镍–磷–硫酸钡化学复合镀层的研制
2011-11-22KARTHIKEYANVENKATACHALAMSRINIVASANNARAYANAN
KARTHIKEYAN S, VENKATACHALAM G, SRINIVASAN K N, NARAYANAN S
镍–磷–硫酸钡化学复合镀层的研制
KARTHIKEYAN S*, VENKATACHALAM G, SRINIVASAN K N, NARAYANAN S
在由硫酸镍31 g/L、次磷酸钠22 g/L、乙醇酸30 g/L、乙酸钠13 g/L、二甲胺0.8 g/L、全氟乙基碘化铵0.1 g/L及重晶石(即硫酸钡)0 ~ 25 g/L组成的稳定镀液中,采用化学镀的方法在低碳钢上制备了Ni–P–BaSO4复合镀层。其表面形貌采用扫描电镜进行分析,耐蚀性以动电位极化及电化学阻抗谱测试。镍–磷合金基体中掺入硫酸钡,提高了化学镀层的显微硬度。相对于纯Ni–P合金镀层,Ni–P–BaSO4复合镀层具有更小的磨损率。硫酸钡颗粒的存在使得复合镀层的耐蚀性明显降低。
化学镀;镍磷合金;硫酸钡;复合镀层;耐蚀性;显微硬度;耐磨性
1 Introduction
The utility of nickel coatings for chemical engineering applications is different which has been now well established[1]. There is no doubt that the unique properties of nickel coatings such as hardness, resistance to corrosion and wear, etc., have created their trend of modern surface engineering[2-5]. The ability to deposit fine composite particles with electrolytic nickel plating is a major step in the extension of various processes for surface engineering[6]. The main objective of incorporation of composite particles is to improve mechanical properties. Various composite coatings such as Ni–P–Al2O3[1], Ni–P–SiC[5], Ni–P–diamond[7], Ni–P-MoS2[6], Ni–P–ZrO2–Al2O3[8], etc., have been reported in the literatures.
Barium sulfate (BaSO4), commonly referred to as barite, is suitable for many diverse uses because of its high specific gravity (4.5), opaqueness to X-rays, inertness and whiteness. Recently, barite has been incorporated into polymers to form electrically insulating epoxy composites with X-ray attenuation properties. On account of the novel properties, it can be expected that barium sulfate particles will have extensive applications. However, as far as we know, no concrete reports available for the incorporation of barite in electroless deposition and hence the present study. This paper addresses the incorporation of BaSO4into electroless Ni–P matrix. The properties of the composite coating were evaluated by Vickers hardness test, Taber abraser, AC impedance and potentiodynamic polarization methods. SEM micrographs have been described for determining the surface morphology of coatings. The electrolyte used was aqueous 3.5% NaCl solution.
2 Experimental
The bath used in the present study had the following composition of A.R. grade chemicals.

Before addition, BaSO4particles were mixed with a small amount of wetting agent as propitiatory surfactant. A mild steel with dimensions 3 cm × 5 cm × 0.6 cm was used as substrate for the codeposition. Prior to plating, the substrate was subjected to pretreatment such as degreasing by trichloroethylene and acid pickling in 10% HCl at room temperature. After pretreatment, the samples were introduced in electroless plating bath. The bath was mechanically agitated with 200 r/min. The deposit thickness was measured using a X-ray fluorescence (XRF) spectroscope (AMI, USA). The determination of phosphorus in the deposits of Ni–P is based on completely precipitating phosphorus as ammonium phosphomolybdate, dissolving the precipitate in concentrated HNO3solution and estimating the excess HNO3by back titration with standard NaOH solution. Nickel was analyzed by complexometric titration using EDTA with murexide as indicator.
The content of BaSO4particles in a composite coating was calculated by the equation:
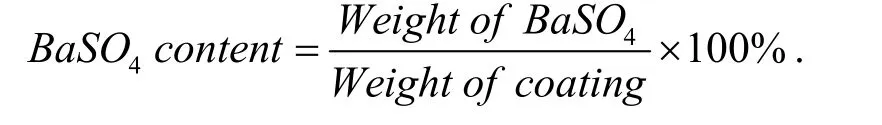
The weight of BaSO4particles in the composite coatings was precisely measured by the chemical method described below. The conventional Ni–P–BaSO4composite coatings were put into a HNO3solution separately and reacted till they were completely dissolved. BaSO4particles do not react with HNO3, so they precipitated out in the solution. The whole solution was placed for two weeks in order to make BaSO4particles totally precipitate. Then the BaSO4particles were separated and dried at 90 °C for 20 h. Finally the dried BaSO4particles were weighed using an electronic balance with an accuracy of 0.01 mg.
The Vickers hardness measurement for electroless Ni–P–BaSO4in as plated condition (without heat treatment) was evaluated at a load of 100 g with a diamond pyramid indenter technique where the time of indentation was 15 s. The results were an average of 10 times measurements. Taber abrasion resistance with CS-10 abrasive wheel having a load of 1 kg for 1 000 cycles was performed on both Ni–P and Ni–P–BaSO4deposits. For the studies of corrosion, EG&G Princeton Applied Research Model 6310 was used for potentiodynamic polarization and AC impedance measurements. To that extent 1 cm2electrolessly deposited Ni–P and Ni–P–BaSO4were used as working electrodes. 4 cm2of platinum and saturated calomel electrode (SCE) were used as counter and reference electrodes, respectively. The electrolyte was 3.5% NaCl. SEM micrographs have been described for determining the surface morphology of coatings at a magnification of × 3 000.
3 Results and discussion
3. 1 Chemical deposition
It can be seen from the Table 1 that there isincreasing concentration of dispersoid in the matrix as the content of barite in the electroless bath is increased. In the present system studied, the increasing concentration of particulate cause a decrease in the percentage of nickel and phosphorus content in deposit. 15 g/L of BaSO4is the recommended dosages for the bath. Beyond these concentrations, collision among the moving particles inhibits the plating process.
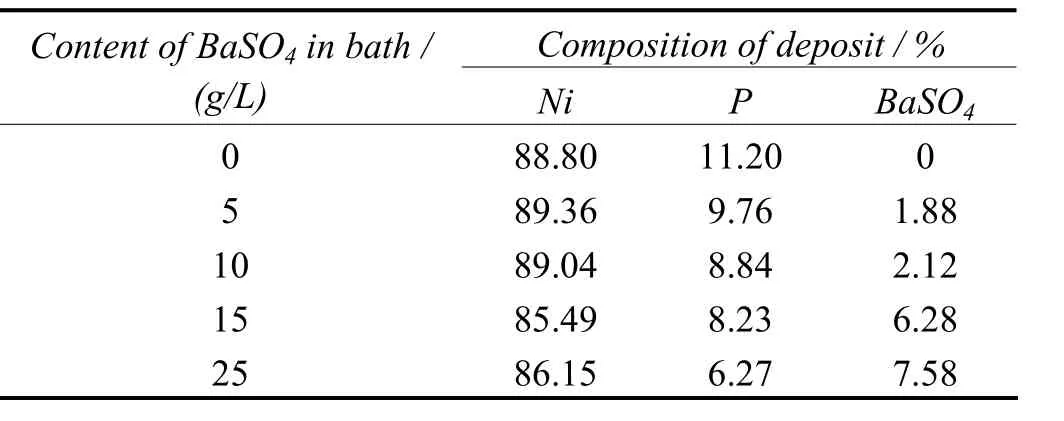
Table 1 Effect of mass concentration of BaSO4 particles in bath on chemical composition of deposit表1 镀液中硫酸钡颗粒的质量分数对镀层化学成分的影响
3. 2 Microhardness
The results of microhardness testing obtained for Ni–P–BaSO4composite coatings are presented in Table 2. It is observed from the Table 2 that the microhardness values are more dependent on the barite particle contents in deposits.
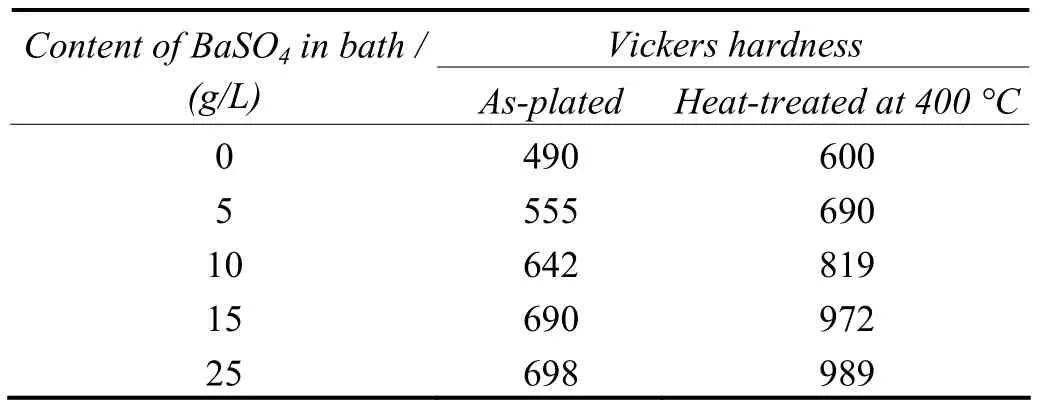
Table 2 Effect of mass concentration of BaSO4 particles in bath on coating microhardness表2 镀液中硫酸钡颗粒的质量分数对镀层显微硬度的影响
The coating prepared with 15 g/L BaSO4in bath has higher microhardness than a pure Ni–P alloy coating both in as-plated and heat-treated conditions. The increased microhardness in the case of composite coatings after heat treatment is ascribed to the precipitation of intermetallic compound phases induced by BaSO4particles[9]. This increase in microhardness is related to microstructural changes in the matrix. The introduction of BaSO4particles in electroless Ni–P coating, with microhardness of 690 to 989 HV, increases the resistance to plastic deformation which results in an increase of microhardness. Also the composite is believed to have a large amount of Ni3P phase which is responsible for the hardened film. Thus the higher hardness value of the coating containing Ni–8.23% P–6.28% BaSO4can be attributed to both the hard barite particles and to the Ni3P rich matrix[9].
3. 3 Wear resistance
The results of wear resistance measurement for the composite coatings are presented in Table 3. The wear resistance of the Ni–P–BaSO4composite coating prepared with 15 g/L BaSO4in bath is found to be higher than the pure Ni–P alloy coating, suggesting that the improved performance is more due to the incorporation of BaSO4. The wear resistance of the deposits are greatly enhanced after heat treatment.
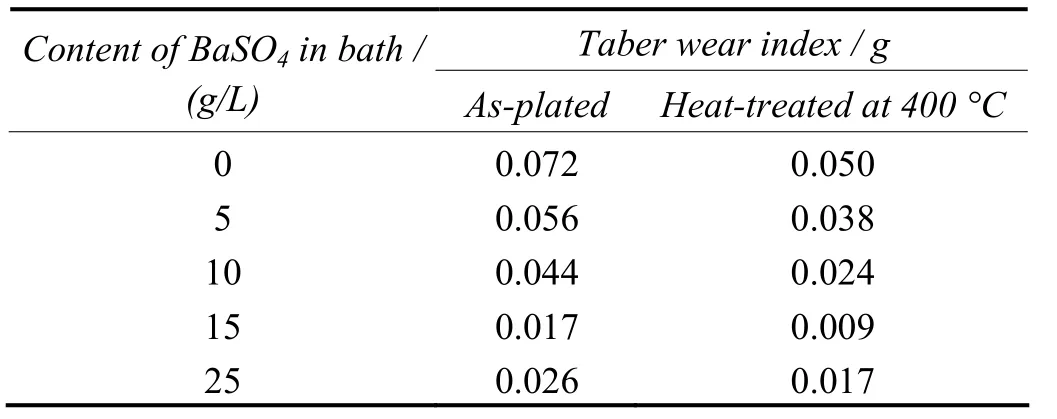
Table 3 Effect of mass concentration of BaSO4 particles in bath on abrasion resistance of coatings表3 镀液中硫酸钡颗粒的质量分数对镀层耐磨性的影响
The reason for this is that the entire amorphous portion of deposited nickel got converted into crystalline nickel and nickel phosphides[9]. This implies that a higher densification of the matrix results in more firmly incorporated particles. The formation of more amount of Ni3P precipitates in the case of higher phosphorus content will also enhance the wear resistance[10-11]. In addition, if any chemical bonding between the barite particle and nickel–phosphorus metal matrix had taken place, interdiffusion might have enhanced the integrity between the particle and deposit.
3. 4 Corrosion resistance
Table 4 and 5 gives the corrosion resistance properties of Ni–P–BaSO4and Ni–P deposits evaluated through potentiodynamic polarization and AC impedance measurements.
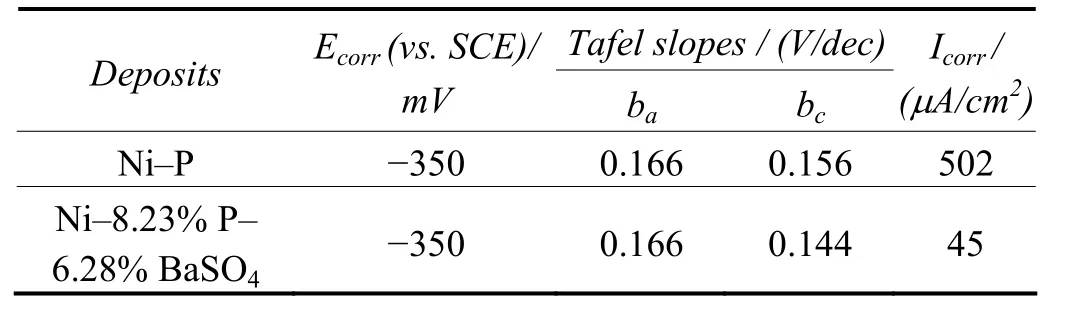
Table 4 Evaluation of corrosion resistance of deposits in 3.5% NaCl solution by potentiodynamic polarization studies表4 采用动电位极化方法评价镀层在3.5%氯化钠溶液中的耐蚀性能
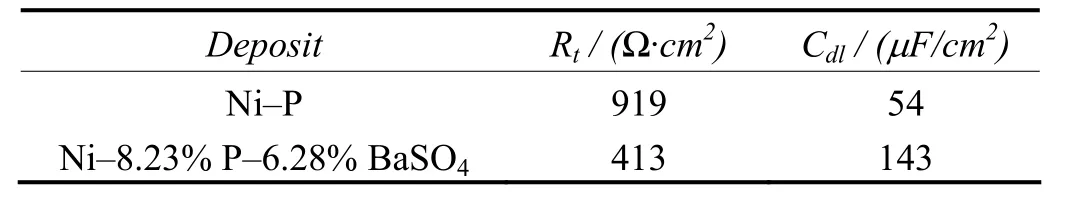
Table 5 Evaluation of corrosion resistance of deposits in 3.5% NaCl solution by AC impedance measurement表5 采用交流阻抗法评价镀层在3.5%氯化钠溶液中的耐蚀性能
Polarization studies clearly shows that the Ni–8.23% P–6.28% BaSO4deposit has less corrosion resistance than Ni–11.2% P. The AC impedance measurement also clearly indicates the inferior performance of composite coatings compared with Ni–P deposits in NaCl environments. The poor corrosion resistance of the composite coating is attributed to the porous BaSO4particles incorporation in the coating.
3. 5 Surface morphology
The SEM photographs are presented in Figure 1a and 1b taken for Ni–P and Ni–P–BaSO4coatings under heat-treated conditions.
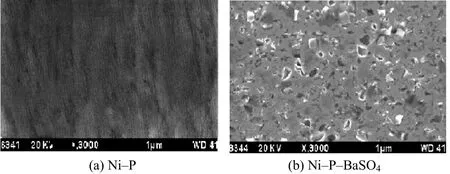
Figure 1 SEM images of electrolessly deposited coatings图1 化学镀层的扫描电镜照片
In the case of Ni–P–BaSO4composite coating shown in Figure 2, a porous structure is seen, with particle coalescence. The incorporation of barite seems to be due to mechanical and little owing to the electrophoretic effect of the hydrophobic particles. As the particles strike the surface, they are included in the Ni–P alloy being deposited and become a part of the cermet. There is no molecular bond between the particles and the metal matrix. The composite is developed mechanically by the effect of settling and impinging of the particles upon the substrate metal and subsequent envelopment of the particles by the matrix material, as they are codepositing. Evenly keeping the particles in suspension and rotating the object to be coated within resonance by bath agitation can ensure uniformity of composition for the composites.
However, the poor corrosion resistance of Ni–P–BaSO4coatings can be attributed to the incorporation of porous barite particles into Ni–P matrix.
4 Conclusions
(1) A suitable bath has been optimized based on nickel sulfate, sodium hypophosphite and sodium acetate to form electroless Ni–P–BaSO4composite coatings.
(2) The microhardness and wear resistance of electroless Ni–P–BaSO4composite coatings are better than electroless Ni–P coatings both in as-plated and heat-treated conditions.
(3) Ni–P coatings showed better corrosion resistant than electroless Ni–P–BaSO4composite coatings.
(4) SEM studies proves the incorporation of BaSO4in Ni–P matrix.
[1] AGARWALA R C, AGARWALA V, Electroless alloy/composite coatings: A review [J]. Sādhanā, 2003, 28 (3/4): 475-493.
[2] DATTA P K, BEDINGFIELD P B, LEWIS D B, et al. Structure and phase changes accompanying treatment of electroless Ni–B alloy coating [C] // Proceedings of 2nd International Electroless Nickel Conference, 1991: 139-153.
[3] CSANÁDY Á, RÖHLICH P, SZABÓ D. Proceedings of 8th European Congress on Electron Microscopy [C]. [S.l.]: European Microscopy Society, 1984: 913.
[4] NARAYAN R, PANDEY A. Electroless Ni–PTFE composite coatings [C] // Proceedings of 12th National Convention of Metallurgists and Material Scientists. 1997: 98-106.
[5] KARTHIKEYAN S, SRINIVASAN K N, VASUDEVAN T, et al. Characterization of electroless Ni–P–SiC composite coatings [J]. Bulletin of Electrochemistry, 2001, 17 (3): 127.
[6] SRINIVASAN K N, JOHN S, KARTHIKEYAN S, et al. Studies on electroless nickel–phosphorous molybdenum sulphide composite coatings [J]. Transactions of the Metal Finishers’ Association of India [J]. 2003, 12 (3/4): 143-147.
[7] REDDY V V N, RAMAMOORTHY B, NAIR P K. Studies on electroless Ni–P/diamond composite coatings [C] // Proceedings of 18th AIMTDR Conference. Kharagpur: Indian Institute of Technology, 1998: 440-444.
[8] SHARMA S B, R. C AGARWALA R C, AGARWALA V, et al. Wear and friction behaviour of Ni–P–ZrO2–Al2O3composite electroless coatings [R]. International Conference on Mechanical Engineering 2001. Dhaka: Bangladesh University of Engineering & Technology, 2001.
[9] XINMIN H, ZONGANG D. The wear characteristics of Ni–P–SiC composite coatings [J]. Transactions of the Institute of Metal Finishing, 1992, 70 (2): 84-86.
[10] KARTHIKEYAN S, NEELAKANDAN M A. Characteristics of electroless Ni–P–graphite composite coatings [J]. Electroplating & Finishing, 2006, 25 (4): 1-4.
[11] IZZARD M, DENNIS J K. Deposition and properties of electroless nickel/graphite coatings [J]. Transactions of the Institute of Metal Finishing, 1987, 65 (3): 85-89.
Development of electroless Ni–P–BaSO4composite coatings
S. Karthikeyan1,*, G. Venkatachalam2, K. N. Srinivasan3,S. Narayanan4
(1. Materials Division, School of Advanced Sciences, VIT University, Vellore-632014, Tamilnadu, India; 2. Design Division, School of Mechanical and Building Sciences, VIT University, Vellore-632014, Tamilnadu, India; 3. IMF Division, Central Electrochemical Research Institute (CECRI), Karaikudi-630006, Tamilnadu, India; 4. Manufacturing Division, School of Mechanical and Building Sciences, VIT University, Vellore-632014, India)
Ni–P–BaSO4composite coatings were prepared on mild steel by electroless plating from a stable bath containing nickel sulfate 31 g/L, sodium hypophosphite 22 g/L, glycolic acid 30 g/L, sodium acetate 13 g/L, dimethylamine 0.8 g/L, perfluoroethyl ammonium iodide 0.1 g/L, and barite (BaSO4) particles 0-25 g/L. The surface morphology of the composite coatings was analyzed by SEM, and the corrosion resistance by potentiodynamic polarization and electrochemical impedance spectroscopy. The incorporation of BaSO4into Ni–P alloy matrix increases the microhardness of the electroless coatings. Ni–P–BaSO4composite coating exhibits a distinctly small wear rate as compared with pure Ni–P alloy coating. The presence of barite particles in the deposit significantly reduces the corrosion resistance of the composite coatings.
electroless plating; nickel–phosphorus alloy; barium sulfate; composite coating; corrosion resistance; microhardness; wear resistance
TQ153.12
A
1004 – 227X (2011) 03 – 0031 – 04
date:2010–08–13 Revised date: 2010–09–29
S. Karthikeyan, (E-mail) skarthikeyanphd@yahoo.co.in, drskarthikeyanphd@gmail.com.
Biography:S. Karthikeyan (1975–), male, graduated with gold medal in M. Sc (Chemistry) from Alagappa University, Karaikudi, India and obtained his PhD degree in industrial chemistry (electroless plating) from the same university in 2002. He has published 34 research papers in leading national and international journals and seminars. He is having 11 years teaching and research experience. He has been awarded young scientist fellowship for the year 2005-06 by TNSCST, India. His research interest includes electroless plating of metals and composites, electroplating and corrosion inhibition.
[ 编辑:温靖邦 ]
