线状和树枝状银纳米结构、形成机理及表面增强拉曼散射性质
2010-12-12吴馨洲裴梅山王庐岩李肖男陶绪堂
吴馨洲 裴梅山 王庐岩, 李肖男 陶绪堂
(1济南大学化学化工学院,山东省氟化学化工材料重点实验室,济南 250022; 2山东大学晶体材料国家重点实验室,济南 250100)
Silver nanoparticles,especially one⁃dimensional nanostruc⁃tures,have drawn particular attention due to their highest elec⁃trical,thermal conductivities and strong surface plasmon reso⁃nance,which make them attractive for use in biochemistry as nanoscale biomaterials[1-5].For instance,Thorpe et al.[6]synthe⁃sized a composite electrode structure comprised of silver nanowires and carbon nanotubes for use as cathode catalysts in alkaline fuel cell.They found that silver nanowires had similar performance at a much lower catalyst loading than the bulk samples.
In the past few years,many wet⁃chemical methods,like soft template,non-template,seedless and ionic liquid processes, have been developed for the synthesis of one dimensional sil⁃ver nanostructures[7-10].And their properties such as catalysis, SERS,and plasmon resonances have been investigated[5-6,11-12]. However,these methods have their own disadvantages,such as relatively high temperature,expensive and commercially un⁃available reagents,complicated operations,and low⁃yield.Suh et al.[10]prepared silver nanowires in the presence ofN⁃alkyl imidazolium based ionic liquids.The obtained straight wires are about 200 nm in width and up to about 15 mm in length with no or few nanoparticles.But this method requires expen⁃sive reagents and complicated operations.Therefore,there is still a need to develop a more straightforward procedure for fabricating silver nanowires.Monodispersed silver nanowires were synthesized under oil⁃bath heating in high yield(>90% without isolation)using bubbling of air through a reagent solu⁃tion from ca 20℃to the boiling point of ethylene glycol(EG) (198℃)for 20 min by Tsuji et al.[13].Nevertheless,this method is limited due to relatively high temperature and the bubbling of oxygen molecules is indispensable.Most recently,Saha et al.[14]prepared single crystalline micron⁃sized rectangular silver bar using polyacrylamide(PAM)and silver nitrate(AgNO3)by a hydrothermal process.But this method requires relatively high⁃er temperature(above 107℃)and the PAM aqueous solution needs to be mature for 7 days.And the main products are rect⁃angular silver bars,and this method has a low yield of silver bars according to the SEM images.
Herein,we describe a simple one⁃step method to prepare tan⁃gled silver nanowires as well as the dendritic structures,using PAM as the stabilizer and soft template at room temperature in large quantities.The structures obtained in our system are all in tangled morphology,which is different from those silver nanow⁃ires reported before.Moreover,PAM,being widely used in pe⁃troleum exploitation,water treatment,textile dyeing and chemi⁃cal industry,is cheaper and easy to be purchased.It plays the key role in the formation of wire⁃like structures.The growth mechanism concerning the tangled sliver nanowires are pro⁃posed and detailed investigated.Further,the study on the SERS properties indicates that such anisotropic structures are suitable substrates for probing PATP and probably other analytes.
1 Experimental
1.1 Materials
Acrylamide(AM,99.0%),Polyacrylamide(PAM,≥99.0%, Mw≈5000000)are purchased from Tianjin Kermel Reagent Co.Ascorbic acid(Vc,99.7%)and silver nitrate(AgNO3, 99.8%)are purchased respectively from Shanghai Reagent Co..4⁃aminothiophenol(PATP,97%)is purchased from Alfa Aesar China(Tianjin)Co.,Ltd..All chemicals are used without fur⁃ther purification.The secondary distilled water is used for all solution preparation and experiments.
1.2 Synthesis of silver products
A typical procedure to synthesize the silver tangled nanow⁃ires is as follows:2 mL of 10 mmol·L-1PAM([PAM]denotes concentrations calculated in terms of moles of the repeating unit of PAM,with a molecular weight of 71 g·mol-1,per liter of solution),1 mL of 10 mmol·L-1silver nitrate,1 mL of 10 mmol·L-1ascorbic acid are added to 6 mL of water.The final concentrations of PAM,ascorbic acid,and AgNO3are 2,1,and 1 mmol·L-1,respectively.The solution is stirred for several seconds and aged for 24 h at 25℃without any stirring.All samples are sealed in glass tubes and left at certain temperature for further study.
1.3 Characterization of silver products
TEM observations are performed with a JEM⁃100CX II(JE⁃OL,Japan)electron microscope operated at an accelerating voltage of 100 kV.To prepare TEM samples,the reacted mix⁃tures are dispersed in water under sonication and centrifuged at 5500 r·min-1for 15 min.Then the upper solution containing unreduced ions and unbound molecules is removed.Such ob⁃tained samples are redispersed in water.A little drop of result⁃ing dispersion is put onto a Formvar⁃covered copper grid(230 meshes)and followed by drying naturally in the air at room temperature for TEM measurement.For the UV(HP 8453E UV⁃Vis spectrometer,US)measurements,the suspension obtained above is placed in a 1 cm light path quartz cell,and spectra are recorded at room temperature.Raman measurements are made with a Renishaw System 1000 Raman imaging microscope (Renishaw Plc,U.K.)equipped with 25 mW(632.8 nm)He⁃Ne laser(model 127⁃25RP,Spectra⁃Physics,USA)and a Pelti⁃er⁃cooled CCD detector(Renishaw,576 pixels×384 pix⁃els).A 50×objective(numerical aperture=0.80)mounted on an Olympus BH⁃2 microscope(Japan)is used to focus the laser onto a spot approximately 1 μm in diameter and collect the back⁃scattered light from the sample.To analyze the SERS ac⁃tivities of these samples,40 μL of these concentrated colloids is directly cast on the clean glass slide and let dry in air.Final⁃ly,10 μL of a 24 mmol·L-1ethanol solution of PATP is cast onto the colloid films formed on the glass slide,and allowing the solvent to evaporate.
2 Results and discussion
Fig.1(A-C)presents representative SEM and TEM images of the silver products obtained at a PAM concentration of 2 mmol·L-1,which apparently consists of tangled wire⁃like struc⁃tures as the main products with width ranging from 50 to 100 nm.Fig.1D shows a small amount of structures in the products, with some short wires and some aggregated quasi⁃spherical nanostructures.The quasi⁃spherical nanoparticles connect with the short wires(arrows in Fig.1D)with the tendency of form⁃ing tangled silver nanostructures.And the sizes of the sliver quasi⁃spherical nanostructures range from 50 to 100 nm in di⁃ameter,which is similar to the width of the wires.
To investigate the growing mechanism of tangled silver nanostructures,different products obtained from the same sam⁃ple after certain reaction time are shown in Fig.2.The probable formation mechanism can be described in Fig.3,path A.PAM plays the key role in the formation of such silver nanowires as the capping agent as well as the soft template.In the reaction process,the ascorbic acid acts as the reducing agent.Nucle⁃ation first occurs in aqueous solution and small particles are formed(Fig.2A).Then the amide groups of PAM molecules are adsorbed on the surfaces of silver nanoparticles simultane⁃ously.On the basis of Flory⁃Krigbaum′s theory of dilute solu⁃tion[15],PAM molecules dissolved in the aqueous solution are mainly in the form of the cloud of chain segments(see Fig.3). The nascent nuclei and small nanoparticles would be arranged side by side along the polymer chains due to the presence of numerous amide groups on PAM chain(Fig.2(B-F)).This is helpful to the anisotropic growth of silver particles and then tangled silver nanowires can be formed through particles at⁃taching with each other.This can further be verified by Fig. 1D,where it can be seen that some wires are formed through aggregating of some quasi⁃spherical particles and short wires (denoted by arrows in Fig.1D).Saha et al.[14]demonstrated that when reaction solution containing PAM and AgNO3was heat⁃ed at a temperature of about 237℃,thermal degradation of am⁃ide bonds of acryl amide to carboxylic acids occur,accompa⁃nied by the release of ammonia.Then ammonium ions got re⁃placed by silver ions and reduced the silver ions attached to the PAM to form a silver nanoparticles assembly along the PAM chain.After oriented attachment and Ostwald ripening,single crystalline micron⁃sized rectangular silver bars with smooth sur⁃face were produced.According to Saha,the Ostwald ripening was the key to produce the rectangular smooth silver bars,so the longer reaction time(7 d)was necessary and the yield was relatively lower.In our system,silver tangled nanowires in⁃stead of rectangular silver bars are the main products due to the fast reaction rate and oriented attachment with the shorted reac⁃tion time and higher yield.
According to the mechanism,PAM is the key factor in the synthesis of the tangled silver nanowires.To test this,further experiments are done to study the effects of concentrations of different components and AM(monomer of PAM)as the cap⁃ping agent on the products.
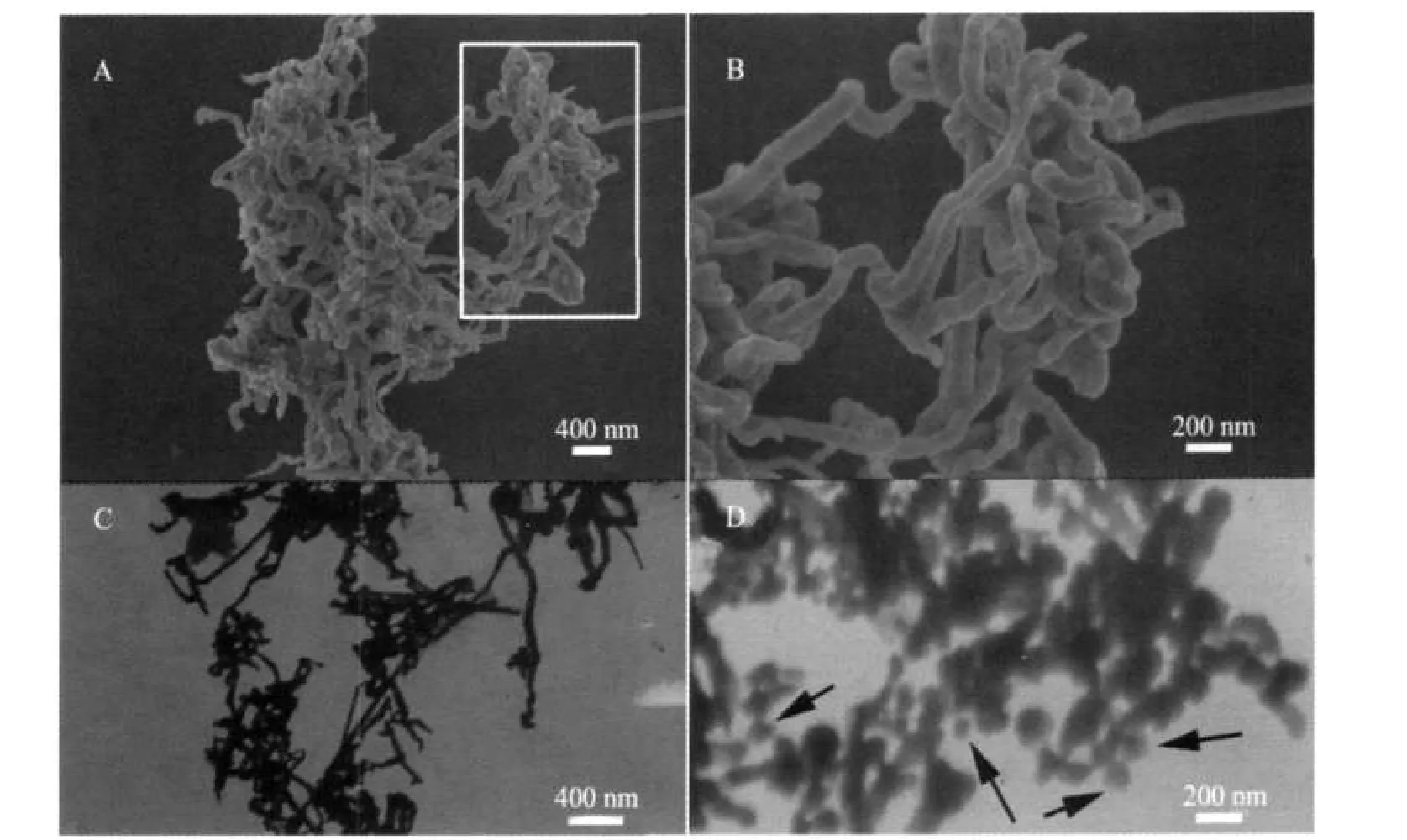
Fig.1 SEM(A,B)and TEM(C)images of silver tangled nanostructures from PAM(2 mmol·L-1)⁃Vc(1 mmol·L-1)⁃AgNO3(1 mmol·L-1)aqueous solution at 25℃B is a magnified image marked by a white rectangle in A.D represents a few products obtained from the same reaction system with the tendency of forming structures like C.
With the concentration of ascorbic acid increasing to 10 mmol·L-1,dendritic silver structures are produced(Fig.4A).A higher reducing agent concentration enhances the reduction rate of silver nitrate and results in the fast formation of more sliver nuclei,and such effect is disadvantageous to the growing of sliver nanostructures.The result tells us that the concentra⁃tion of ascorbic acid has great influence on the silver morpholo⁃gy.A little lower concentration of ascorbic acid is helpful to the anisotropic growth of silver nanostructures.Therefore,it can be concluded that too fast reduction process is unfavorable to silver tangled nanowires.These obtained dendritic silver structures(Fig.4A)further support our mechanism.As shown in Fig.3,path B,small particles,inside of the cloud of chain segments,connect each other rapidly due to the faster growth rate and lead to formation of the core with the diameter range from 800 nm to 2 μm.Wang et al.[16]reported that dendritic sil⁃ver nanostructures were synthesized very easily by dropping a droplet of AgNO3⁃HF solution on silicon wafers without any capping agent and surfactant.They explained the structural evolution by the oriented attachment⁃based aggregation mecha⁃nism,which can also be used to explain the formation of den⁃dritic silver nanostructures in this work(insert in Fig.4A). With prolonging reaction duration,the concentrations of the sil⁃ver salt and reduction agent decrease,the reaction process is dominated by a non⁃equilibrium condition(under kinetic fac⁃tor)due to a high silver ion concentration[16],so silver dendrites (outside the core)are formed.And with increasing reaction time and the consumption of the silver ions,the reaction pro⁃cess was dominated by a quasi⁃equilibrium or equilibrium con⁃dition(thermodynamic factor)[16].The branches(denoted by ar⁃rows in insert Fig.4A)become less and shorter.

Fig.2 TEM images of silver products obtained from the samples of Fig.1 for monitoring the tangled nanowires evolution over timet/min:(A)1,(B)60,(C)180,(D)300,(E)420,(F)540
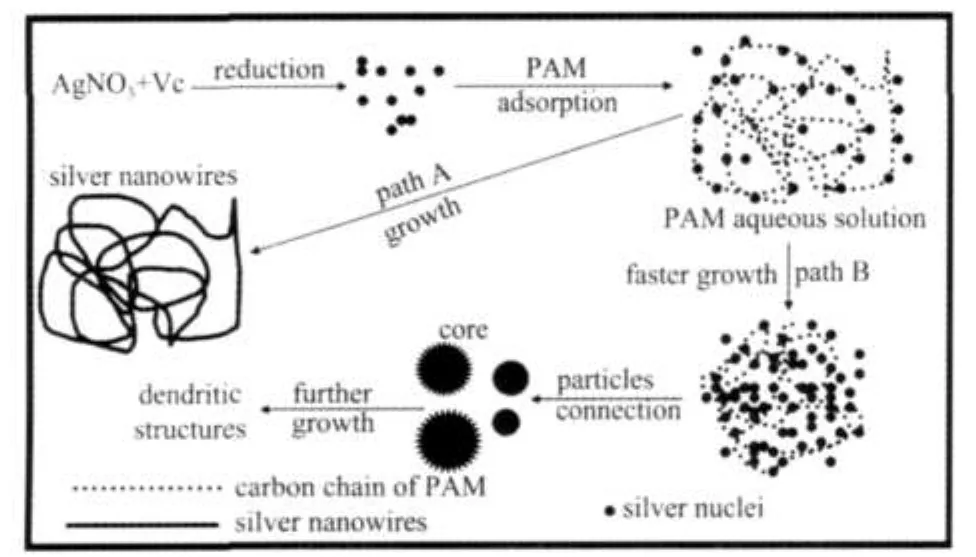
Fig.3 Schematic illustration of formation mechanism of tangled and dendritic silver nanostructures
The growth mechanism of tangled nanowires is further certi⁃fied by the products from the AM⁃assistant method.AM(2 mmol·L-1)is introduced into the reaction system instead of PAM as the capping agent.Compared to the polyacrylamide, amide(the monomer of PAM)without the long carbon chain can not act as the template,so the silver products are dominat⁃ed by branched particles with long acuminate branches of more than 300 nm(Fig.4B).Increasing the ascorbic acid concentra⁃tion to 3 mmol·L-1,flower⁃like particles with short branches (about 50 nm)appear as the main products,as can be seen in Fig.4C.Moreover the number of the short branches on one sil⁃ver particle decreases and the central parts of the structures shrink.As we know,metals like Ag,Au,Pt,Pb,and Pd have a face⁃centered cubic(fcc)structure,which leads to no crystallograph⁃ic driving force for anisotropic growth[17].Indeed,atoms of these metals should assemble to form faceted spheres to mini⁃mize their surface energy[17].Therefore spherical core can be easily formed.Nevertheless the appearance of these branched structures demonstrates that amide group can adsorb on silver surface,which indirectly demonstrates the proposed mecha⁃nism mentioned above.

Fig.4 TEM images of silver nanoproducts from different AgNO3(1 mmol·L-1)reaction systems at 25℃(A)PAM(2 mmol·L-1)-Vc(10 mmol/L);(B)AM(2 mmol·L-1)-Vc(1 mmol· L-1);(C)AM(2 mmol·L-1)-Vc(3 mmol/L);(D)Vc(1 mmol·L-1).The image inserted in A represents the magnified part of A.
Additionally,the effect of PAM concentration on the prod⁃uct is also discussed.When PAM concentrations are changed from 0 to 0.05 mmol·L-1,the products are dendritic silver nanostructures.With PAM concentration increases from 0.1 to 2 mmol·L-1,the quantity of nanowires is also increased and fi⁃nally nanowires are the main product(Fig.1).Products of sys⁃tems with PAM concentration increasing to 8 mmol·L-1,are the same as those obtained from system containing PAM of 2 mmol·L-1.With the higher concentration of PAM,the PAM template dominates and directs the growth to form silver tan⁃gled nanostructures.These results are consistent with the mech⁃anism mentioned above.
As can be seen from Fig.5,a large amount of long straight wires(the maximum length is about 7 μm)accompanied with tangled wires are obtained by stirring the reaction system.The stirring process makes the chains of PAM relatively extend, thus resulting to the formation of straight wires.
Fig.6 shows the absorption spectra of the silver structures presented in Fig.1 and Fig.4A,respectively.It is well known that UV⁃Vis absorption spectra of silver nanostructures depend strongly on their shapes and sizes[18].The main optical response of spherical silver nanoparticles with diameters of 20-40 nm and 40-90 nm often exhibits a single absorption band around 410 and 480 nm attributed to the surface plasma resonance,re⁃spectively[19].While anisotropic metal particles could give rise to two or more surface plasmon resonance(SPR)bands[20].

Fig.5 TEM image of silver nanoproducts from PAM(2 mmol·L-1)⁃Vc(1 mmol·L-1)⁃AgNO3(1 mmol·L-1)aqueous solution under stirring for 24 h at 25℃
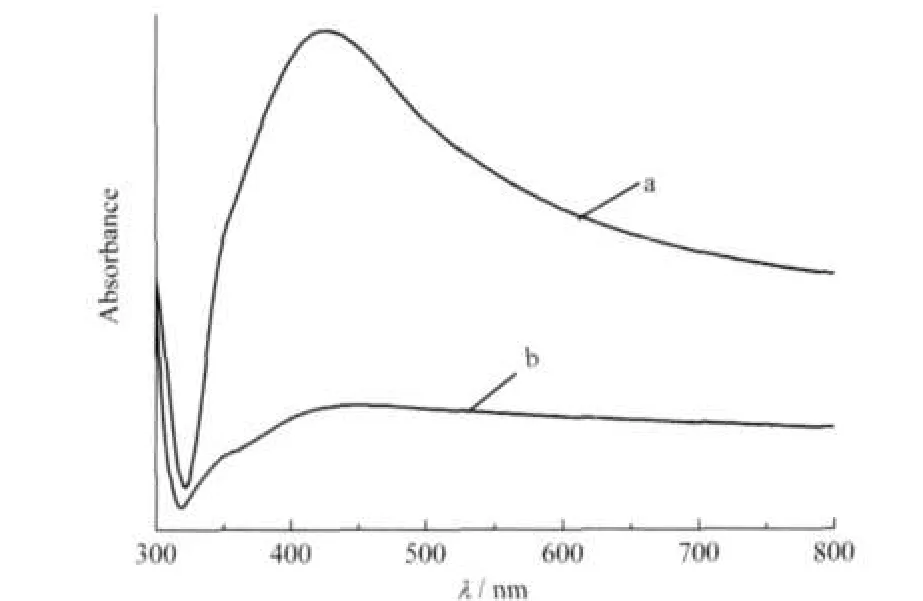
Fig.6 UV⁃Vis absorption spectra of tangled silver nanowires(a)and dendritic nanostructures(b)obtained from different AgNO3(1 mmol·L-1)reaction systems at 25℃(a)PAM(2 mmol·L-1)⁃Vc(1 mmol·L-1); (b)PAM(2 mmol·L-1)⁃Vc(10 mmol·L-1)
The absorption spectrum(Fig.6a)of silver nanowires shows a shoulder peak at around 350 nm and an evident peak centered at 410 nm with a long tail extending to 800 nm.Gao et al.[21]syn⁃thesized uniform silver nanowires with an average length of 6 mm and diameter of 70 nm via PVP⁃assisted(polyvinylpyrrol⁃idone,PVP⁃K30)polyol reduction.They explained this tail band to the overlapping of the in⁃plane quadrupole and dipole resonance modes of nanowires with peaks at 445 and 514 nm, respectively[21].In our work,the peak(located at 410 nm)exhib⁃its a broad full⁃width at half⁃maximum of about 100 nm,which could be attributed to the existence of a broad distribution in size and morphology(as can be seen in Fig.1)for these silver structures.Moreover,the shoulder peak at about 350 nm which is attributed to the transversal modes could be considered as the optical signature of relatively long silver nanowires[22].And it is in good accordance with our TEM images.
The spectrum in Fig.6b displays a broad plasmon band cen⁃tered at about 420 nm for sliver dendritic structures.The peak at 420 nm is attributed to the out⁃of⁃plane dipole resonance,but the expected longitudinal plasmon band does not appear,nei⁃ther.This may be explained by considering that the silver den⁃dritic structures do not adopt a uniform morphology(Fig.4A), which signifcantly can decrease the intensity of the longitudi⁃nal plasmon band,leading to the disappearance of the band[23].
To investigate the SERS sensitivity of the silver nanowires substrates,the Raman spectra of the PATP molecules adsorbed on the surface of silver nanowires as well as silver dendritic structures are measured.All the obtained SERS spectra of PATP are in agreement with those in the literature[24].It should be noted that without silver colloids,no detectable spectrum could be obtained when the same amount of PATP is dropped on the glass slide(Fig.7a).And noticeable changes in the fre⁃quency shift and relative intensity of the bands can be ob⁃served from the SERS spectra on different silver substrates,in⁃dicating that the thiol group in PATP directly contacts with the silver surfaces.The SERS spectra obtained from the silver den⁃dritic structures(Fig.7b)and silver nanowires(Fig.7c)are dom⁃inated with theb2modes(in⁃plane,out of⁃phase modes)locat⁃ed at 1438,1389,1142,1189,and 1003 cm-1.Recent study has shown that the apparently selective enhancement of the non⁃to⁃lally symmetric b2modes could be ascribed to the surface cata⁃lytic reaction of adsorbed PATP molecules to form the aromat⁃ic azo compound[25].Moreover the enhancement of a1vibration⁃al modes(in⁃plane,in⁃phase modes),such as v(C—C)and v(C—S)at 1577 and 1077 cm-1,is also apparent.The apparent enhancement of a1modes in the SERS spectra may imply that the enhancement via an electromagnetic(EM)mechanism is significant.The better enhancement ability of sample is sup⁃posed to be closely related to its unique tangled structure be⁃cause the branches on the particles made the surface of them highly curved[26].In principle,high curvature features on the surface(lightening rod effect)could cause very large enhance⁃ment[24].In contrast with the silver dendritic structures,the SERS intensity of nanowires is stronger.According to Xia et al.[27],high surface areas and many sharp edges could serve as great substrates for SERS detection.From Fig.1A and Fig.4A, the tangled nanowires have larger surfaces areas than the den⁃dritic structures with the same amount of silver atoms,because the dendritic structures have relatively larger cores.Although it is difficult to calculate the enhancement factors from these data because of the complex particle shapes,the strong Raman sig⁃nals enabled by the particles indicate that these tangled silver structures are active SERS substrates.
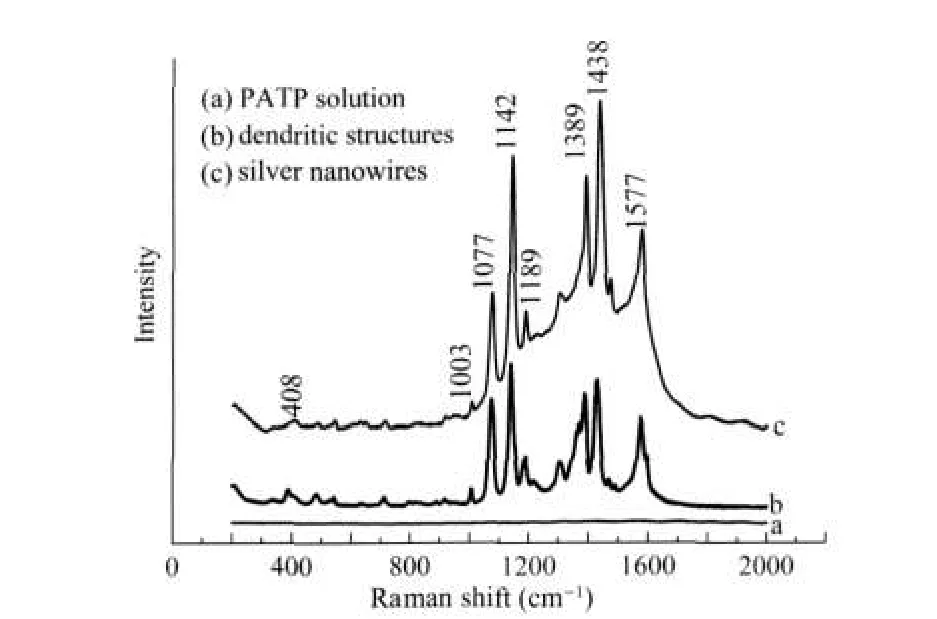
Fig.7 Comparison of normal Raman spectrum and SERS spectra of PATP(a)normal Raman spectrum of solid sample;(b)and(c)are SERS spectra of PATP(0.024 mol·L-1)on the dendritic structures and silver nanowires, respectively.
3 Conclusions
At mild conditions,a large yield of tangled silver nanowires and dendritic structures are synthesized from PAM aqueous solution under different concentrations of ascorbic acid.PAM provides a useful soft template for the growth of tangled silver nanowires.At the initial reaction stage,silver nuclei are formed and adsorbed by PAM with a tangled structure.As the reaction time is prolonged,the nanoparticles contact with each other and grow along the polymer chain,leading to the formation of tangled silver nanowires.When the reducer concentration become higher or small AM molecule is used to replace PAM as the capping agent,it is disadvantageous for silver particles to anisotropically grow along the soft template, so dendritic or branched nanostructures can be obtained. Raman measurements show silver nanowires and dendritic structures are active SERS substrates for probing PATP and probably other analytes.The tangled structures will provide new structural diversity for the applications in biological tagging,optoelectronics,SERS,and catalysis.
1 Yao,H.J.;Liu,J.;Duan,J.L.;Hou,M.D.;Sun,Y.M.;Mo,D.; Chen,Y.F.;Xue,Z.H.Acta Phys.⁃Chim.Sin.,2007,23:489 [姚会军,刘 杰,段敬来,侯明东,孙友梅,莫 丹,陈艳峰,薛智浩.物理化学学报,2007,23:489]
2 Fu,X.F.;Zou,H.M.;Zhou,L.;Zhou,Z.K.;Yu,X.F.;Hao,Z. H.Acta Phys.⁃Chim.Sin.,2008,24:781 [付小锋,邹化民,周 利,周张凯,喻学峰,郝中华.物理化学学报,2008,24:781]
3 Chi,G.J.;Yao,S.W.;Fan,J.;Zhang,W.G.;Wang,H.Z.Acta Phys.⁃Chim.Sin.,2002,18:532 [迟广俊,姚素薇,范 君,张卫国,王宏智.物理化学学报,2002,18:532]
4 Xia,Y.;Yang,P.;Sun,Y.;Wu,Y.;Mayers,B.;Gates,B.;Yin, Y.;Kim,F.;Yan,H.Adv.Mater.,2003,15:353
5 Sarkar,R.;Kumbhakar,P.;Mitra,A.K.;Ganeev,R.A.Curr. Appl.Phys.,2010,10:853
6 Kostowskyj,M.A.;Gilliama,R.J.;Kirkb,D.W.;Thorpe,S.J. Int.J.Hydrog.Energy,2008,33:5773
7 Gao,Y.;Jiang,P.;Liu,D.F.;Yuan,H.J.;Yan,X.Q.;Zhou,Z. P.;Wang,J.X.;Song,L.;Liu,L.F.;Zhou,W.Y.;Wang,G.; Wang,C.Y.;Xie,S.S.Chem.Phys.Lett.,2003,380:146
8 Jiang,Z.Y.;Xie,Z.X.;Zhang,S.H.;Xie,S.Y.;Huang,R.B.; Zheng,L.S.Chem.Phys.Lett.,2003,374:645
9 Chen,C.;Wang,L.;Yu,H.;Jiang,G.;Yang,Q.;Zhou,J.;Xiang W.;Zhang,J.Mater.Chem.Phys.,2008,107:13
10 Kim,T.Y.;Kim,W.J.;Hong,S.H.;Kim,J.E.;Suh,K.S. Angew.Chem.Int.Edit.,2009,48:3806
11 Sanders,A.W.;Routenberg,D.A.;Wiley,B.J.;Xia,Y.; Dufresne,E.R.;Reed,M.A.Nano Lett.,2006,6:1822
12 Kang,T.;Yoon,I.;Jeon,K.S.;Choi,W.;Lee,Y.;Seo,K.;Yoo, Y.;Park,Q.H.;Ihee,H.;Suh,Y.D.;Kim,B.J.Phys.Chem.C, 2009,113:7492
13 Tanga,X.;Tsuji,M.;Jiang,P.;Nishio,M.;Jang,S.M.;Yoon,S. H.Colloids and Surfaces A,2009,338:36
14 Mondal,B.;Majumdar,D.;Saha,S.K.J.Mater.Res.,2010,25: 383
15 Krigbaum,W.R.;Geyme,D.O.J.Am.Chem.Soc.,1959,81: 1859
16 Ye,W.;Shen,C.;Tian,J.;Wang,C.;Hui,C.;Gao,H.Solid State Sci.,2009,11:1088.
17 Chen,J.;Wiley,B.J.;Xia,Y.Langmuir,2007,23:4120
18 Caswell,K.K.;Bender,C.M.;Murphy,C.J.Nano Lett.,2003, 3:667
19 Mdluli,P.S.;Revaprasadu,N.Mater.Lett.,2009,63:447
20 Brennan,M.E.;Whelan,A.M.;Kelly,J.M.;Blau,W.J.Synth. Met.,2005,154:205
21 Gao,Y.;Jiang,P.;Song,L.;Liu,L.;Yan,X.;Zhou,Z.;Liu,D.; Wang,J.;Yuan,H.;Zhang,Z.;Zhao,X.;Dou,X.;Zhou,W.; Wang,G.;Xie,S.J.Phys.D⁃Appl.Phys.,2005,38:1061
22 Sun,Y.;Yin,Y.;Mayers,B.T.;Herricks,T.;Xia,Y.Chem. Mater.,2002,14:4736
23 Zhang,J.;Liu,K.;Dai,Z.;Feng,Y.;Bao,J.;Mo,X.Mater. Chem.Phys.,2006,100:313
24 Zou,X.;Ying,E.;Dong,S.J.Colloid Interface Sci.,2007,306: 307
25 Wu,D.Y.;Liu,X.M.;Huang,Y.F.;Ren,B.;Xu,X.;Tian,Z.Q. J.Phys.Chem.B,2009,113:18212
26 Jana,N.R.;Pal,T.Adv.Mater.,2007,19:1761
27 Wang,Y.;Camargo,P.H.C.;Skrabalak,S.E.;Gu,H.;Xia,Y. Langmuir,2008,24:12042
