L, et al. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience. 2012;201:297-306.
[13] Zakaria MM, Hajipour B, Khodadadi A, et al. Ameliorating effects of dexpanthenol in cerebral ischaemia reperfusion induced injury in rat brain. J Pak Med Assoc. 2011;61(9):889-892.
[14] Yang C, Wang H, Zhang S, et al. Neuroprotective effects of NKN on focal cerebral ischemia in rats. Turk Neurosurg. 2012;22(1):1-6.
[15] Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet. 2008;371(9624):1612-1623.
[16] Warlow CP. Epidemiology of stroke. Lancet. 1998;352 Suppl 3:SIII1-4.
[17] Bora KS, Sharma A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm Biol. 2011;49(12):1216-1223.
[18] Bora KS, Arora S, Shri R. Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. J Ethnopharmacol. 2011;137(3):1360-1365.
[19] Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32 Suppl:S22-27.
[20] Kim DW, Lee SH, Jeong MS, et al. Transduced Tat-SAG fusion protein protects against oxidative stress and brain ischemic insult. Free Radic Biol Med. 2010;48(7):969-977.
[21] Chen H, Yoshioka H, Kim GS, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14(8):1505-1517.
[22] Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38(11):1433-1444.
[23] Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391-1401.
[24] Yang Q, Dong H, Deng J, et al. Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg. 2011;112(4):931-937.
[25] Chrissobolis S, Miller AA, Drummond GR, et al. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci. 2011;16:1733-1745.
[26] Qi J, Hong ZY, Xin H, et al. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol Pharm Bull. 2010; 33(12):1958-1964.
[27] Kuang X, Yao Y, Du JR, et al. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006;1102(1):145-153.
[28] Yoshioka H, Niizuma K, Katsu M, et al. Consistent injury to medium spiny neurons and white matter in the mouse striatum after prolonged transient global cerebral ischemia. J Neurotrauma. 2011;28(4):649-660.
[29] Zhang P, Li W, Li L, et al. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience. 2012;201:297-306.
[30] Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265-275.
[31] Guo H, Hu LM, Wang SX, et al. Neuroprotective effects of scutellarin against hypoxic-ischemic-induced cerebral injury via augmentation of antioxidant defense capacity. Chin J Physiol. 2011;54(6):399-405.
[32] He Y, Qu S, Wang J, et al. Neuroprotective effects of osthole pretreatment against traumatic brain injury in rats. Brain Res. 2012;1433:127-136.
[33] Chen Y, Zhou J, Li J, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36-45.
[34] Ye N, Liu S, Lin Y, et al. Protective effects of intraperitoneal injection of TAT-SOD against focal cerebral ischemia/reperfusion injury in rats. Life Sci. 2011; 89(23-24):868-874.
Cite this article as:Neural Regen Res. 2012;7(22):1722-1728.
Bailiu Ya☆, Ph.D., Lecturer, Department of Physiology, Jining Medical University, Jining 272067, Shandong Province, China
Lan Zhang, Ph.D., Associate Professor, Department of Pharmacology, Xuanwu Hospital of Capital Medical University, Beijing Geriatric Medical Research Center, Key Laboratory for Neurodegenerative Diseases of Ministry of Education, Beijing 100053, China
Bailiu Ya and Lan Zhang contributed equally to this paper.
Lin Li, M.D., Ph.D., Professor, Department of Pharmacology, Xuanwu Hospital of Capital Medical University, Beijing Geriatric Medical Research Center, Key Laboratory for Neurodegenerative Diseases of Ministry of Education,
Beijing 100053, China
linli97@hotmail.com
2012-03-15
2012-06-19
(N20111216003/YJ)
Ya BL, Zhang L, Zhang L, Li YL, Li L. 5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia. Neural Regen Res. 2012;7(22):1722-1728.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.22.007
We thank the staff of Animal Laboratory, Xuanwu Hospital of Capital Medical University, China for animal care.
(Edited by Wang F, He ML/Su LL/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Early hyperbaric oxygen therapy inhibits aquaporin 4 and adrenocorticotropic hormone expression in the pituitary gland of rabbits with blast-induced craniocerebral injury**★
- Transgene expression and differentiation of baculovirus-transduced adipose-derived stem cells from dystrophin-utrophin double knock-out mouse********☆
- Thrombospondins and synaptogenesis***★
- Stem cell transplantation for treating Duchenne muscular dystrophy A Web of Science-based literature analysis*
- Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro***☆
- Precision radiotherapy for brain tumors A 10-year bibliometric analysis☆
