Afferent and efferent pathways in the visual system of the freshwater snail Planorbarius corneus
2011-12-25OksanaTuchinaValeryZhukovVictorBennoMeyerRochow
Oksana P Tuchina, Valery V Zhukov, Victor Benno Meyer-Rochow,*
(1. School of Engineering and Science, Jacobs University, Bremen D-28759, Germany; 2. Department of Agricultural and Soil Ecology, Faculty of Bioresources and Natural Usage, Kaliningrad State Technical University, Kaliningrad 236000, Russia)
Afferent and efferent pathways in the visual system of the freshwater snailPlanorbarius corneus
Oksana P Tuchina1, Valery V Zhukov2, Victor Benno Meyer-Rochow1,*
(1.School of Engineering and Science,Jacobs University,BremenD-28759,Germany; 2.Department of Agricultural and Soil Ecology,Faculty of Bioresources and Natural Usage,Kaliningrad State Technical University,Kaliningrad236000,Russia)
Afferent and efferent neural elements of the retina and central ganglia in the freshwater snailPlanorbarius corneuswere labelled using retrograde transport of neurobiotin through the optic nerve. Axons of at least some photoreceptor cells become direct contributors to the optic nerve as no synaptic junctions could be detected. The processes enter the cerebral ganglion and form a dense bundle of thin afferent fibres, the so-called optical neuropil. Efferent neurons were revealed in all ganglia, except the buccal ones. Some of the ascending axons branch in the cerebral ganglia, cross the cerebro-cerebral commissure, reach the contralateral eye and form arborizations in the eye cup. Some efferent neurons send axons to different peripheral nerves as well:n.n. intestinalis, pallialis dexter, pallialis sinister internus et externus.Serotonin- and FMRF-amide-ergic fibres were revealed in the optic nerve. These fibres belong to those central neurons which send their axons to the ipsilateral eye only. They form abundant varicoses in the eye cup and nuclear layer of the retina, and possibly help to regulate retinal sensitivity to light.
Gastropoda; Nervous system; Eye; Retrograde transport; Serotonin; FMRF-amide
Retrograde transport of dyes like cobalt chloride and neurobiotin through the optic nerves of the freshwater snailLymnaea stagnalishas shown the existence of neurons, which form connections between the two eyes and some internal organs (Zaitzeva, 1987; Zhukov & Tuchina, 2008). Moreover there are several central neurons, sending processes to both eyes (Tuchina et al, 2010). In order to investigate whether a connectivity pattern like that ofL. stagnaliswas present in other pulmonate gastropods we carried out research on the freshwater snailPlanorbarius corneus. This mollusc has well-developed camera-type eyes with a single lens (Paton & Kater, 1972), and its retinas contain many microvillar photoreceptors (Zhukov et al, 2002), whose central pathways are still not known.
The number of photoreceptor cells (Zhukov et al,2002; Bobkova et al, 2004) and the optical properties of the lens (Zhukov, 1993; Gál et al, 2004) inP. corneussuggest that eyes of this mollusc are to be involved in vision, although the corresponding behavioural reactions are not as evident as inL. stagnalis(Vakoljuk & Zhukov, 2000).
In the present work, we focused not only on the central visual pathways inP. corneus, but also on the efferent projections from brain/CNS to the eyes and the presence of some putative neurotransmitters, like 5-HT and FMRF-amide, in the retina as well. Serotonin- and FMRF-amide-ergetic fibres are known to control the light sensitivity in eyes of the marine species, such asAplysia californica(Corrent et al, 1978; Eskin & Maresh, 1982; Takahasi et al, 1989) andBulla gouldiana(Jacklet et al, 1987); and it is also known that serotonin is present in the eyes ofL. stagnalisas well, where it seemingly plays the similar role as in the marine snails (Zhukov et al, 2006).
1 Material and Methods
1.1 Animals
Adult specimens ofP. corneus(n=40), collected from ponds around Bremen (Germany) during the period from autumn 2009 to spring 2010, were kept in aquaria at room temperature under 12L:12D light conditions and fed with cabbage or dandelion leaves twice a week. Before the experiments, the snails were immobilized and then dissected under a binocular microscope (Zeiss Stemi DV4, Göttingen, Germany).
1.2 Backfilling
The brain (or isolated eye with its optic nerve in the case of backfilling) was removed and transferred to a paraffin-filled Petri dish with phosphate-buffered saline (0.1 mol PBS, pH 7.4). All of the peripheral nerves except those we were interested in for backfilling liken. opticus, n. pallialis sinister internus,externus, n. pallialis dexterandn. intestinaliswere cut at the side close to the corresponding ganglia to avoid penetration of the labelling solution. The cut ends of the nerves, which we were interested in, were placed in a tight vaseline pool, filled with distilled water, and exposed for approx. 1 min. The water was then replaced with labelling solution, 10% Neurobiotin (Sigma-Aldrich Inc., St.-Louis, MO). In some samples, Rhodamine B (Sigma-Aldrich Inc.) was added to the neurobiotin solution in order to visualize the ganglia under confocal microscopy. After 24−48 h (the duration of exposure depended on the thickness of the nerve) of exposure to neurobiotin, the samples were fixed 3 h at room temperature or overnight at 4℃ in 4% freshly made paraformaldehyde (PFA) in PBS, then washed at least 5 times in PBS (all further steps at 4℃, on a shaker) and finally cleaned with forceps.
Next, brain samples were put in blocking solution with 3% albumin fraction V (BSA) and 0.05% Triton X (TrX) in PBS for approx. 12 h; then washed several times with cold 0.1% BSA and 0.05% TrX in PBS; and exposed to antibody solution (Streptavidin-FITC or –Cy3: Sigma-Aldrich), diluted at 1:000 with 0.1% BSA and 0.05% TrX in PBS, for approx. 12 h. The samples then underwent another wash in cold PBS buffer, then they were cleaned in a graded series of ethanol (50, 70, 90, 2 × 100%, 10 min each), methylsalicilated (2−3 min) and finally embedded in Permount. Whole mount samples were observed under a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Jena, Germany), using LSM Image Browser and appropriate Zeiss software to make a series of confocal sections. Dichroic mirror HFT 488/543, emission filters LP 560 for Cy3 and BP 505-530 for FITC were used. The results obtained were processed using Adobe Photoshop CS (Adobe System Incorporated, San Jose, California, USA) and pen tablet Wacom Intuos3 (Wacom Co., Ltd, Saitama, Japan) for the preparation of schematic drawings. In some experiments simultaneous backfillings through both optic nerves were carried out (double labelling). In these cases one optic nerve was exposed to neurobiotin and another to lucifer yellow (LY) filter LP 475. Exposure to LY was performed the same way as to neurobiotin. For the experiments in which neurobiotin backfilling was combined with immunocytochemical staining to reveal putative neurotransmitters, the procedure for neurobiotin developing was the same as described above.
1.3 Immunocytochemistry
To identify neurotransmitters, the brain with optic nerves and eyes were removed, fixed in 10% freshly made PFA in PBS (pH 7.4) overnight at 4°C or 2−3 h at room temperature. When the immunocytochemistry procedure was combined with retrograde transport of neurobiotin through the optic nerve, backfilling with neurobiotin was carried out before the fixation. After the fixation all further steps were carried out on a shaker, at 4°C. The samples were washed in PBS, 5 times for 10 min, and incubated in 3% BSA overnight as described above for neurobiotin; then washed in 0.1% BSA with 0.05% TrX in PBS and exposed to primary antibody solution (polyclonal rabbit anti-serotonin, Sigma Aldrich Inc., dilution 1:400, or rabbit anti-FMRF-amide, ImmunoStar, Mudson, WI, USA, dilution 1:400) for 2−3 days. Subsequently, the samples were washed again in 0.1% BSA with 0.05% TrX in PBS and put into secondary antibody solution (goat anti-rabbit antibodies-FITC conjugate, Sigma Aldrich Inc., dilution 1:1000) overnight. The whole mount samples were then washed in PBS buffer, cleaned in a graded series of ethanol (50, 70, 90, 2 × 100%, 10 min each), methylsalicilated (2−3 min), embedded in Permount and finally observed under a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Jena, Germany).
For frozen sections, right after incubation in the secondary antibody, the samples were washed in PBS buffer, transferred to the molds, filled with freezing medium (Jung, Leica Microsystems, Nussloch, Germany), then freezed in liquid nitrogen for several minutes and cut on a Leica CM 1900 microtome (Leica Microsystems, Nussloch, Germany). Section thickness was 60 µm. The sections were then air-dried on microscope slides for 20-30 minutes, washed with PBS and dehydratated in a graded series of ethanol (50, 70, 90, 2 × 100%, 2 min each). In some samples prodipium iodide (Sigma Aldrich Inc.) was added prior to the dehydratation with ethanol in order to identify the nuclei of the cells. The samples were then methylsalicilated (2 min), embedded in Permount and finally observed under the confocal microscope.
2 Results
2.1 Backfilling through the optic nerve
Neurobiotin, transferred through the optic nerve ofP. corneusto the central nervous system (CNS), labelled neuronal bodies in all ganglia except the buccal ones. The distribution of labelled neurons is shown in Tab.1. More neuronal bodies and their processes were identified in case of backfillings through the right optic nerve than the left one (Fig. 1). Stained bundles of fibres pass through the right and left sides of the CNS and it seems that they do not converge to each other, not even in the visceral ganglion (Fig. 2A). However, transit fibres form thin arborizations in all ganglia (Fig. 2B).
Fibres from the optic nerve formed two overlapping bundles of axons (neuropils) in the middle of the ipsilateral cerebral ganglion (Fig. 2C, D). One neuropil was small, but very dense, and located close to the input of the optic nerve, while another one, which was larger but more diffuse had a more central location. Double labelling with neurobiotin and lucifer yellow revealed that these two neuropils were formed by different fibres (Fig. 3A). Only one large neuropil, which was not dense, could be seen in the contralateral cerebral ganglion, and it is connected to the symmetrical neuropil from theipsilateral ganglion via several axons in the cerebrocerebral commissure (Fig. 3B). Two or three medium to large-sized neurons and several smaller ones were stained in the cerebral ganglia as well. The biggest number of labelled neurons was found on the dorsal surface of the pleural ganglia, especially the ipsilateral one. Axons of these cells joined in the bunch of transit fibres and went further to the cerebral and probably parietal ganglia. At least one axon from both sides of the circumoesophageal ring entered the pedal ganglion through the pleuro-pedal commissure. In the right parietal ganglion only a few transit fibres contributing (came from or went) to then. pallialis dexterand only one neuron was stained (Fig. 3C).
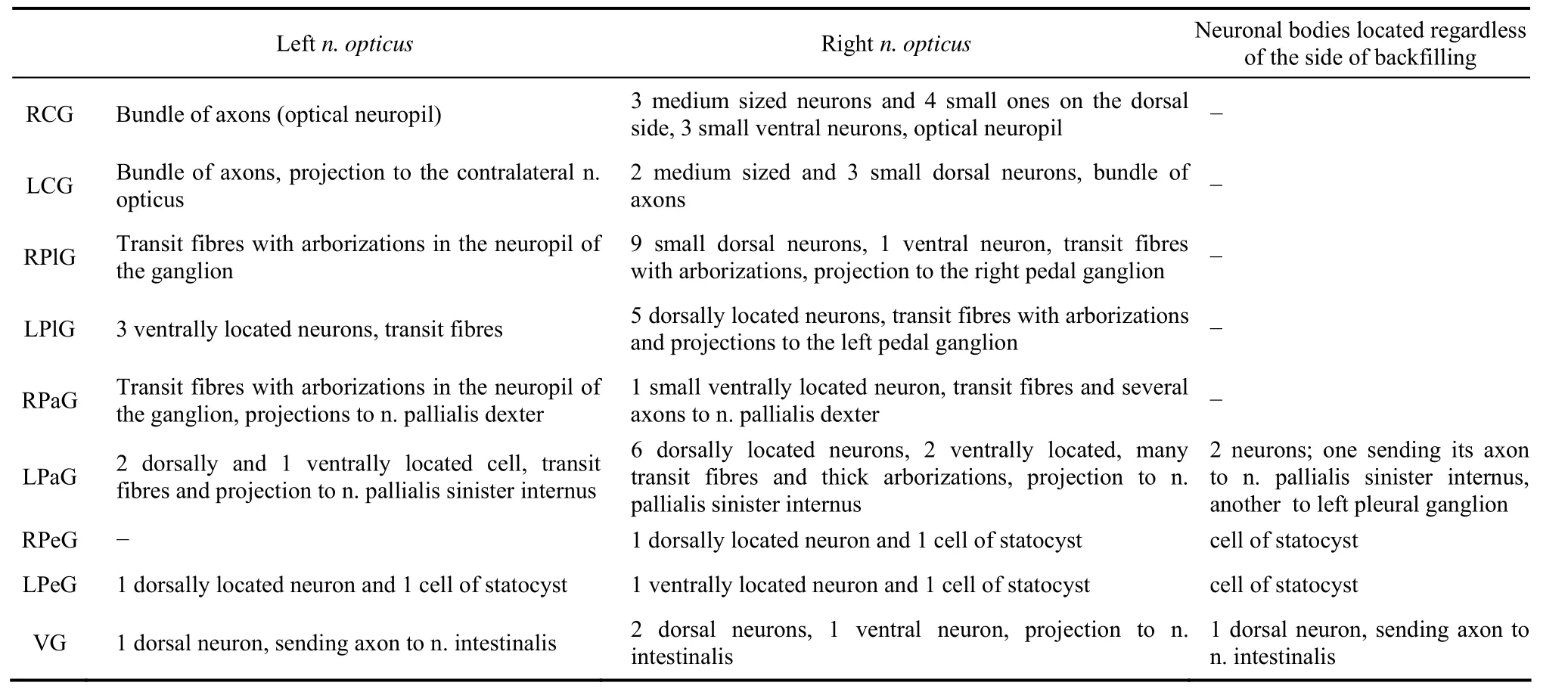
Tab. 1 Central neurons and fibres, revealed with backfilling through the optic nerve (left and right) in different ganglia of Planorbarius corneus (n=13)
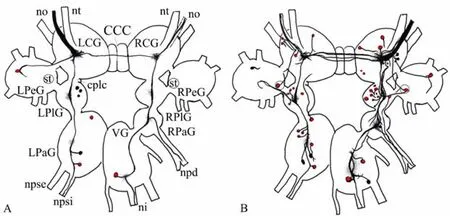
Fig. 1 Schematic drawings of the central visual pathways in Planorbarius corneus
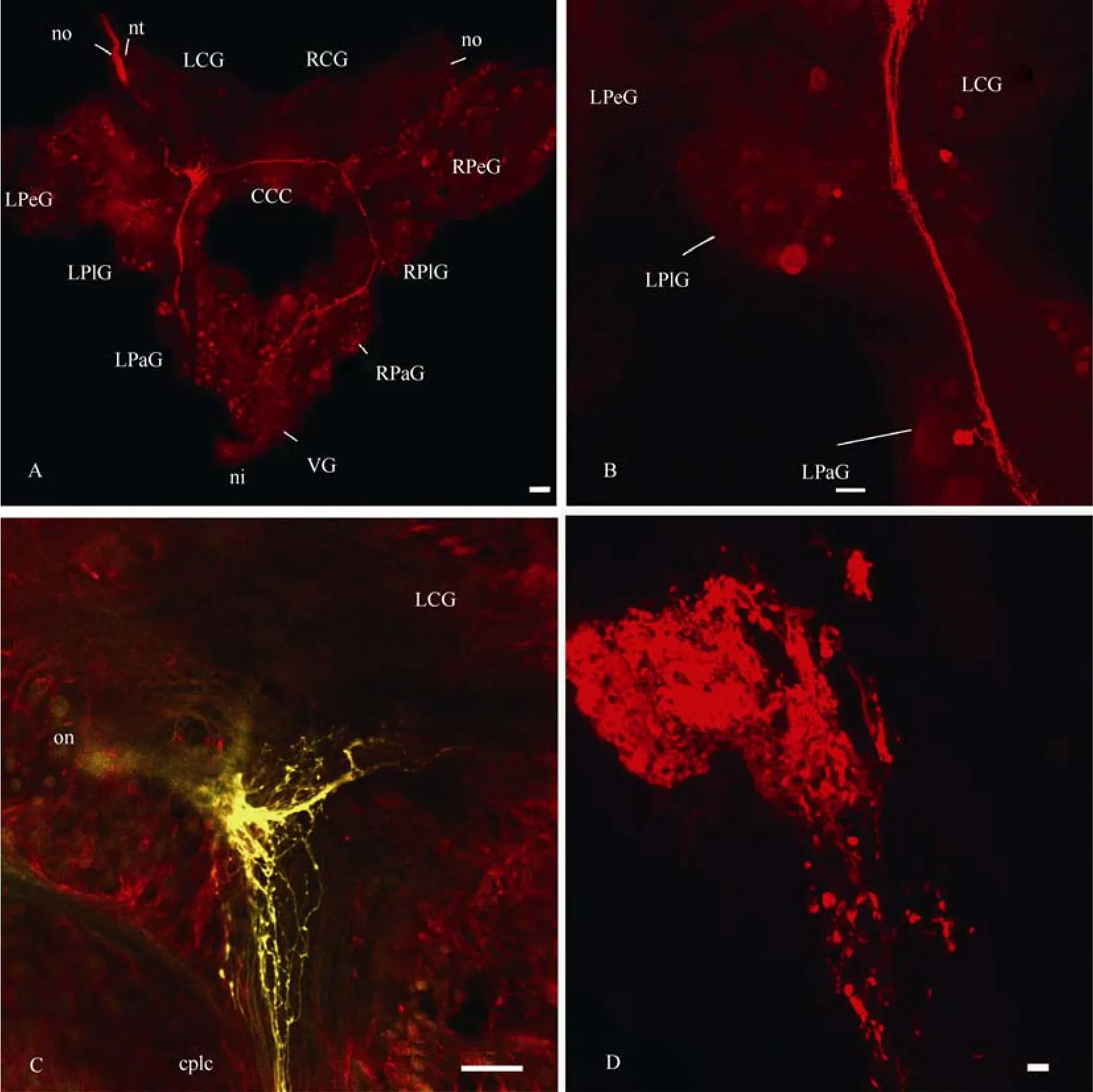
Fig. 2 Confocal micrographs of the central visual pathways in Planorbarius corneus
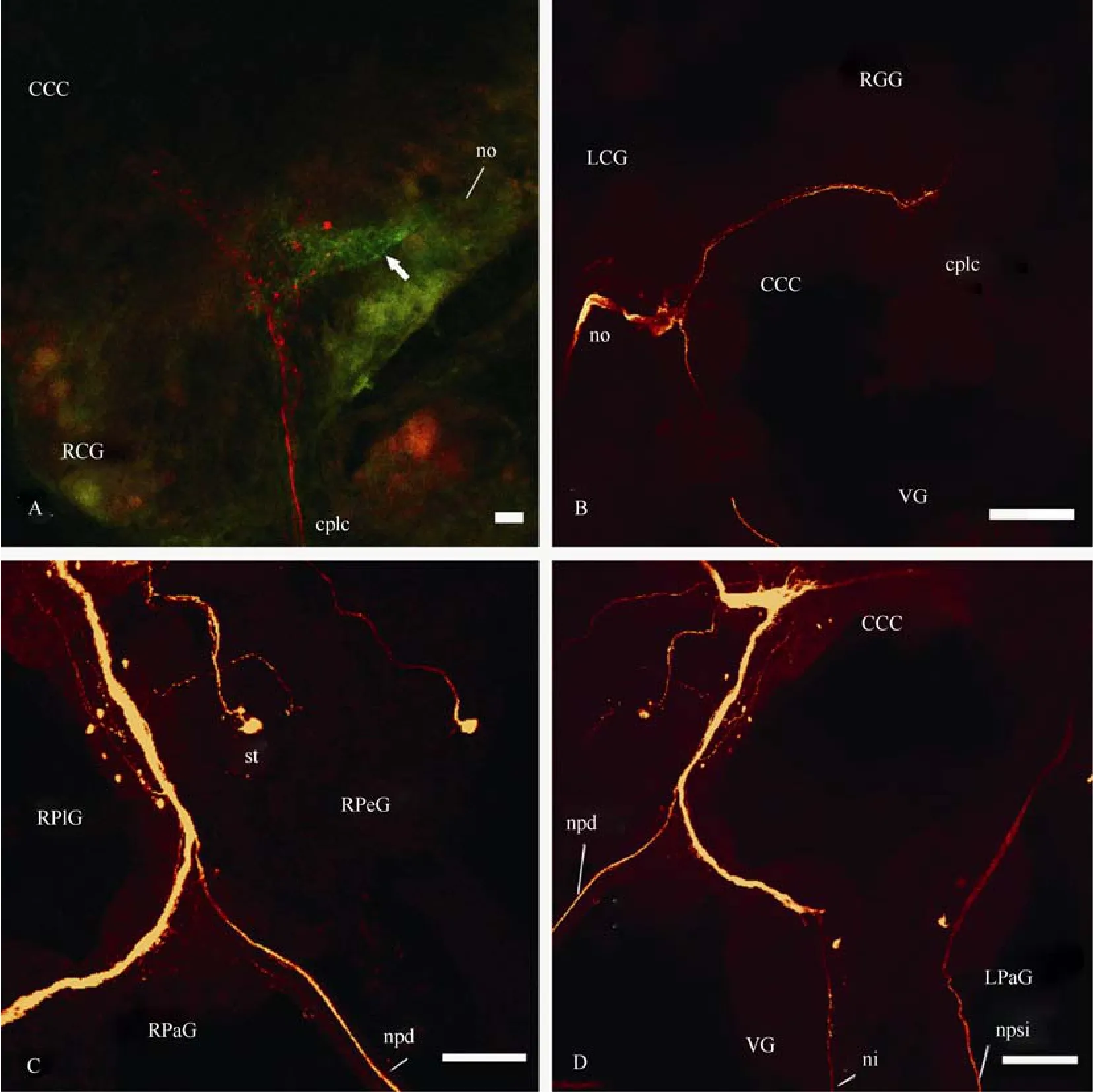
Fig. 3 Confocal micrographs of the central visual pathways in Planorbarius corneus
In the left parietal ganglion, which was bigger than the right one (contrary toL. stagnalis), we revealed several quite large as well as some smaller neurons, whose axons jointly formed a bundle in the parietal neuropil. Axons of these cells ascended to the left cerebral ganglion where they joined the cerebral neuropil. Some axons passed along then. pallialis sinister internus(Fig. 3D). In the unpaired visceral ganglion, three neurons were labelled; their fibres rise to the right cerebral ganglion. The ascending axon of one neuron, which is labelled on the ventral side of the visceral ganglion, forms arborization in the right cerebral ganglion and goes further through the cerebro-cerebral commissure (Fig. 4A). At least one axon from the visceral neuropil joins then. intestinalis. In each pedal ganglion one cell from the statocyst was stained through then. staticus, and one neuron was revealed through the cerebro-pedal connective (Fig. 4B). Some neurons of the visceral, left parietal and probably other ganglia send their axons to both optic nerves and, thus, connect the eyes with each other. As a result of this, one or two stained fibres are found in the contralateral optic nerve (Fig. 4C, D); they branch in the eye cup, but probably do not penetrate deep into the retina. Backfilling with neurobiotin through the optic nerve to the eye labelled photoreceptive cells, including microvillus-bearing parts (Fig. 10C, D), but no cell bodies were stained outside the retina.
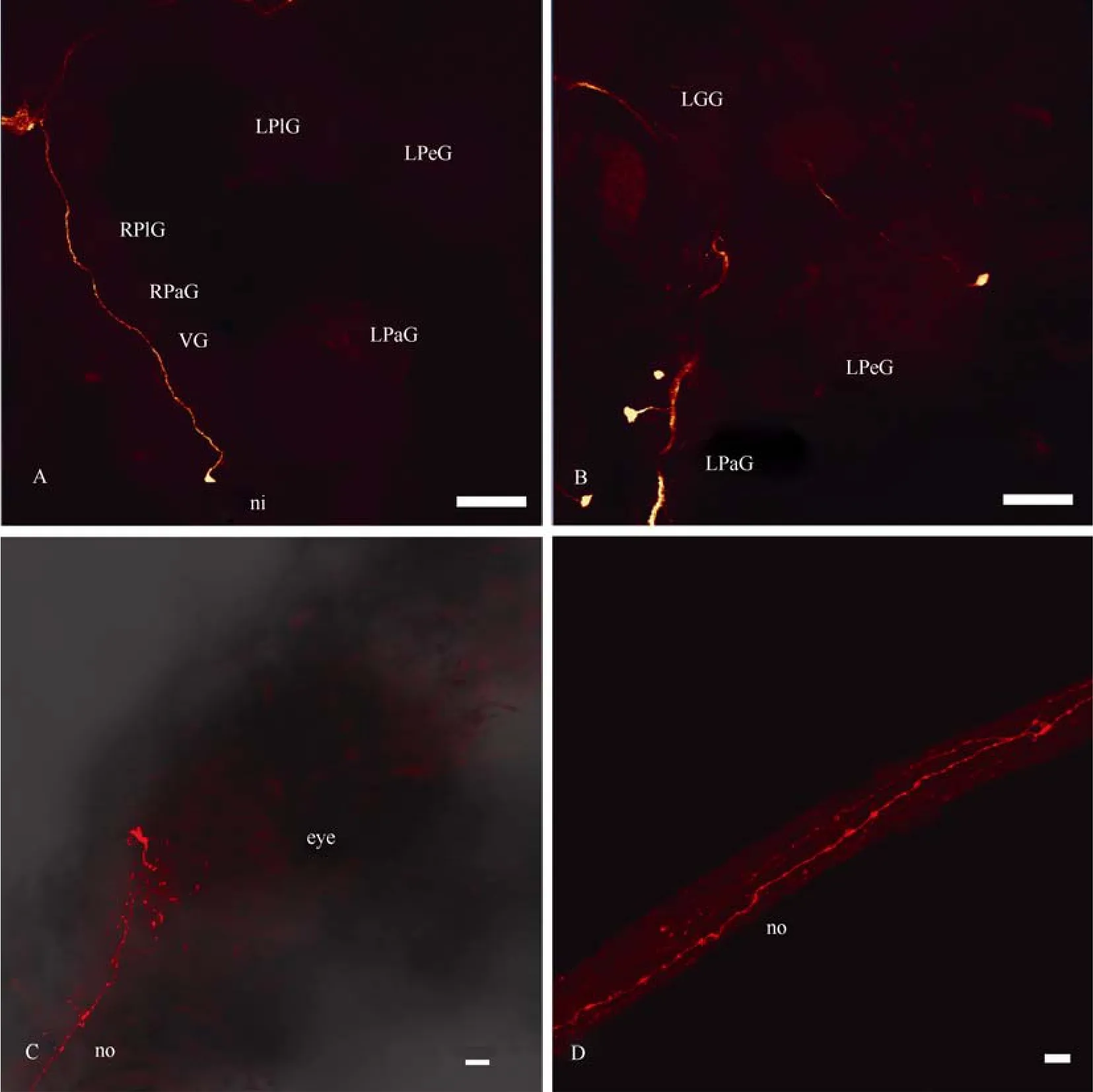
Fig. 4 Confocal micrographs of the central visual pathways in Planorbarius corneus
2.2 Backfilling with neurobiotin through n. pallialis sinister internus, n. pallialis sinister externus, n. pallialis dexter and n. intestinalis
These nerves were chosen, since they contain fibres, stained by backfilling with neurobiotin through the optic nerve. Many more neuronal bodies and axons were mapped through these nerves than through the optic one (Fig.5). Staining revealed projections to the nerves of cerebral, pedal, parietal and visceral ganglia. In case of backfilling throughn.pallialis dexterandn. pallialis sinister externussome fibres in then. opticuswere also labelled (Tab. 2).
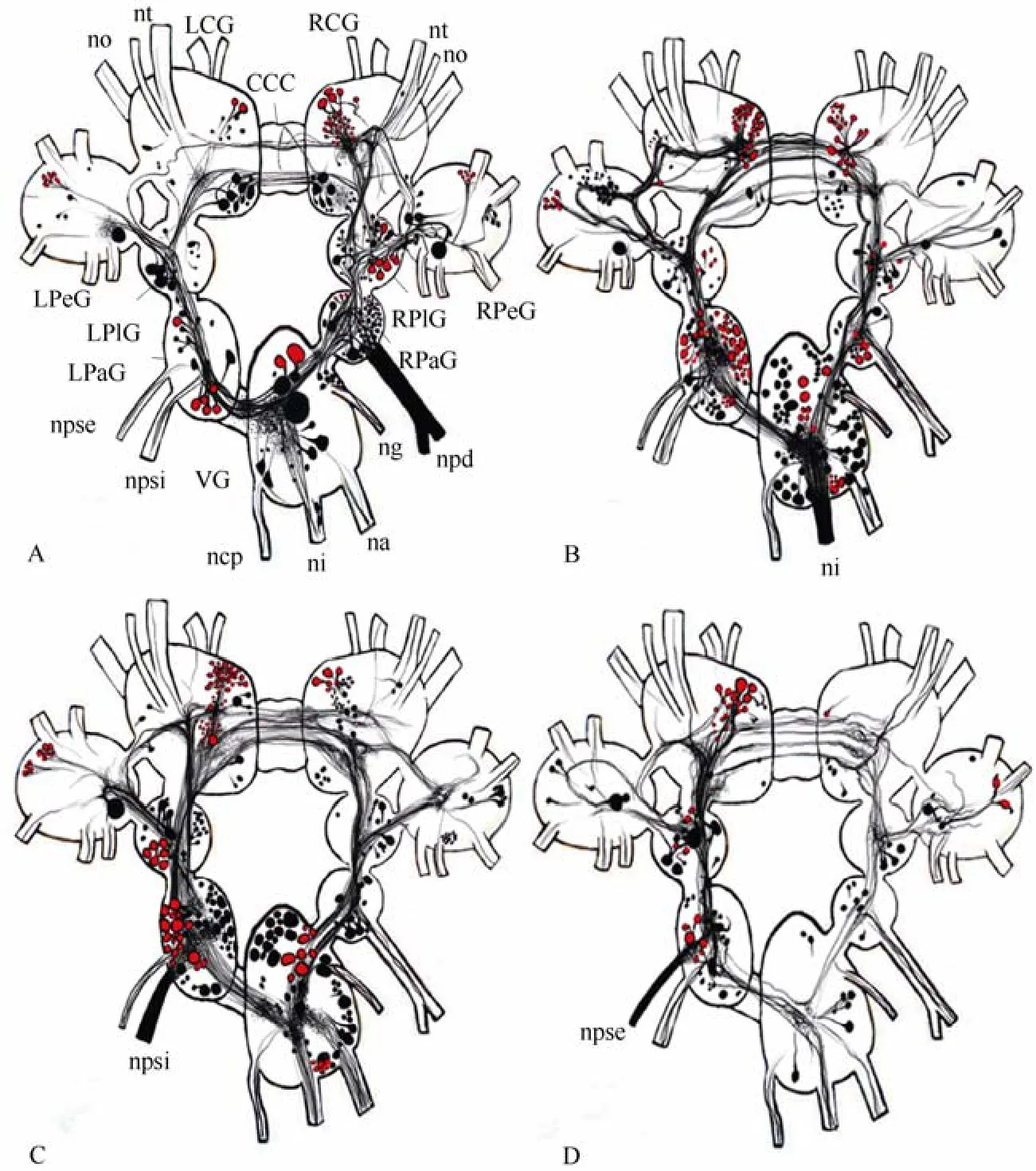
Fig. 5 Schematic drawings of the central pathways revealed through parietal and visceral nerves in Planorbarius corneus

Tab. 2 Projections of n. pallialis sinister externus et internus, n. intestinalis and n. pallialis dexter into different nerves
Fibres in the cerebro-cerebral commissure were labelled through all nerves (n. pallialis sinister internus et externus, n. pallialis dexteras well asn. intestinalis). However, their topographies were different. The upper bundle of fibres (marked with arrows in Figs. 6C, 7A, 9A) connects symmetrical clusters of neurons in the mesocerebra of the cerebral ganglia, while the lower bundle (Fig.6A) connects groups of neurons in the caudal regions of the ganglia. Most fibres in the cerebrocerebral commissure were stained through then.intestinalisandn. pallialis sinister internus.
Backfilling through then. pallialis sinister externusrevealed four thin bundles of axons, and staining through then. pallialis dexterturned up two well-defined bundles. Labelled fibres form a triangle-like structure in the cerebral neuropil (marked with arrows in Figs. 6B, 8A, B), from which several processes lead into then. tentacularisandn. opticus.
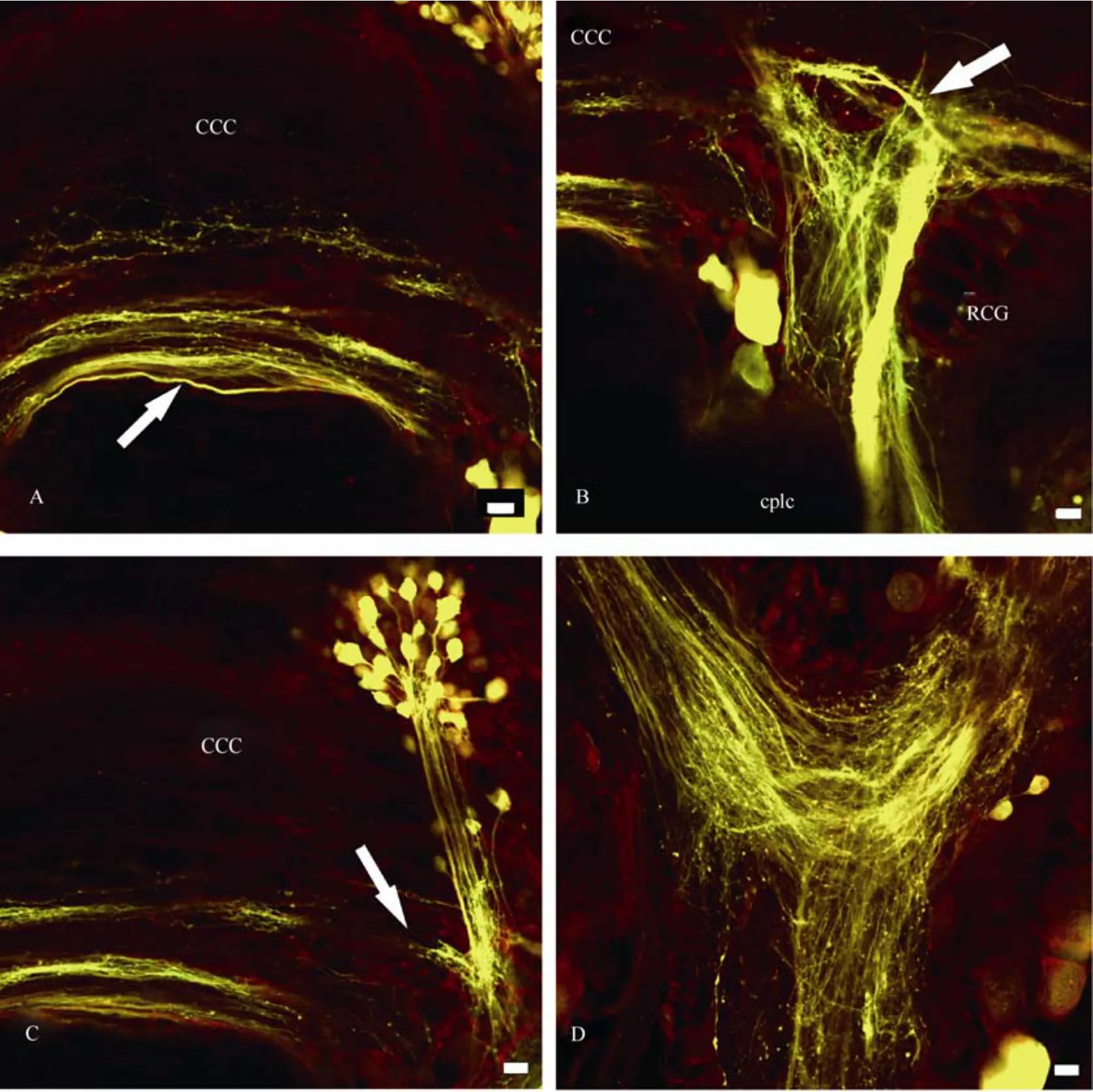
Fig. 6 Confocal micrographs of the central pathways of n. pallialis dexter in Planorbarius corneus
From the cerebral ganglia, transit fibres went to pedal, pleural, and parietal ganglia on each side of the CNS and finally join in the visceral ganglion (Fig.6D, 7D, 8C, 9D). Thus, the projections form a closed ring and send processes into most of the peripheral nerves, including pedal, parietal and visceral nerves. It is to be noted that transit fibres form two well-defined bundles of axons (Fig.6B, 9B).
The same groups of neurons were labelled through various nerves in the pedal ganglia (Fig. 7B, 8D, 9C). Primarily, all these are two groups of small neurons, which seem to have a symmetrical location and voluminous cells near the cerebro-pleural connective. Groups of neurons send their processes mostly to the corresponding pleural ganglion; large cells send processes to some pedal nerves as well as to the corresponding, i.e., ipsilateral, cerebral and pleural ganglia.
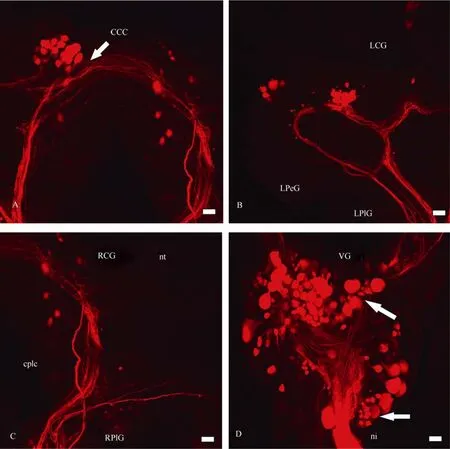
Fig. 7 Confocal micrographs of the central pathways of n. intestinalis in Planorbarius corneus
Numerous transit fibres and neurons were mapped in both pleural ganglia (Fig. 8B, 9B), in case ofn. pallialis dexterespecially in the right pleural ganglion, but in the case ofn. pallialis sinister externus et internusin the left one. Groups of cells, which seem to have a symmetrical location in the right and left pleural ganglia, were stained through then. pallialis dexter, and some cells of this group were likely stained through other nerves: one cell in the left pleural ganglion and two cells in the right one in case of backfillings through the intestinal nerve, and 2-3 cells in each pleural ganglion in case of the staining of left pallial nerves.
As for the parietal ganglia, the largest amount of neurons and fibres in the left parietal ganglion was labelled through then. pallialis sinister internus. Firstly, there is a large group of dorsally located cells close to the site where the pallial nerves enter the ganglion; then there are several ventrally located cells in the caudal region of the ganglion and one more group of neurons, which is located in the proximal region of the ganglion. The latter group can be defined in the case of backfilling through then. intestinalisas well. Neuronal structures in the right parietal ganglion were stained more clearly after backfilling through then. pallialis dexter. However, the topography of the transit fibres and thin axons can be distinguished more easily in case of backfillings through other nerves.
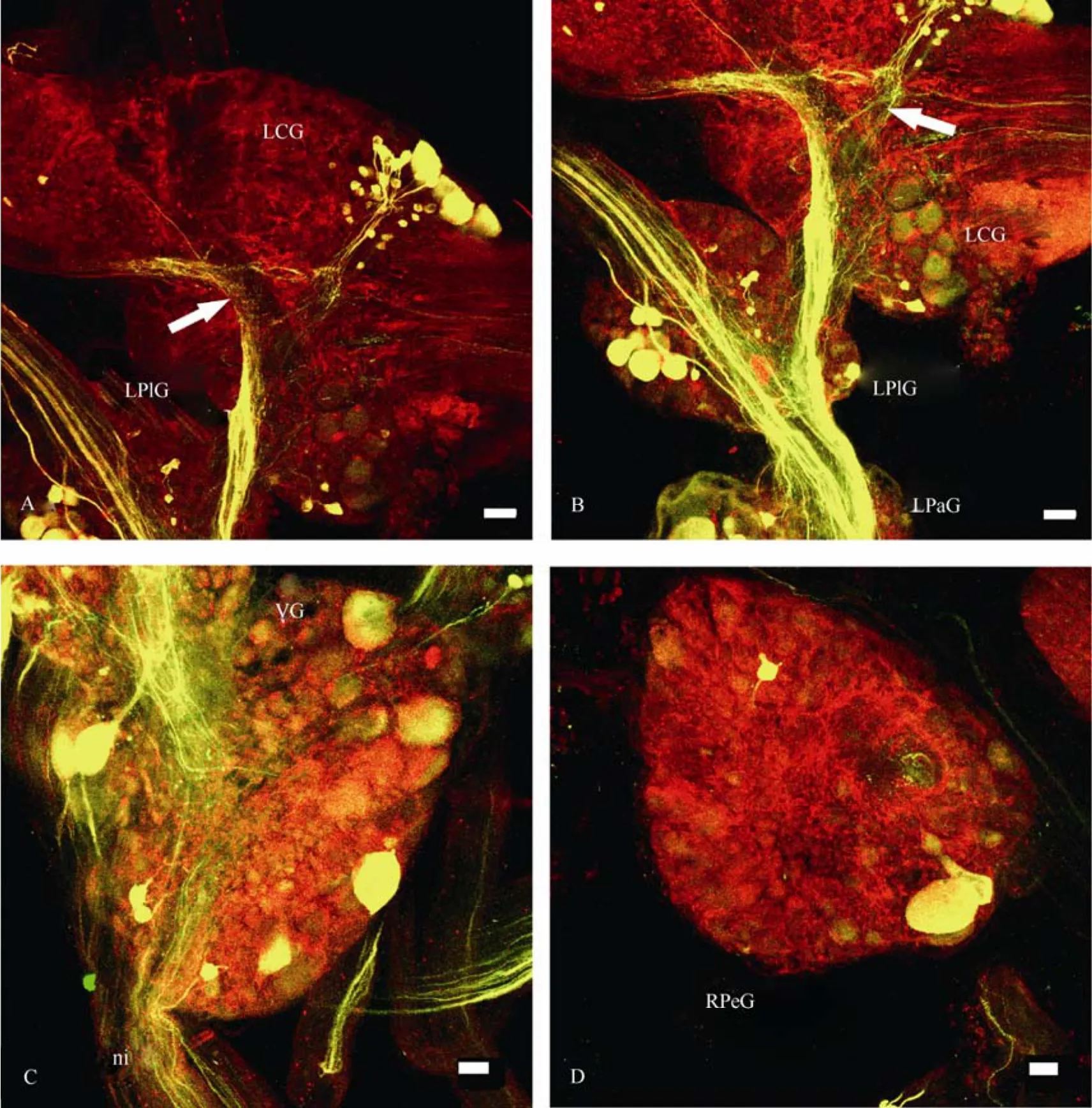
Fig. 8 Confocal micrographs of the central pathways of n. pallialis sinister internus in Planorbarius corneus
Numerous neurons and fibres were labelled in the unpaired visceral ganglion (Fig. 6D, 7D, 8C, 9D). Two identical groups of dorsally located neurons were stained through then. intestinalisandn. pallialis sinister internus(marked with arrows on Fig.7D and 9D). Projections enter all visceral nerves, but they become more apparent through retrograde transport along then. pallialis sinister internusandn. intestinalis.
2.3 Immunocytochemistry
Serotonin- and FMRF-amide-ergic fibres were revealed immunocytochemically in the CNS, optic nerve and the eye ofP. corneus(Tab.3). Several 5HT-ergic fibres with varicoses were mapped in the optic nerve, and branches with many varicoses were abundant in the eye cup (Fig. 10). The stained branches were seen mostly at the base of the nuclear layer of the retina (Fig. 10C). It is difficult to follow the thin serotoninergic fibres (labelled in the optic nerve) down to the CNS. They end somewhere in the mid region of the ipsilateral cerebral ganglion. Retrograde transport of neurobiotin through the optic nerve together with an application of serotonin antibodies allowed us to compare central visual pathways with 5HT-ergic neurons and fibres (Fig. 11). The two do not seem to coincide.
As for FMRF-amide, there are many thin FMRF-amide-ergic fibres with varicoses in the sheath of the CNS and the optic nerve in particular (Fig.12A, B). These fibres enter the eye capsule and then form a varicosed branch there. FMRF-ergic fibres differ from those stained with neurobiotin through the contralateral optic nerve (Fig.12B), although their central visual pathways can also be revealed in all of the ganglia (Fig.12C, D). Only fibres, but no 5HT- or FMRF-amidimmunoreactive cell bodies within the eye cup were revealed by staining.
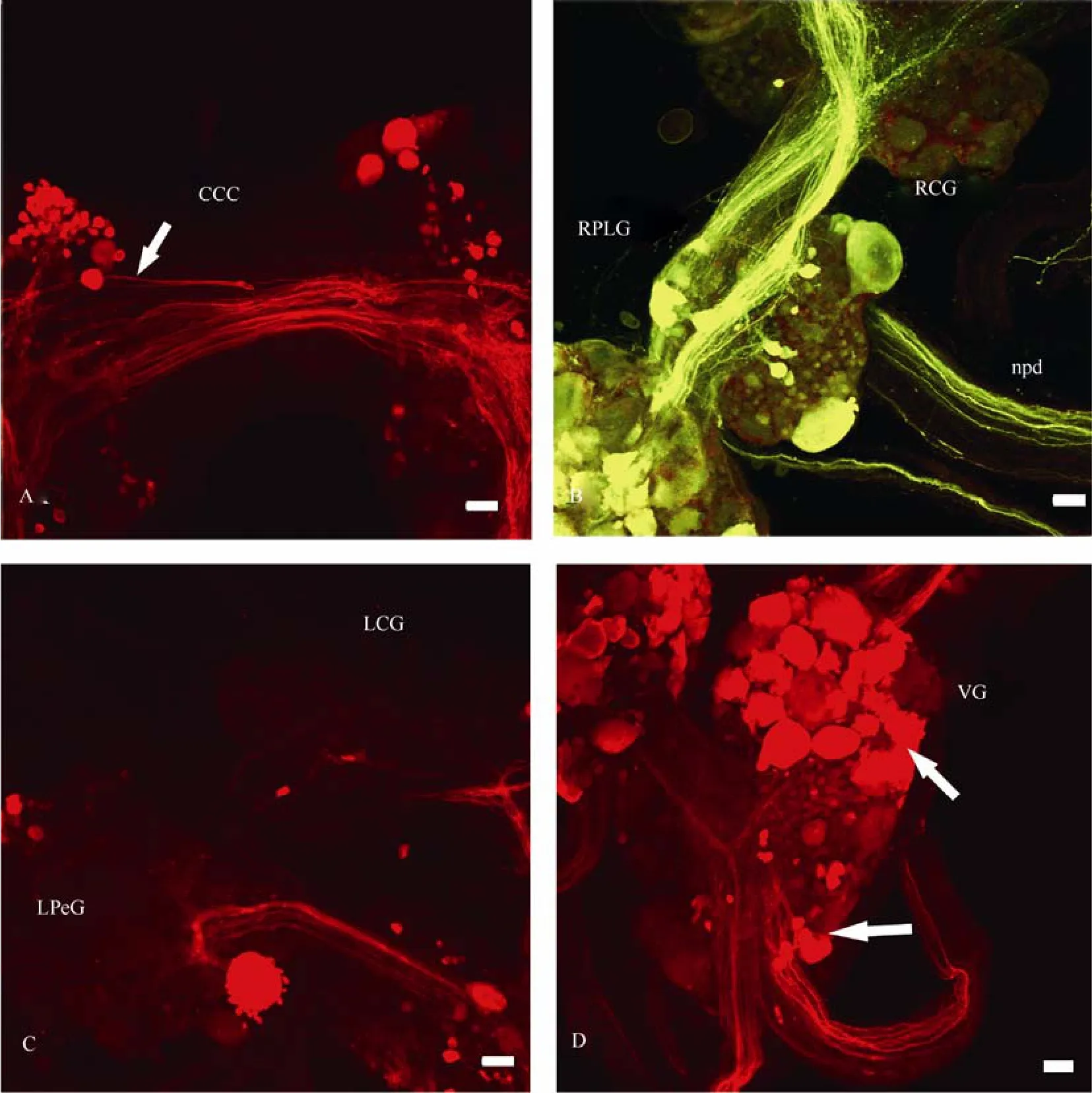
Fig. 9 Confocal micrographs of the central pathways of n. pallialis sinister externus in Planorbarius corneus
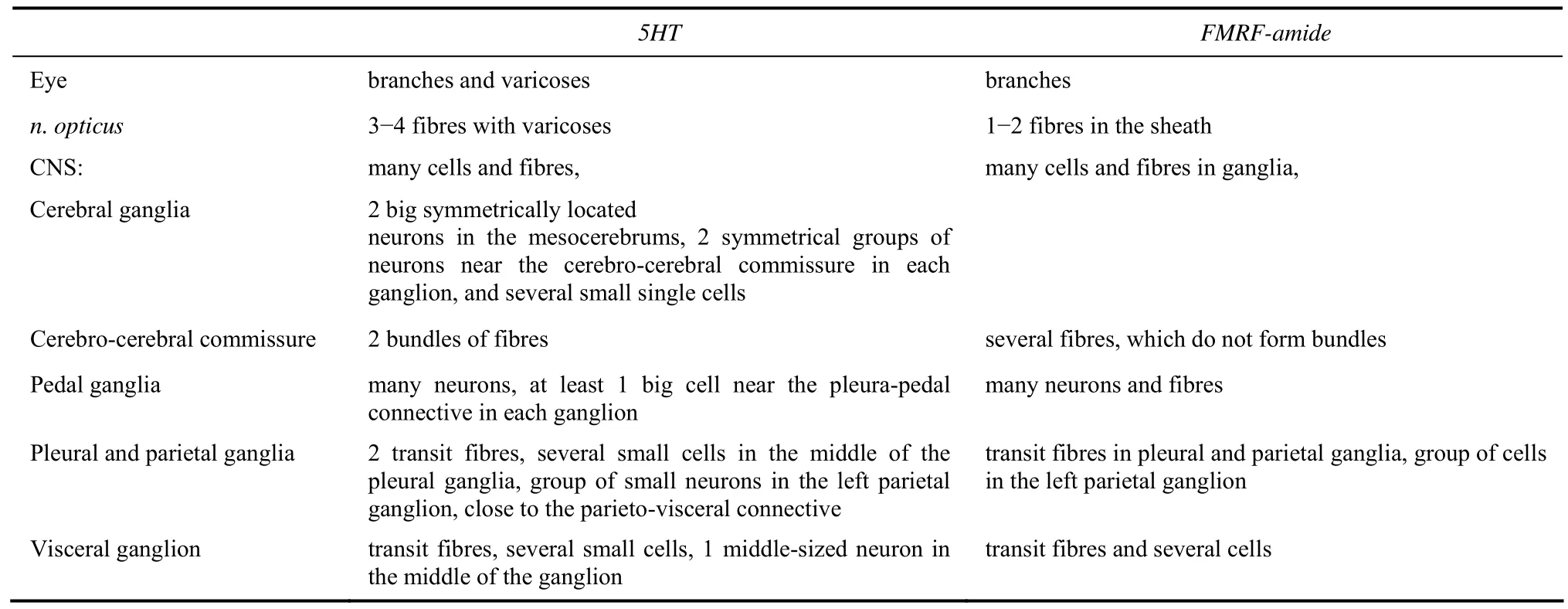
Tab. 3 Localization of the serotonin- and FMRF-amide-ergic cells and fibres in the eye optic nerve and central neural system of Planorbarius corneus
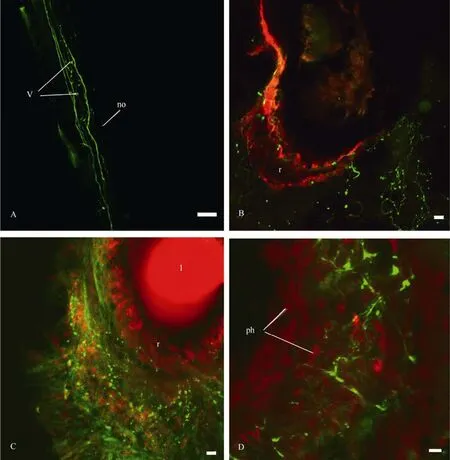
Fig. 10 Distribution of 5HT-fibres and varicoses in the optic nerve (A) and the eye (B, C and D) of Planorbarius corneus
3 Discussion
3.1 Earlier findings
In earlier investigations on the visual pathways in gastropods, cobalt compounds were generally used (Jacklet et al, 1982; Zaitseva et al, 1982; Ovchinnikov, 1986). These tracers (as well as lucifer yellow and rhodamine-dextran) label central neurons and fibres only in the cerebral ganglia possibly because of the small diameter of the fibres in the optic nerve. For example, retrograde transport of rhodamine-dextran through the pedal nerves inL. stagnalisresults in the visualization of numerous neurons and fibres, stained throughout the oesophageal ring (Kononenko, Zhukov, 2005), while backfilling through the optic nerve allowed us to reveal only the optic neuropil(Zhukov & Tuchina, 2006).To reveal more details, neuronal processes can be labelled using horseradish peroxidase (HRP) (Olson & Jacklet, 1985; Lacroixetal, 1991), but the best results forL. stagnalisandP. corneuswere achieved with neurobiotin (Zhukov & Tuchina, 2008). Double labelling with neurobiotin and lucifer yellow allowed us to distinguish (a) afferent fibres, which stem from the ipsilateral optic nerve and form the optical neuropil in the cerebral ganglion, and (b) efferent fibres, which come from the contralateral nerve which seemingly belong to the neurons of visceral and left parietal ganglia. Efferent fibres interact with afferent ones by forming arborizations and varicoses in the optical neuropil.
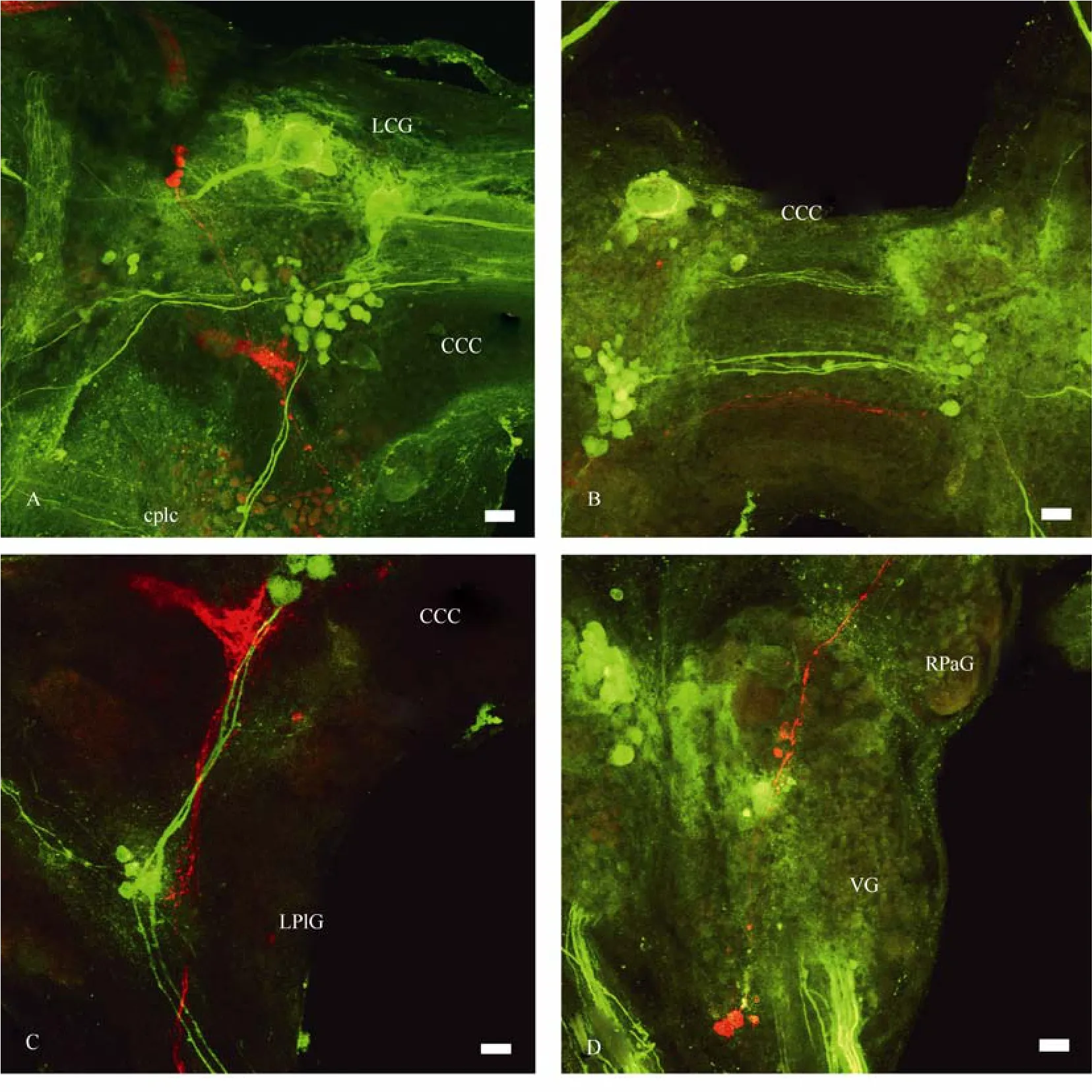
Fig. 11 Distribution of the central visual pathways (red) and 5HT-ergic neurons and fibres (green) in the CNS of Planorbarius corneus
The optical neuropil inP. corneus, formed by afferent fibers of the optic nerve, is a dense bundle of thin axons, i.e., processes of photoreceptor cells, which seems to be a very characteristic structure of gastropods. Its presence inL. stagnalisandHelix lucorumhas already been verified by Zhukov & Tuchina (2008) and Ovchinnikov (1986), respectively. In the land snailHelixsp. axons of photoreceptors synapse with secondary neurons in an enlargement of the optic nerve, and, thus, the optical neuropil, labelled in the ipsilateral cerebral ganglion in this snail, is formed by processes of secondary cells quite unlike the situation of the freshwater snailsL. stagnalisandP. corneus.
3.2 Pathways of photoreceptor neurons
Although some neurons were discovered in the retina ofL. stagnalis(Bobkova, 1998), we still possess little information about their function (since the presence of a circadian oscillator in the eye ofL. stagnalisis still under investigation). Most of the photoreceptors send their axons directly to the optic nerve, allowing us to use backfilling as a method of identification for the central visual pathways (Zhukov, 2007). Based on the results from the present work obtained through anterograde transport, we suggest that the optical neuropil inP. corneusis also formed mainly by processes of the photoreceptive cells.
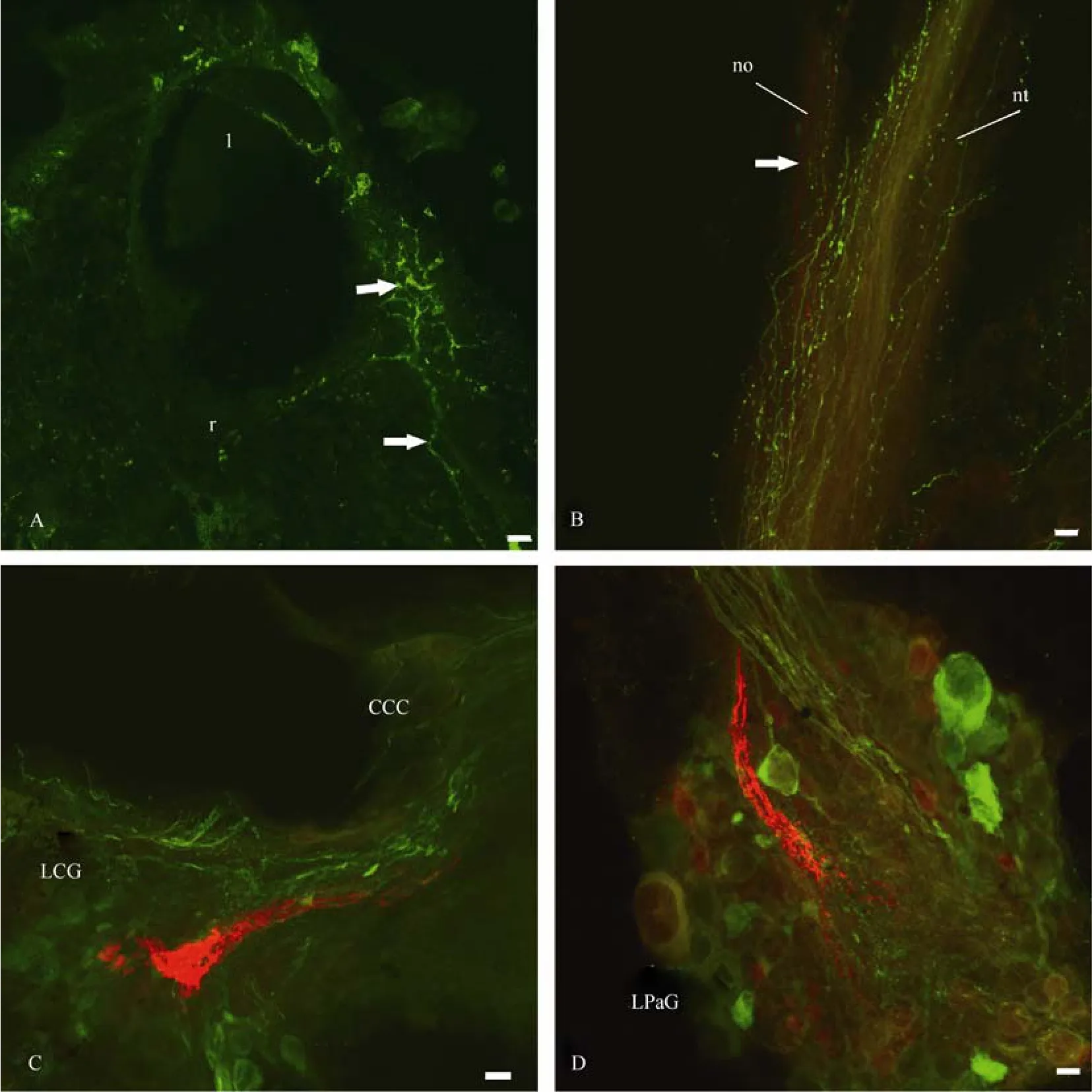
Fig. 12 Distribution of the central visual pathways (red) and FMRF-amide-ergic neurons and fibres (green) in the eye (A), optic nerve (B) and CNS (C and D) of Planorbarius corneus.
The number of cells in the retina of some of the snails mentioned above is quite high. For example, it is about 2,500 inL. stagnalis(Gal et al, 2004), around 5,000-7,000 inA. californica(Jacklet, 1969; 1973) and approximately 1,000 inB. gouldiana(Jacklet, Colquhoun, 1983). Based on the density of the apices of the photoreceptors in the retina ofP. corneus, the number of photoreceptor cells in the eye of this snail ranges from several hundreds to more than two thousand (Zhukov et al, 2002; Gál et al, 2004), which allows us to estimate that there have to be numerous synaptic contacts in the optical neuropil.
As for the marine speciesAplysia californicaandBula gouldiana, the localization of the afferent visual pathways is still under investigation. In addition to the photoreceptor cells in the retinas of these two species, there are also neurons of a circadian oscillator (Block & McMahon, 1984; Block et al, 1984; Jacklet, 1984). Axons of these neurons as well as axons of the photoreceptive cells form the optic nerve. Olson & Jacklet (1985) and other authors (Lacroix et al, 1991) were trying to separate these two kinds of fibres and mark them with HRP, precursors of serotonin or3Н-leucine, but a clear answer about the exact localization of the afferent fibres has not been achieved. Most likely the plexus of fibres, which was stained in the lateral region of the cerebral ganglion in the aforementioned two species, is formed at least partially by afferent optical fibres, comparable to the optical neuropil of freshwater snails. Labelling of the CNS ofAplysiathrough the optic nerve revealed cell bodies and fibres in almost all of the ganglia, and these stained fibres form arborizations in the neuropils of the ganglia. Although authors like Olson & Jacklet suggested that these projections belonged to the circadian oscillator system and their widespread distribution in the brain ofAplysiawas due to the need to adjust a variety of functions to the light cycle, we think that the visual pathways (i.e., processes of photoreceptors) may contribute as well.
Staining of the CNS through the optic nerve inP. corneusrevealed bodies of neurons that send their axons to the optic nerve and are likely to represent efferents of the eye. Similar neurons were labelled in the cerebral ganglia ofAplysia(Olson & Jacklet, 1985) andHelix(Ovchinnikov, 1986). InAplysiathese neurons form two groups, which innervate the eye through the main and additional optic nerves. The overall distribution of the labelled neurons in the CNS ofP. corneusis similar to that described inL. stagnalis(Zhukov & Tuchina, 2008; Tuchina et al, 2010). First of all, the labelled neurons are located in all of the ganglia except the buccal ones. The most remarkable cells were identified in the parietal and visceral ganglia, since their axons reach the retinas of both eyes. One of the presumed functions of these cells is to compare information on light conditions reaching the right and left eyes, and thus to identify the direction of the light source.P. corneus, just likeL. stagnalis, exhibits a positive phototaxis (Zhukov et al, 2002).
The eyes ofB. gouldianaare functionally connected, since the electrical activity of the retinal cells in one eye can be registered in the contralateral optic nerve, and this feature is probably important for the synchronization of both circadian oscillators (Roberts & Block, 1985). But the morphological basis for these connections is still missing: the central pathways, which were labelled with HRP, can be followed only through the cerebro-cerebral commissure towards the contralateral eye (Lacroix et al, 1991). Since HRP-stained fibres are also found in the pleural ganglion and in the pleura-parietal connective, there are probably some neurons, which are able to connect both eyes as inL. stagnalisandP. corneus. InH. crassicornis, whose retina is rather simple, the eyes are connected through the neurons of the optic ganglion, i.e. secondary neurons (Alkon, 1973). Interestingly, direct connections between the eyes ofAplysia,which like those ofBulla gouldianahave circadian oscillators, in accordance with Olson & Jacklet (1985), were not revealed. However, in the related snailBursatella leachii pleiicircadian oscillators have been reported to be highly synchronized (Roberts et al, 1987)
3.3 Neuronal projections
Another remarkable feature in the topography of the central visual pathways inP. corneusis projections to the pedal ganglia and particularly to the statocysts, something that had also been shown inL. stagnalis(Tuchina et al, 2010), these connections probably provide the basis for phototaxis (Vakoljuk & Zhukov, 2000) and visuo-vestibular associative learning (Sakakibara et al, 1998; Sakakibara, 2006). It is to be noted that behavioural patterns inP. corneusare poorly studied, but we suggest that interactions between visual and vestibular information processing could be important for the orientation of these snails towards the light, i.e., the surface of their aquatic habitat, for breathing.
As inL. stagnalis, some of the labelled central neurons inP. corneussend their processes along peripheral nerves. By using retrograde transport of neurobiotin through these nerves we were able to reveal projections to the optic nerve only forn. pallialis dexter internus,but most likely such projections also occur with regard to then. pallialis dexter externusandn. intestinalisalso. The neurons, which connect different nerves, are very typical for the CNS ofL. stagnalis, where they form mononeuronal reflex arcs (Zaitseva, 1982; 1987). Such neurons can be expected to be present in the neural system ofBullaandAplysia, since after the backfilling of the optic nerve some stained fibres are found in peripheral nerves. InAplysiasuch fibres were presented on the right and left sides of then. tentacularisandn. frontolabialis superior(Olson & Jacklet, 1985). We conclude that mononeuronal connections between different peripheral parts of the body are rather typical in gastropods.
3.4 Efferent innervation
Efferent innervations of the retina are well known from arthropods, but they are present in cephalopods as well. In octopus the bodies of the efferent dopamineergic neurons are located in the optical lobe; they are electrically coupled with photoreceptors and are able to change the responses of the photoreceptor cells, as well as control the screening pigment migration (Suzuki, Tasaki, 1983; Gleadall et al, 1993).
Observations on efferent innervations of the retina inCheliceratainvolved: spiders (Yamashita, 1990; Uehara et al, 1993), scorpions (Fleissner, Schliwa, 1978) and the horseshoe crabLimulus polyphemus(Calman, Battelle, 1991). In the scorpionAndroctonus australisthere is a stem of 10-20 central neurons, which is located near the oesophagus, each neuron of this group sending processes to both eyes (Fleissner & Fleissner, 2002). The electrical activity of these cells depends on the phase of the circadian cycle. Depending on the timing of the cycle a different amount of octopamine is secreted in order to modulate the screening pigment migration and, thus, the state of light adaptation of the eye (Fleissner & Fleissner, 1978, 2002). In the spiderArgiope sp.efferent innervations of the retina are achieved by neurons of the protocerebrum, which modulate the light sensitivity of the photoreceptors, and probably exert an effect on synthesis and degradation of photoreceptor membranes (Yamashita & Tateda, 1981). The electrical activity of these protocerebrum neurons changes according to the illumination of the retina (Yamashita, 1990). Moreover, they themselves are light-sensitive (Yamashita & Tateda, 1983; Yamashita, 2002).
The efferent innervations of the lateral eye retina inLimulushave been studied in great details. Some of the efferent neurons, which are located in the cheliceral ganglia, innervate both eyes (Calman & Battelle, 1991). Other cells, located in the protocerebrum in separate groups, are connected to each other through the commissure and probably affect both eyes as well (Saitoet al, 2004). The efferent fibres of the retina contain octopamine (Battelle et al, 1982), which changes the light sensitivity of ommatidia in two ways (Barlow et al, 1977): (a) migration of screening pigments and (b) packing of the photoreceptor membrane (folds). Both lead to changes in the receptor’s sensitivity to light (Chamberlain & Barlow, 1979; Barlow, 1985; Battelle, 1984; 2006).
3.5 Functional interpretations
The described structural and positional similarities of efferent visual cells in freshwater gastropods and inChelicerataallow us to suggest that these cells carry out analogous functions. ForL. stagnaliswe suggested that such cells, receiving information from dermal photoreceptors, can be involved in the regulation of retinal light sensitivity (Tuchina et al, 2010), but published data on the presence of dermal photoreception inP. corneusdo not exist.
There is still very little information on neurotransmitters, which can perform efferent modulations of the retinal cells in gastropods. There is no GABA-, octopamine- or histamine-immunoreactivity in the eye or in the optic nerve ofLymnaea(Zhukov, 2007), and a similar lack of information was reported forBullaby Michel et al (2000). However, as shown forAplysia, the cerebral ganglion of this mollusc modulates the performance of the ocular pacemaker (Jacklet, 1984), and the putative transmitter here is serotonin, since it alone can phaseshift the rhythm (Corrent et al, 1978). Serotonin mimics ganglion attachment in case of experiments with the isolated eye (Nadakarukaren & Likey, 1986). Serotonin was also found to modulate responses of B-photoreceptors inHermissenda; it significantly increases the amplitude and duration of the photoresponse evoked by light flashes with constant intensity (Crow & Bridge, 1985). The authors also suspected that serotonergic interneurons were activated by inputs from statocyst hair cells inHermissendaand may then interact with B-photoreceptors. It was shown that exogenous serotonin can change the amplitude of the electroretinogram (ERG) and the light sensitivity of the isolated eye inLymnaea(Zhukov et al, 2006) and that it is present in fibres of the optic nerve in this snail species.
In the optic nerve ofBulla, efferent fibres contain FMRF-amide and it was shown that this peptide suppresses the CAP activity, similar to what serotonin does in the eye ofAplysia(Jacklet et al, 1987). In the optic nerve ofAplysiano FMRF-amide-ergic fibres were revealed, and only exogenous FMRF-amide modulates the influence of light and serotonin on the circadian oscillator in the eye (Colwell et al, 1992).
We were interested whether serotonin or/and FMRF-amide are involved in the processing of the visual information inPlanorbarius, and we found that both neurotransmitters are represented in the optic nerves, as well as in the eyes in this species. Serotonin- and FMRF-amide-ergic efferent fibres in the eye ofP. corneusbelong to neurons other than those, which send their processes in both optic nerves, and, thus, the chemical nature of these parietal and visceral neurons remains unclear. The presence of fibres, showing immunoreactivity to serotonin and FMRF-amide in the retina ofP. corneus, allows us to suggest that these fibres play a modulatory role in adjusting retinal sensitivity levels to photic stimulation. Obviously, to confirm or reject this hypothesis requires further study.
Thus, we propose that the central neurons, which were identified inP. corneus, can control the adaptational state of the retina. It is hard to discuss their connections with the circadian pacemaker since there is no information on the localization of the latter inP. corneus. But we found that besides the described central neurons, retinal cells can also be influenced by 5-HT and FMRF-amideergic-like fibres, which was not reported in previous studies.
Acknowledgements:We wish to thank Prof. H.-J. Pflueger, Dr Natalia Kononenko and Heike Wolfenberg for their advice, overall help with the project and an opportunity for O.T. to study immunohistochemichal methods at the Free University Berlin (Germany).
Alkon D. Neural organization of molluscan visual system. 1973 [J].Gen Physiol,61: 444-461.
Barlow RBJ, Bolanowski SJJ, Brachman ML. 1977. Efferent optic nerve fibres mediate circadian rhythm in theLimuluseye [J].Science197: 86-89.
Barlow RB, Kaplan E, Renninger GH, Saito T. 1985. Efferent control of circadian rhythms in theLimuluslateral eye [J].Neurosci Res, 2: 65-78.
Battelle BA, Evans JA, Chamberlain SC. 1982. Efferent fibres toLimuluseyes synthesizeand release octopamine [J].Science216: 1250-1252.
Battelle BA. 1984. Efferent innervation toLimuluseyes [J].Trends Neurosci, 7: 277-282.
Battelle BA. 2006. The eyes ofLimulus polyphemus(Xiphosura,Chelicerata) and their afferent and efferent projections [J].Arth Struct Develop, 35: 261-274.
Block GD, McMahon DG, Wallace SF, Friesen WO. 1984. Cellular analysis of theBullaocular circadian pacemaker system. I. A model for retinal organization [J].Comp Physiol,A155: 365-378.
Block GD, McMahon DG. 1984. Cellular analysis of the Bulla ocular circadian pacemaker system. III. Localization of the circadian pacemaker system [J].Comp Physiol,A155: 387-395.
Bobkova MV. 1998. Structural and functional organization of peripheral part of the visual system of the common pond snailLymnaea stagnalis[J].Evol Biochem Physiol,34(6): 531-546.
Bobkova MV, Gál J, Zhukov VV, Shepeleva IP, Meyer-Rochow VB. 2004. Variations in the retinal designs of pulmonate snails (Mollusca,Gastropoda): squaring phylogenetic background and ecophysiological needs (I) [J].Invert Biol,123(2): 101-115.
Calman BG & Battelle BA. 1991. Central origin of the efferent neurons projecting to the eyes ofLimulus polyphemus[J]. Vis Neurosci6(5): 481-95.
Chamberlain SC, Barlow RB. 1979. Light and efferent activity control rhabdom turnover inLimulusphotoreceptors [J].Science,V206: 361-363.
Colwell CS, Khalsa SB, Block GD. 1992. FMRF-amide modulates the action of phase shifting agents on the ocular circadian pacemakers ofAplysiaandBulla[J].Comp Physiol,170A: 211-215.
Corrent G, McAdoo D, Eskin A. 1978. Serotonin shifts the phase of the circadian rhythm from theAplysiaeye [J].Science,202: 977-979.
Crow T & Bridge MC. 1985. Serotonin modulates photoresponses inHermissendatype-B photoreceptors [J].Neurosci Lett60(1): 83-8.
Eskin A, Maresh RD. 1982. Serotonin or electrical optic nerve stimulation increases the photosensitivity of theAplysiaeye [J].Comp Biochem Physiol,73C: 27-31.
Fleissner G, Fleissner G. 1978. The optic nerve mediates the circadian pigment migration in the median eyes of the scorpion [J].Comp Biochem Physiol,61A: 69-71.
Fleissner G, Fleissner G. 2002. Retinal Circadian Rhythms. In: Biological Rhythms. Ed. Vinod Kumar [M]. Narosa Publishing House. New Dehli. 71-82.
Fleissner G, Schliwa M. 1977. Neurosecretory Fibres in the Median Eyes of the Scorpion,Androctonus australisL [J].Cell Tiss Res178(2): 189-198.
Gál J, Bobkova MV, Zhukov VV, Shepeleva IP, Meyer-Rochow VB. 2004. Fixed focal-length optics in pulmonate snails (Mollusca,Gastropoda): squaring phylogenetic background and ecophysiological needs (II) [J].Invert Biol,123(2): 116-127.
Gleadall IG, Ohtsu K, Gleadall E, Tsukahara Y. 1993. Screeningpigment migration in the octopus retina includes control by dopaminergic efferents [J].Exp Biol,185: 1-16.
Jacklet JW. 1969. Electrophysiological organization of the eye ofAplysia[J].Gen Physiol,53: 21-42.
Jacklet JW. 1973. Neuronal population interactions in a circadian rhythm inAplysia. In Neurobiology of Invertebrates (ad. J.Salánki) [M]. Akademiai Kiado, Budapest. 363-380.
Jacklet JW, Rolerson C. 1982. Electrical activity and structure of retinal cells of theAplysiaeye: II. Photoreceptors [J].Exp Biol,99: 381-395.
Jacklet JW, Schuster L, Rolerson C. 1982. Electrical activity and structure of retinal cells of theAplysiaeye: I. Secondary neurons [J].Exp Biol,99: 369-380.
Jacklet JW, Colquhoun W. 1983. Ultrastructure of photoreceptors and circadian pacemaker neurons in the eye of a gastropod,Bulla[J].Neurocytol,12: 673-696.
Jacklet JW. 1984. Neural organization and cellular mechanisms of circadian pacemakers [J].Int Rev Cytol,89: 251-294.
Jacklet JW, Klose M, Goldberg M. 1987. FMRF amide-like immunoreactive efferent fibres and FMRF amide suppression of pacemaker neurons in eyes ofBulla[J].Neurobiol,18: 433-449.
Kononenko NL, Zhukov VV. 2005. Neuroanatomical and immunocytochemical study of the head retractor muscle (HRM) innervation in the pond snail,Lymnaea stagnalisL [J].Zool,108(3): 217-237.
Lacroix L, Strack S, Olson L, Jacklet JW. 1991. Axons of circadian pacemaker neurons in the eye ofBullaproject to the central nervous system and contralateral eye [J].Comp Biochem Physiol,98A: 383-391.
Michel S, Schoch K, Stevenson PA. 2000. Amine and amino acid transmitters in the eye of the molluscBulla gouldiana: An immunocytochemical study [J].Comp Neurol425: 244–256.
Nadakavukaren JJ, Lickey ME, Jordan WP. 1986. Regulation of the circadian clock in theAplysiaeye: mimicry of neural action by serotonin [J].Neurosci6:14-21.
Olson LM, Jacklet JW. 1985. The circadian pacemaker in theAplysiaeye sends axons throughout the central nervous system [J].Neurosci,5: 3214-3227.
Ovchinnikov AV. 1986. Morphological characteristics of chemosensory, visual and statocyst pathways in the snailHelix lucorum[J].Neurophysiol,18(1): 1-9.
Patton ML, Kater SB. 1972. Electrotonic conduction in the optic nerves of planorbid snails [J].Exp Biol,56: 695-702.
Roberts MH, Block GD. 1985. Analysis of mutual circadian pacemaker coupling between the two eyes ofBulla[J].Biol Rhythm,1(1): 55-75.
Roberts MH, Block GD, Lusska AE. 1987. Comparative studies of circadian pacemaker coupling inOpisthobranch molluscs[J].Brain Res,423(1-2): 286-292.
Roberts MH, Moore RY. 1987. Localization of neuropeptides in efferent terminals of the eye in the marine snail,Bulla gouldiana[J].Cell Tiss Res,248(1): 67-73.
Sakakibara M, Kawai R, Kobayashi S, Horikoshi T. 1998. Associative learning of visual and vestibular stimuli inLymnaea[J].Neurobiol Learning and Memory,69: 1-12.
Sakakibara M. 2006. Comparative study of visuo-vestibular conditioning inLymnaea stagnalis[J].Biol Bull,210: 298-307.
Suzuki H, Tasaki K. 1983. Inhibitory retinal efferents from dopaminergic cells in the optic lobe of the Octopus [J].Vision Res,23: 451-457.
Takahasi JS, Nelson DE, Eskin A. 1989. Immunocytochemical localization of serotonergic fibres innervating the ocular circadian system ofAplysia[J].Neurosci,28: 139-147.
Tuchina OP, Zhukov VV, Meyer-Rochow VB. Central and peripheral neuronal pathways revealed by backfilling with neurobiotin in the optic, tentacular and small labial nerves ofLymnaea stagnalis[J].Acta Zool(in press).
Uehara A, Uehara K, Ogawa KE. 1993. Efferent fibres and daily rhabdomal changes in the anteromedial eye of the liphistiid spider,Heptathela kimurai[J].Cell Tiss Res272: 517-522.
Vakoljuk IA, Zhukov VV. 2000. The study ofLymnaea stagnalisphotoreception from phototaxis manifestation [J].Evol Biochem Physiol,36(5): 544-550.
Yamashita S. 1990. Efferent optic nerve impulses in response to illumination of single eyes of orb weaving spiders [J].Vision Res,30(6): 817-821.
Yamashita S, Tateda H. 1981. Efferent neural control in the eyes of orb weaving spiders [J].Comp Physiol,143: 477-483.
Yamashita S, Tateda H. 1983. Cerebral photosensitive neurons in the orb weaving spiders,Argiope bruennichiiandA. amoena[J].Comp Physiol,150: 467-472.
Yamashita S. 2002. Efferent innervation of photoreceptors in spiders [J].Microscop Res Tech,58: 356-364
Zaitseva OV. 1982. Sensory elements in the central nervous system of the snailLymnaea stagnalis(pleuro-visceral loop of the ganglia) [J].Zh Evol Biohim Phisiol,18(5): 482-490 (in Russian).
Zaitseva OV, Kovalev VA, Sokolov VA. 1982. Investigation of the cerebral region of the visual system in pulmonate mollusks [J].Neirofiziol,14(2): 179-184 (in Russian).
Zaitseva OV. 1987. The central elements of the tentacular and osphradial sensory systems of the pond snailLymnaea stagnalis[J].Sens Sist,1(2): 154-165 (in Russian).
Zhukov VV. 1993. Some optical properties of lenses of freshwater pulmonate snailsLymnaea stagnalis L.andPlanorbarius corneus L[J].Sens Sist,7(2): 17-24 (in Russian).
Zhukov VV, Bobkova MV, Vakoljuk IA. 2002. Eye structure and vision in the freshwater pulmonate molluscPlanorbarius corneus[J].Evol Biochem Physiol,38(4): 419-430.
Zhukov VV, Kononenko NL, Panormov IB, Borissenko SL. 2006. Serotonin changes the electrical responses to light of the eye ofLymnaea stagnalis[J].Sens Sist,20(4): 270-278 (in Russian).
Zhukov VV, Tuchina OP. 2006. Topographical asymmetry of efferent neurons in visual system of freshwater gastropodLymnaea stagnalis. Abstr. International conference on functional neuromorphology. SPb (in Russian).
Zhukov VV. 2007. On the problem of retinal transmitters of the freshwater molluscLymnaea stagnalis[J].Evol Biochem Physiol,43(5): 524-532.
Zhukov VV, Tuchina OP. 2008. Structure of visual pathways in the nervous system of freshwater pulmonate mollusks [J].Evol Biochem Physiol,44(3): 341-353.
淡水蜗牛视觉系统的输入与输出通路
Oksana P Tuchina¹, Valery V Zhukov², Victor Benno Meyer-Rochow1,*
(1.School of Engineering and Science,Jacobs University,BremenD-28759,Germany; 2.Department of Agricultural and Soil Ecology,Faculty of Bioresources and Natural Usage,Kaliningrad State Technical University,Kaliningrad236000,Russia)
通过神经生物素在视神经上的逆行性传输对淡水蜗牛(Planorbarius corneus)视网膜及中央神经节的输入、输出神经元进行标记。由于没有发现突触联系, 所以至少一部分光感受细胞的轴突可被视为直接参与形成视神经。这些神经元的轴突进入大脑神经节形成密集的细传入神经纤维束-视神经堆。传出神经元则存在于除颊部以外的所有神经节。一些上行轴突在大脑神经节处分叉, 通过脑-脑联合, 到达对侧眼并在眼杯处形成分枝。部分传出神经元的轴突也投射于不同的外周神经, 如:n.n. intestinalis, pallialis dexter, pallialis sinister internus et externus。五羟色胺能纤维和FMRF-酰胺能纤维均存在于视神经上, 且这些纤维隶属于只投射在同侧眼的中央神经元。它们形成了位于眼杯处的丰富曲张结构及视网膜核心层, 并且可能有助于调节视网膜对光的敏感性。
腹足纲; 神经系统; 眼; 逆行性传输; 五羟色胺; FMRF-酰胺
Q42; Q954.67; Q959.212
A
0254-5853-(2011)04-0403-18
2011-02-15;接受日期:2011-06-09
10.3724/SP.J.1141.2011.04403
date: 2011-02-15; Accepted date: 2011-06-09
*Corresponding author (通信作者), Tel: +49-421-2003242, E-mail: b.meyer-rochow@jacobs-university.de
猜你喜欢
杂志排行
Zoological Research的其它文章
- 悬尾应激对小鼠空间记忆及其反转学习的损伤效应
- Behavioral migration diversity of the Yangtze River Japanese Eel, Anguilla japonica, based on otolith Sr/Ca ratios
- Visual modeling reveals cryptic aspect in egg mimicry of Himalayan Cuckoo (Cuculus saturatus) on its host Blyth’s Leaf Warbler (Phylloscopus reguloides)
- Notch signaling dependent differentiation of cholangiocyte-like cells from rhesus monkey embryonic stem cells
- Metabolism and thermoregulation between Mrs Hume’s Pheasant (Syrmaticus humiae) and Elliot’s Pheasant (S. ellioti)
- Localization of stationary pronuclei during conjugation of Paramecium as indicated by immunofluorescence staining
