Effects of urethane on the response properties of visual cortical neurons in young adult and old cats
2011-12-25PENGQingSongZHOUJunSHIXiaMingHUAGuoPengHUATianMiao
PENG Qing-Song, ZHOU Jun, SHI Xia-Ming, HUA Guo-Peng, HUA Tian-Miao
(School of Life Science, Anhui Normal University, Anhui Wuhu 241000, China)
Effects of urethane on the response properties of visual cortical neurons in young adult and old cats
PENG Qing-Song, ZHOU Jun, SHI Xia-Ming, HUA Guo-Peng, HUA Tian-Miao*
(School of Life Science,Anhui Normal University,Anhui Wuhu241000,China)
Previous studies have shown that visual cortical neurons in old mammals exhibit higher spontaneous activity, higher responsiveness to visual stimuli, and lower selectivity for stimulus orientations and motion directions than did neurons in young adult counterparts. However, whether the responsive difference in cortical neurons between young and old animals resulted from different effects induced by anesthetics has remained unclear. To clarify this issue, we recorded the response properties of individual neurons in the primary visual cortex of old and young adult cats while systematically varying the anesthesia level of urethane, a widely used anesthetic in physiology experiments. Our results showed that cumulatively administrating 50 mg and 100 mg of urethane upon the minimal level of urethane required to anesthetize an old or young adult cat did not significantly alter the degree of neuronal response selectivity for stimulus orientations and motion directions nor significantly change the visually-driven response and spontaneous activity of neurons in old and young adult cats. Cumulatively administrating 150 mg of urethane decreased neuronal responsiveness similarly in both age groups. Therefore, urethane appears to exert similar effects on neuronal response properties of old and young adult animals.
Urethane; Anesthesia effects; Neuronal response properties; Primary visual cortex; Young adult cats; Old cats
Anesthetic effects of various drug agents have been reported frequently, but few authors have focused on whether anesthesia exerts different effects on young and old subjects. Clinical observations indicate that the anesthetic dosage needed for induction and maintenance of anesthesia varies with patient age. In general, young subjects need a higher dose than older subjects (Hilton et al, 1986; Scheepstra et al, 1989; Larsson & Wahlstrom,1998; Segal et al, 2002), while amobarbital, propofol, thiopentone, isoflurane, and nitrous oxide exert a larger anesthesia effect on older subjects than younger subjects, such as more severely impaired short and long term memory as well as reduced vigilance and competence in motion detection (Nadstawek et al, 1989; Culley et al, 2003). After being anesthetized, old subjects took longer time to wake up or return to baseline EEG status (Scheepstra et al, 1989; Segal et al, 2002). Some authors suggest that maintaining anesthesia might alter the sensitivity of the central nervous system of old individuals to anesthetics. For example, old animals might develop an acute tolerance after repeated exposure to propofol whereas young animals do not. Therefore, old brains might be less sensitive to anesthesia than young ones (Larsson & Wahlstrom, 1996, 1998).
Although some observations on different behavior response of young adult and aged individuals to anesthetics have been conducted, no study has systematically examined the effects of different anesthesia level on the single-cell response in old and young adult brains. A recent study observed the anesthetic effects of isoflurane and halothane on the response properties of single neurons in the visual cortex of normal young adult animals (Villeneuve & Casanova, 2003). This study reported that increasing the concentration of anesthetics could decrease the animal’s heart rate, expired CO2and neuronal responsiveness, but did not significantly change neuronal selectivity for stimulus orientations, motion directions and signal-to-noise ratio. Urethane is a anesthetic widely used in cat visual electrophysiology experiments (Shou et al, 1996; He et al, 2005; Hua et al, 2006, 2009). Impacts of different urethane dosage on the neuronal response properties in young and old subjects has not been documented anywhere, yet is a concern in the interpretation of data from experiments using young and old anesthetized animals. To clarify this concern, we compared effects of different anesthesia levels of urethane on the responsiveness of single V1 neurons between old and young adult cats.
1 Materials and Methods
1.1 Subjects
Subjects included six young adult cats (2 - 3 years old) and four old cats (12 - 14 years old). The body weight of all cats ranged from 2 to 3 kg. All cats were examined ophthalmoscopically to confirm they had no optical or retinal problems that would impair their visual functions. All experiments were conducted strictly in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1.2 Preparation for extracellular recording
The preparation for extracellular single-unit recording was carried out as previously described (Hua et al, 2006, 2010). Briefly, cats were pre-anesthetized with ketamine HCl (40 mg/kg, im) and xylazine (2 mg/kg, im). Local anesthesia was by way of Lidocaine (1%) application to all incisions for surgical entry to the skull of cats. After intubation of intravenous and tracheal cannulae, cats were placed in a stereotaxic apparatus. Pupils were maximally dilated with atropine (1%), and appropriate contact lenses were used to protect the corneas. A mixture of urethane [20 mg/(kg·h)] and gallamine triethiodide [10 mg/(h·kg body weight) ]was infused intravenously to maintain anesthesia and eye muscle paralysis. Animals were artificially respired, and expired CO2was maintained at approximately 4%. To assess the level of anesthesia, ECG (electrocardiograph) and heart rate (180 - 220 beats/s) were monitored throughout the experiment. A small hole was drilled in the skull at a position 4 - 8 mm posterior to the ear bars and 0 - 4 mm lateral to the midline. A glass-coated tungsten microelectrode (impedance 3 - 5 MΩ) was positioned over area 17 according to stereotaxic coordinates of the cat brain and advanced using a hydraulic micromanipulator. After the preparation was completed, the optic discs of the two eyes were reflected on a tangent screen positioned 114 cm from the retina, and the central areas for both eyes were located.
1.3 Visual stimulation
Visual stimuli were drifting sinusoidal gratings, written in MATLAB and shown on a CRT monitor (1024×768, 85 Hz) 57 cm from the animal’s eyes. Once a single unit was isolated, the cell’s receptive field center was carefully located by consecutively presenting computer-generated light spots on the CRT. We selected optimal stimulus size and temporal and spatial frequency of gratings for each cell by presenting a series of stimulus packages. Each stimulus was presented monocularly to the dominant eye. A neuron’s tuning response to stimulus orientations and motion directions was obtained by presenting a stimulus package of gratings moving in 24 different motion directions (0 - 360° scale with an increment of 15°). The orientation of each drifting stimulus was orthogonal to its direction of motion. Before each stimulus presentation, 1-second spontaneous activities were acquired, while mean luminance was shown on the CRT. Each stimulus repeated 4 - 6 times with a 5-minute interval between trials for functional recovery of the recorded neuron. The presentation duration of each stimulus was less than 5 seconds based on the neuron’s optimal temporal frequency. The stimulus mean luminance and contrast were fixed at 19 cd/m2and 80%, respectively, and the environment luminance on the cornea was 0.1 lux.
1.4 Experimental procedure
After a single unit was isolated, the orientation tuning curve of the neuron was recorded repeatedly (4 - 6 times). We then cumulatively increased the anesthetic level of urethane (from 50 mg, 100 mg to 150 mg) by intravenous injection in one dose (each dose contained 50 mg urethane in 3 mL 0.9% physiological saline), while observing the effects of the increased anesthetic level on the same neuron’s response properties. Extracellular recordings and heart rate sampling was started 5 minutes after each modification of anesthetic level and repeated 3 - 4 times with a 5-minute interval. The testing of each anesthetic level lasted within 30 minutes. After testing all anesthetic levels on each neuron, a solution containing 0.9% physiological saline and 5% glucose or minimal level of urethane (20 mg/hr·kg body weight) and gallamine triethiodide (10 mg/hr·kg body weight) was infused intravenously while the animal’s heart rate and ECG were continuously monitored to assess anesthesia level and functional recovery. After heart rate and ECG returned to the normal range (usually lasting about 6 - 8 h), we repeated the examining procedure on the next neuron.
1.5 Data collection and analysis
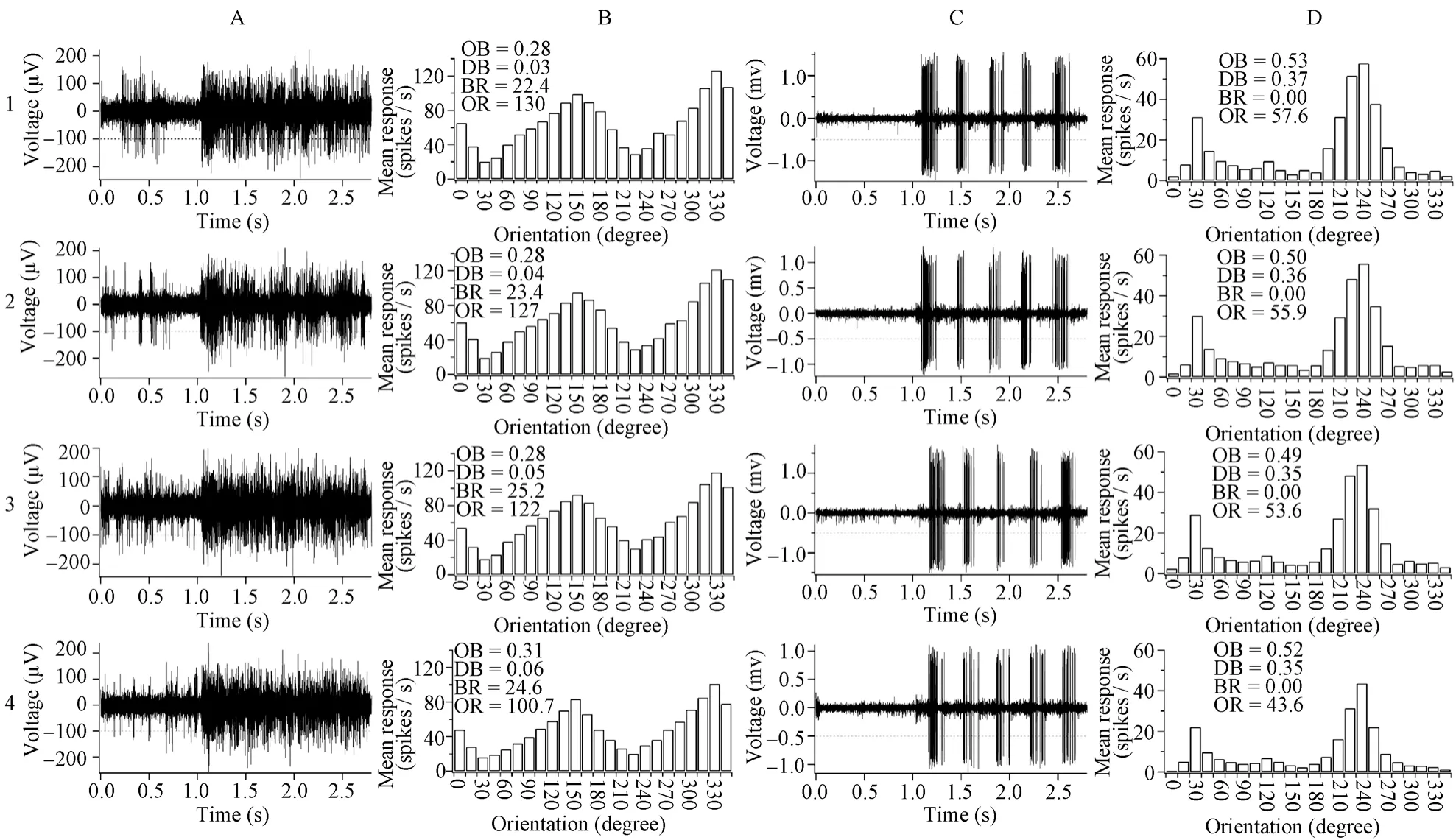
Fig. 1 The voltage trace (panel A&C) of a sample cell’s response to a grating stimulus, with optimal orientation and motion direction (A: 330°; C: 240°) and orientation tuning curve (panel B & D) to stimuli with different orientations and motion directions in old (A & B) and young adult (C & D) cats
The neuron response signal was amplified with a microelectrode amplifier (NIHON KOHDEN, Japan) and differential amplifier (FHC, USA), and action potentials were fed into a window discriminator with audio monitor. The original voltage traces (Fig. 1A,C) were digitized using an acquisition board (National Instruments, USA) controlled by IGOR software (WaveMetrics, USA).
The responses of each neuron to different stimuli were analyzed in real time or stored in the computer for later analysis. The response of a cell to different stimulus orientation and direction were defined as the mean response (spontaneous activity subtracted) corresponding to the time of stimulus modulation, which was used to draw the orientation tuning curve (Fig. 1B,D). The orientation and motion direction selectivity of each cell were calculated (Schmolesky et al, 2000; Hua et al, 2006). Briefly, the responses of each cell to the different stimulus orientations or directions were stored as a series of vectors. The vectors were added and divided by the sum of the absolute values of the vectors. The angle of the resultant vector gave the preferred orientation or direction of the cell. The length of the resultant vector, termed the orientation or direction bias (OB or DB), provided a quantitative measure of the orientation or direction sensitivity of the cell. A cell’s maximal response was defined as the response (subtracted spontaneous activity) to optimal orientation and motion direction.
All values were expressed as mean ± standard deviation. Variations between different anesthetic levels and different age groups were evaluated using analysis of variance (ANOVA), paired t-test, and Chi-square test.
2 Results
A total of 18 neurons from the six young adult cats and 14 neurons from the four old cats were examined (with an additional 13 neurons excluded from data analysis because of escaping during recording). The proportion of simple cells to complex cells was comparable in the two cat groups, with seven simple versus eleven complex in young adult cats and five simple versus nine complex in old cats (χ2(1)=0.034,P=0.854). Therefore, data from the two types of cells were combined for statistical analysis.
2.1 Heart rate change
Tab. 1 shows the average heart rate changes after each anesthetic level modification and heart rate recovery at different times from dosage change. On the whole, the heart rate remained within a normal range of 180 - 210 pulses/s at different dosage of urethane in both old and young adult cats. The heart rate change was also similar for the two age groups. However, unlike previous reports (Villeneuve & Casanova, 2003), the heart rate observed in this study actually increased with anesthetic level of urethane, especially at high anesthetic levels (pairedt-test:P<0.05). This heart rate increase lasted at least 5 hours before recovery to the initial heart rate level.
2.2 Neuronal response properties
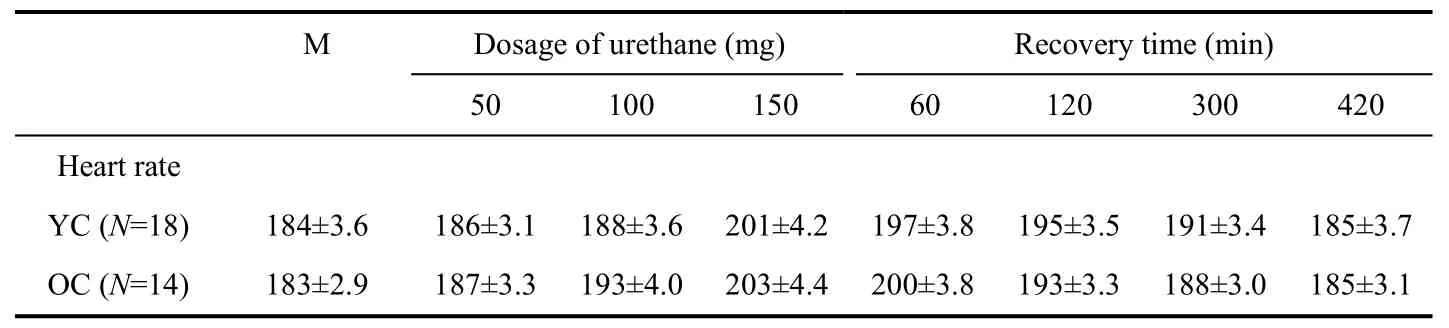
Tab. 1 Heart rate change 5 minutes after each anesthesia level modification and heart rate recovery
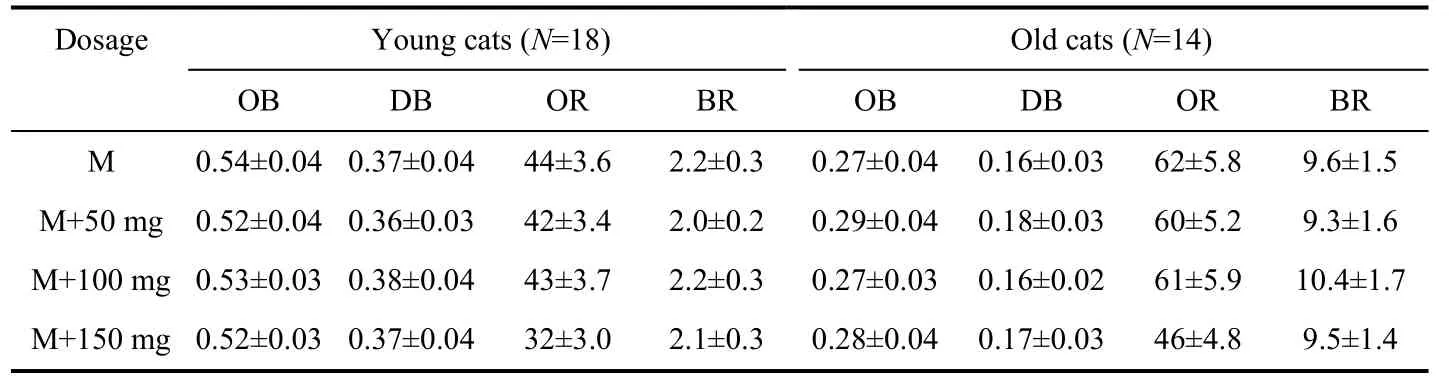
Tab. 2 Changes in neuronal response properties at different anesthetic levels of urethane
Tab. 2 shows average neuronal response changes after each anesthesia level modification. Statistical analysis indicated that cumulatively administrating 50 mg or 100 mg urethane did not significantly change the degree of neuronal selectivity for stimulus orientations, directions, visually evoked response, or baseline response (Fig. 2A - D) in either old or young adult cats (pairedttest,P>0.05). However, the neuron’s visually-driven response at 150 mg of urethane significantly decreased (averagely decreased by 27.3% for the young cats and 25.8% for the old cats) when compared with that at minimal anesthetic level (Fig. 2C) (pairedt-test,P<0.05). Even under the highest level of anesthesia, the neuron’s spontaneous activity and selectivity for stimulus orientations and motion directions did not show significant change compared with minimal anesthesia level (pairedt-test,P>0.05). Therefore, the effects of urethane on single neuron’s response at different anesthesia levels were similar in both age groups of cats. As shown by the ANOVA analysis, the neuronal selectivity for stimulus orientations and motion directions at each anesthesia level in old cats significantly decreased compared with that in young cats (OB:F(1,120)=92.637,P<0.0001; DB: F(1,120)=65.576,P<0.0001), whereas the neuronal spontaneous activity (BR) and visually-evoked response (OR) at each anesthesia level in old cats were significantly higher than in young adult cats (OR:F(1,120)=29.414,P<0.0001; BR:F(1,120)=111.813,P<0.0001).
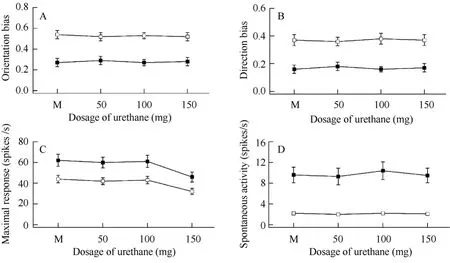
Fig. 2 Changes in neuronal response properties at different dosages of anesthesia for old cats (black square) and young adult cats (white square)
3 Discussion
To study the effects of various anesthetics on human and nonhuman subjects is of great importance in clinical practice and neuroscience research. Many studies have shown that anesthetics not only affect cardiovascular reactions but also brain and neuronal activities (Uhl et al, 1980; Sebel et al, 1986; Tigwell & Sauter, 1992). Some anesthetics, particularly volatiles, may affect or even modulate the activity of GABA receptors, which are, in turn, involved in the shaping of neuron’s receptive field properties (Nakahiro et al, 1989), and thus may modify neuron selectivity for stimulus orientations and motion directions.
Urethane is a widely used anesthetic in animal electrophysiology research. It remains unclear, however, whether different concentrations or dosages of urethane affect cardio-vascular and neuronal activities. The present study showed that the heart rate of both old and young adult cats increased with anesthesia level of urethane, especially at higher anesthetic levels. This observation differed from previous reports that improving the concentration of isoflurane and halothane can suppress heart rate (Villeneuve & Casanova, 2003). This difference may be caused by different anesthetics. It is possible that urethane may exert an excitatory influence on the heart though most anesthetics, such as isoflurane and halothane, display a suppressive effect. Further studies are needed to clarify this possibility. Our results also showed that urethane exerted no significant effects on neuronal selectivity for stimulus orientations and motion directions, which was in agreement with reports on other anesthetics (Villeneuve & Casanova, 2003). Although the visually-evoked response of neurons decreased significantly at high anesthetic level of urethane, neuronal selectivity for stimulus orientations and motion directions was not significantly affected (Fig. 2A - C). This result indicated that urethane might equally affect neuronal response to all stimulus orientations and motion directions and might not be involved in the regulation of intracortical inhibition, especially GABAergic inhibition. Unlike other anesthetics (Villeneuve & Casanova, 2003), urethane shows a mild effect on neuronal response properties. Even at a high dosage (150 mg), urethane exerted a comparable effect on the responsiveness of neurons in old and young adult animals. Therefore, we concluded that the effects of different urethane dosages on the response properties of V1 neurons in young adult and old cats were quite similar. It is unlikely that the previously observed functional declines of visual cortical neurons in old animals (Schmolesky et al, 2000; Mendelson & Wells, 2002; Hua et al, 2006) could result from different anesthetic effects on young adult and old brains.
Culley DJ, Baxter M, Yukhananov R, Crosby G. 2003. The memory effects of general anesthesia persist for weeks in young and aged rats [J].Anesth Analg, 96: 1004-1009, table of contents.
He L, Li X, Hua T, Bao P, Zhou Y. 2005. Degradation of response modulation of visual cortical cells in cats with chronic exposure to morphine [J].Neurosci Lett, 384: 168-171.
Hilton P, Dev VJ, Major E. 1986. Intravenous anaesthesia with propofol and alfentanil. The influence of age and weight [J].Anaesthesia, 41: 640-643.
Hua T, Li G, Tang C, Wang Z, Chang S. 2009. Enhanced adaptation of visual cortical cells to visual stimulation in aged cats [J].Neurosci Lett, 451: 25-28.
Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. 2006. Functional degradation of visual cortical cells in old cats [J].Neurobiol Aging, 27: 155-162.
Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. 2010. Perceptual learning improves contrast sensitivity of V1 neurons in cats [J].Curr Biol, 20: 887-894.
Larsson JE, Wahlstrom G. 1996. Age-dependent development of acute tolerance to propofol and its distribution in a pharmacokinetic compartment-independent rat model [J].Acta Anaesthesiol Scand, 40: 734-740.
Larsson JE, Wahlstrom G. 1998. The influence of age and administration rate on the brain sensitivity to propofol in rats [J].Acta Anaesthesiol Scand, 42: 987-994.
Mendelson JR, Wells EF. 2002. Age-related changes in the visual cortex [J].Vision Res, 42: 695-703.
Nadstawek J, Hausmann D, Bartsch A, Fodisch M, Stoeckel H. 1989. Return of motor and mental functions following enflurane-nitrous oxide anesthesia of 1.3 MAC in various age groups [J].Anasth Intensivther Notfallmed, 24: 293-297.
Nakahiro M, Yeh JZ, Brunner E, Narahashi T. 1989. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons [J].Faseb J, 3: 1850-1854.
Scheepstra GL, Booij LH, Rutten CL, Coenen LG. 1989. Propofol for induction and maintenance of anaesthesia: comparison between younger and older patients [J].Br J Anaesth, 62: 54-60.
Schmolesky MT, Wang Y, Pu M, Leventhal AG. 2000. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys [J].Nat Neurosci, 3: 384-390.
Sebel PS, Ingram DA, Flynn PJ, Rutherfoord CF, Rogers H. 1986. Evoked potentials during isoflurane anaesthesia [J].Br J Anaesth, 58: 580-585.
Segal JB, Moo LR, Hart J, Jr. 2002. The effect of age on rate of functional recovery after intracarotid amobarbital injection [J].Epilepsia, 43: 659-661.
Shou T, Li X, Zhou Y, Hu B. 1996. Adaptation of visually evoked responses of relay cells in the dorsal lateral geniculate nucleus of the cat following prolonged exposure to drifting gratings [J].Vis Neurosci, 13: 605-613.
Tigwell DA, Sauter J. 1992. On the use of isofluorane as an anaesthetic for visual neurophysiology [J]. Exp Brain Res, 88: 224-228.
Uhl RR, Squires KC, Bruce DL, Starr A. 1980. Effect of halothane anesthesia on the human cortical visual evoked response [J].Anesthesiology, 53: 273-276.
Villeneuve MY, Casanova C. 2003. On the use of isoflurane versus halothane in the study of visual response properties of single cells in the primary visual cortex [J].J Neurosci Methods, 129: 19-31.
乌拉坦对青、老年猫视皮层神经元反应特性的影响
彭青松, 周 俊, 施夏明, 化国鹏, 华田苗*
(安徽师范大学 生命科学学院, 安徽 芜湖 241000)
以前的电生理研究结果显示, 老年哺乳动物视皮层细胞的自发反应及对视觉刺激的诱发反应比青年动物的显著增加, 而对光栅刺激的方位和运动方向选择性却显著下降。然而, 这种视皮层细胞功能的老年性改变是否因青、老年猫细胞对不同麻醉水平的敏感性差异引起尚不清楚。为探讨该问题, 以常用的麻醉药——乌拉坦(Urethane)为实验对象, 通过改变其麻醉剂量分别记录青、老年猫初级视皮层细胞对不同方位和运动方向光栅刺激的调谐反应。研究结果显示, 在基础麻醉量的基础上, 累积增加50 mg和100 mg乌拉坦对青、老年猫视皮层细胞的自发反应和诱发反应以及对光栅刺激方位和运动方向的选择性不产生显著影响, 累积增加150 mg乌拉坦会导致青、老年猫视皮层细胞对视觉刺激的反应性下降, 但下降的幅度相似。以上研究结果表明, 不同剂量的乌拉坦对青、老年动物视皮层细胞的反应性具有相似的影响。
乌拉坦; 麻醉影响; 初级视皮层; 神经元反应特性; 青年猫; 老年猫
Q42; R338.8; Q954.671
A
0254-5853-(2011)03-0337-06
2010-10-19;接受日期:2011-02-17
10.3724/SP.J.1141.2011.03337
date: 2010-10-19; Accepted date: 2011-02-17
s: Supported by Natural Science Foundation of Anhui Province (070413138) and the Key Research Foundation of Anhui Province Education Department (KJ2009A167)
*Corresponding author (通信作者), E-mail: tianmiaohua@gmail.com
猜你喜欢
杂志排行
Zoological Research的其它文章
- 白介素1β对大鼠皮层神经元钠电流的急性作用
- 尼罗罗非鱼Orexin前体基因的克隆、组织分布及其在摄食调控中的表达
- Phylogenetic evaluation of the taxonomic status of Papilio maackii and P. syfanius (Lepidoptera: Papilionidae)
- Genetic differentiations between randomly and selectively bred pig populations in Yunnan, China
- 阿魏酸对脂多糖诱导的小鼠小胶质细胞炎性反应的抑制作用
- 棕颈钩嘴鹛分子系统发育及分类关系初探
