低温NH3-SCR催化剂MnOx-CeOx/ACF的SO2中毒机理
2010-12-11沈伯雄
沈伯雄 刘 亭
(南开大学环境科学与工程学院,天津 300071)
低温NH3-SCR催化剂MnOx-CeOx/ACF的SO2中毒机理
沈伯雄*刘 亭
(南开大学环境科学与工程学院,天津 300071)
在二氧化硫(SO2)气氛下,对Fe、Cu和V改性的催化剂MnOx-CeOx/ACF(活性碳纤维)的氨选择性催化还原(NH3-SCR)一氧化氮的低温活性进行研究.实验结果表明:以Cu和V改性催化剂未提高MnOx-CeOx/ACF的低温抗SO2性能;Fe改性MnOx-CeOx/ACF在初始6 h内提高了催化剂的抗SO2性能,但较长时间仍然失活.以N2吸附、X射线光电子能谱(XPS)、傅里叶变换红外(FT-IR)光谱以及热重分析(TGA)等手段对中毒催化剂进行分析.在SO2存在下,催化剂中毒归因于两个方面:一是覆盖于催化剂表面的铵盐类物质,二是SO2与催化剂中的金属氧化物反应生成的金属硫酸盐及亚硫酸盐.在中毒催化剂中硫元素主要以金属硫酸盐及亚硫酸盐的形式存在,其在中毒的MnOx-CeOx/ACF、Fe-MnOx-CeOx/ACF、Cu-MnOx-CeOx/ACF和V-MnOx-CeOx/ACF催化剂中所占比例分别为70.4%、68.9%、86.3%和71.4%(w).进一步揭示了MnOx-CeOx/ACF催化剂在低温SCR条件下的SO2中毒机理.
低温选择性催化还原;MnOx-CeOx/ACF;NO;SO2
Selective catalytic reduction(SCR)of nitrogen oxides(NOx) with ammonia(NH3)has been a well-proven technique to remove NOxin flue gases from stationary sources.The low-temperature SCR process always operates at temperature below 200℃which is the typical temperature of flue gases.The low-temperature SCR unit can be located downstream of the particulate control device and desulfurizer,which results in systems of low energy consumption and retrofitting easily into the existing boiler systems for flue gas cleaning.There is a great interest in the development of low-temperature SCR catalysts.
Manganese-cerium-mixed-oxide catalysts have been reported to be active for low-temperature SCR of NOxwith NH3.Manganese-cerium oxidesprepared by co-precipitation method yielded high NO conversion at 150℃[1-2].Supported manganesecerium oxide catalysts,such as MnOx-CeO2/AC/C[3](AC means activated carbon)and ceria modified MnOx/TiO2[4],have shown high activities for NOxreduction by NH3.Activated carbon fiber (ACF)-supported manganese oxide catalysts were also found to be highly active for NOx-SCR at low temperature[5-6].Marbán et al.[7]carried out a survey of metal oxides(manganese,iron,vanadium,chromium,and nickel oxides)to form active phase on ACF surface and the catalytic activity order was found to be Fe>Mn>V>Cr>Ni.
Flue gases always contain low concentrations of sulfur dioxide (SO2)even after desulfurization.Many researchers concluded that even low concentrations of SO2poisoned the catalysts[8-9].In the presence of SO2,ACF-supported metal oxide catalysts were also deactivated and SO2tolerance of the different metals followed the order:V>Cr>Fe>Mn(at 150℃)[7].Recently,Wu et al.[10]reported that Ce doped Mn/TiO2catalyst exhibited certain SO2resistance in the presence of 0.01%(w)SO2at 150℃,but the catalytic activity also decreased gradually within the reaction time of 6.5 h.Up to now,there were few reports on deactivation mechanism of the catalysts under low-temperature SCR conditions in the presence of SO2.
In this work,the catalytic activity and SO2tolerance of MnOx-CeOx/ACF were investigated under low-temperature SCR conditions.Some transition metals(Fe[5,7,11],V[12-14],and Cu[15-16]),which wereactiveinthelow-temperatureSCRreaction,wereintroduced to modify the catalyst of MnOx-CeOx/ACF.Special attention was focused on the deactivation mechanism of the catalysts based on MnOx-CeOx/ACF by SO2.
1 Experimental
1.1 Materials
HNO3,citricacid(C6H8O7),manganesenitrates(Mn(NO3)2),cerium nitrates(Ce(NO3)3),ferric nitrates(Fe(NO3)3),cupric nitrates (Cu(NO3)2),and ammonium metavanadate(NH4VO3)were of analytical grade and obtained from Tianjin Guangfu Fine Chemical Research Institute,China.Polyacrylonitrile-based ACF was supplied by Jiangsu SuTong Carbon Fiber Co.,China.
1.2 Support treatment
The polyacrylonitrile-based ACF was used as the catalyst support for NOxreduction.The ACF was oxidized with HNO3(30%,w)at 90℃for 2 h at first.After this process,the ACF was washed to be neutral with deionized water and then dried at 105℃for 10 h.The Brunauer-Emmet-Teller(BET)surface areas of ACF before and after HNO3oxidization were 1000 m2·g-1and 550 m2·g-1,respectively.
1.3 Catalyst preparation
The MnOx-CeOx/ACF catalysts with the same loadings of(6%, w)Mn and(6%,w)Ce was synthesized by incipient-wetness impregnation method using Mn(NO3)2and Ce(NO3)3as precursors. The oxidized ACF was impregnated with the aqueous solution of Mn(NO3)2,Ce(NO3)3in citric acid at room temperature for 24 h.The molar ratio of citric acid to metal components(the total moles of manganese and cerium)was 1.0.After the impregnation,the catalysts were dried in a vacuum at 105℃for 3 h followed by calcination in N2stream at 500℃for 3 h.Following the same procedure,the catalyst based on MnOx-CeOx/ACF with the addition of Fe,Cu or V respectively was prepared using corresponding metal precursors,Fe(NO3)3for Fe,Cu(NO3)2for Cu,and NH4VO3for V.In the resulted catalysts M-MnOx-CeOx/ ACF(M=Fe,Cu,or V),the loadings of M element were all 2% (w).
1.4 Catalytic tests
The SCR activities of the catalysts for NOxremoval in the presence of SO2were carried out in a fixed-bed flow reactor. Five gas streams,0.06%NO,0.065%NH3,3.6%O2,0.01%SO2and pure N2in balance were used to simulate the flue gases.In all the runs,the total gas flow rate was maintained at 300 mL· min-1over 1.0 g of the catalysts.The SCR of NO with NH3over all the catalysts in the absence of SO2was stabilized for 1 h at first,and then 0.01%SO2was added into SCR gas conditions. During the measurements,the concentrations of NO at the inlet and outlet of the reactor were monitored by Flue Gas Analyzer (KM900,Kane International ltd.,United Kingdom)equipped with NO and SO2sensors.
1.5 Catalyst characterization
The morphology of the catalysts based on MnOx-CeOx/ACF wasdeterminedon a SS-550 microscope(Shimadzu Corp.,Japan) at 15 keV.
The N2adsorption isotherms were measured at-196℃on an automated gas adsorption system(Tristar 3000,Micrometrics Instrument Corp.,USA).The specific surface area and the adsorption average pore width of each sample were determined through the BET method.The t-plot theory was used to calculate the micropore(<2 nm)surface area and volume.Before the measurements,the samples were dried at 90℃for 2 h and then evacuated at 150℃for 10 h both in a vacuum.
The X-ray photoelectron spectroscopy(XPS)was recorded using a Kratos Axis Ultra DLD spectrometer(Shimadzu Corp., Japan)equipped with a monochromated Al Kαradiation(1486.6 eV)operated at 15 kV and 10 mA.All XPS spectra were recorded using an aperture slot of 300×700 microns with pass energy of 160 and 40 eV for recording survey and high-resolution spectra. The binding energy calibration was checked by the line position of C 1s as an internal reference(284.6 eV).The normal operating pressure in the analysis chamber was controlled to 10-9Pa during the measurement.According to the XPS spectra,the effects of the added transition metal on the oxidation state of elements in the catalysts were obtained.
Fourier transform infrared(FT-IR)spectroscopy was used to analyze the nature of sulfur-containing species formed in the catalysts.The infrared spectra were recorded on a Nicolet Magna-560 infrared spectrometer(Nicolet,USA).The catalysts were pressured into self-supporting wafers and placed in a hightemperature cell with KBr window.A total of 100 co-added scans with a spectral resolution of 4 cm-1were collected over the spectral range 4000-400 cm-1.
Inorderto further identify the sulfur-containing species formed in the catalysts,thermogravimetric analysis(TGA)was carried out using a NETZSCH STA 409 PC Luxx simultaneous analyzer (NETZSCH Instrument Co.,Germany).The samples were heated from room temperature to 1000℃at a heating rate of 5℃·min-1in a dynamic nitrogen atmosphere.The gas flow rate maintained at 40 cm3·min-1.
2 Results and discussion
2.1 Surface morphology of the catalysts
SEM micrographs in Fig.1 show the surface morphologies of the catalysts based on MnOx-CeOx/ACF,and that of ACF after HNO3oxidation and calcination in N2at 500℃for 3 h is also included for comparison.It is observed that the uncovered ACF exhibits smooth surface with some grooves in the axial direction.The supporting of active phases makes the surface of the catalysts rough.For MnOx-CeOx/ACF,the particles of active phase can be noticeably observed on the surface of the catalysts. With the addition of Fe,Cu,or V,much bigger particles of active phases disperse on the surface of the catalysts.Although the active phases deposit on the ACF at such a large scale,the catalysts based on MnOx-CeOx/ACF still retain the same tridimensional shape as that of the uncovered ACF.
2.2 Physical properties of the catalysts
Fig.2 shows the N2adsorption-desorption isotherms for the fresh catalysts based on MnOx-CeOx/ACF.The detailed physical properties of the catalysts are summarized in Table 1.
As shown in Fig.2,the adsorption isotherm of ACF after HNO3oxidization and calcination in N2at 500℃for 3 h is classified as type I according to International Union of Pure and Applied Chemistry(IUPAC)classification.It is evident that most of the pore volume of the materials are filled at the relative pressure below 0.1,which suggests that the materials are highly microporous ones.The t-plot results given in Table 1 indicate that the contribution of micropore surface area to the total surface area of 505.70 m2·g-1is equal to 84.5%.For MnOx-CeOx/ ACF,the sample has a high N2uptake at a low relative pressure and the uptake is remarkably enhanced at the end(p/p0=1.0). The sample exhibits combined adsorption features characteristic to type I and IV behavior,which indicates the presence of both micro and mesoporosity in the catalysts.Table 1 shows that the microporesurfacearea(Smi)accountsfor78.7%ofthetotalsurface area of 285.70 m2·g-1.Compared with the sample of ACF,the decreased values of the surface area value and micropore volume for MnOx-CeOx/ACF can be due to the micropore blocking when manganese and cerium addition into the catalysts.
With the addition of Fe,Cu,or V,the surface area(SBET)and total pore volume(Vt)of the catalysts decrease as in Table 1. Compared with the surface area of 285.70 m2·g-1for MnOx-CeOx/ACF,the surface area decreases to 219.50,197.57,and 191.43 m2·g-1for Fe-MnOx-CeOx/ACF,Cu-MnOx-CeOx/ACF, and V-MnOx-CeOx/ACF,respectively.Fig.2 shows that the adsorption isotherms of the samples with the addition of Fe,Cu or V are close to that of MnOx-CeOx/ACF though a lower adsorptioncapacity over the entire relative pressure range is observed in this case.This can be a consequence of the addition of Fe,Cu or V which increases the pore blocking phenomena.
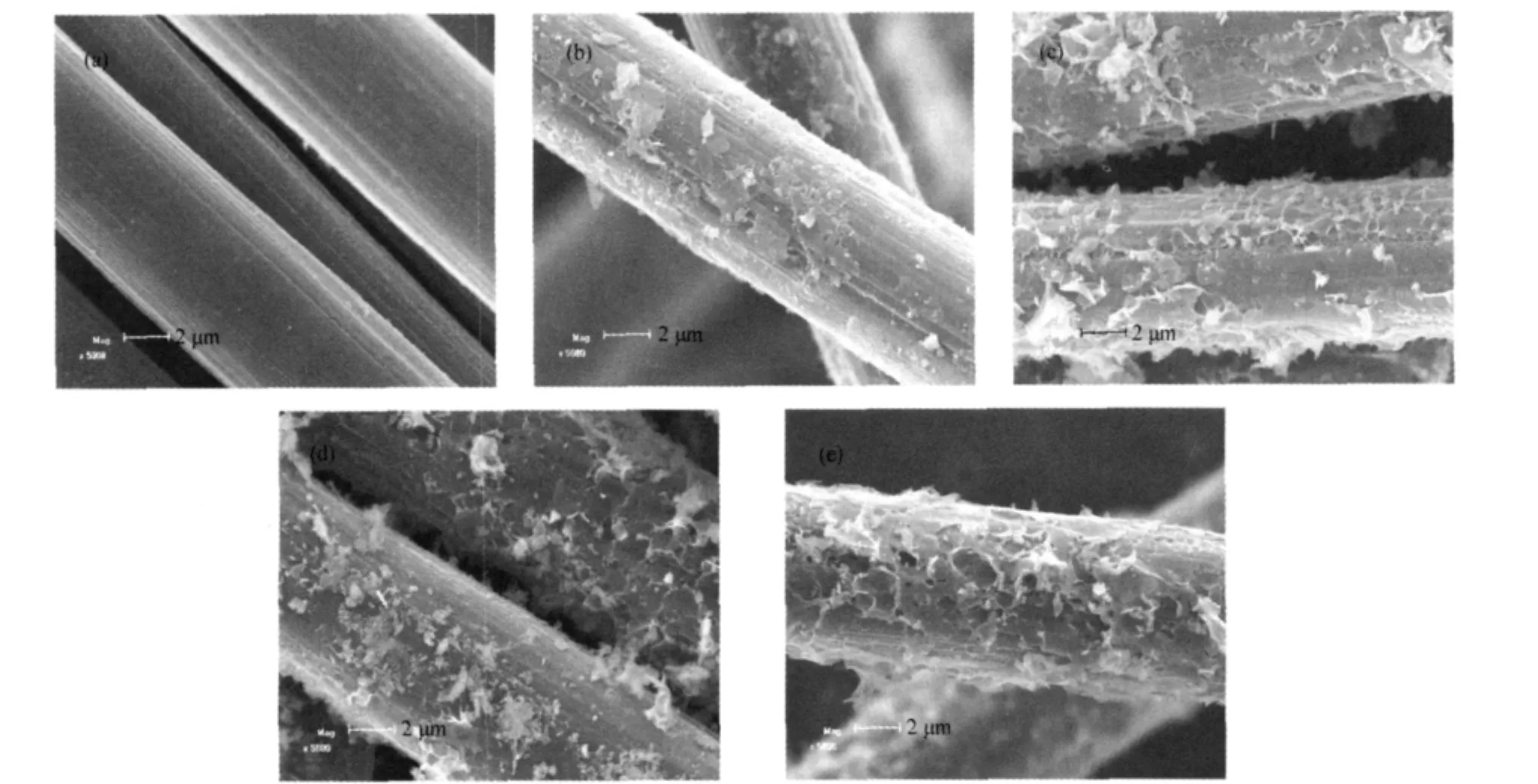
Fig.1 SEM micrographs of various fresh catalysts(a)ACF,(b)MnOx-CeOx/ACF,(c)Fe-MnOx-CeOx/ACF,(d)Cu-MnOx-CeOx/ACF,(e)V-MnOx-CeOx/ACF
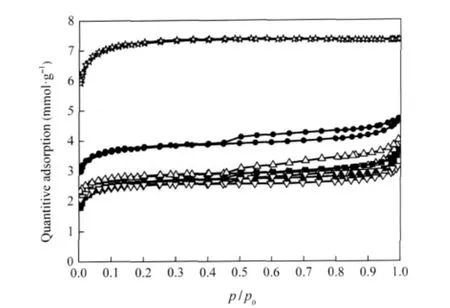
Fig.2 N2adsorption-desorption isotherms of fresh catalysts(★)ACF,(●)MnOx-CeOx/ACF,(△)Fe-MnOx-CeOx/ACF, (■)Cu-MnOx-CeOx/ACF,(▽)V-MnOx-CeOx/ACF
2.3 XPS analysis
XPS was used to investigate the effect of the added Fe,Cu,or V on the oxidation state of Mn and Ce.Mn 2p and Ce 3d XPS spectra for the fresh catalysts are presented in Fig.3.In Mn 2p spectra,the binding energy value of Mn 2p3/2is 641.0 eV for MnOx-CeOx/ACF,indicating that Mn element is mainly in trivalent form(Mn3+)[17].After Fe,Cu,or V was added,the peaks are clearly changed.As shown in Fig.3(a),the peaks of Mn 2p3/2with binding energy between 641.8 and 642.6 eV are found for MMnOx-CeOx/ACF(M=Fe,Cu,or V),which can be attributed to the coexistence of Mn4+and Mn3+species[18].The phenomena that the main peak of Mn 2p3/2shifts to higher binding energies with the addition of Fe,Cu,or V indicate the increase of oxidation state of manganese.Previously reported results also found the chemical shift in Mn 2p line toward higher binding energies in the case of Fe modified catalysts[19].This can be due to the formation of various nonstoichiometric mixed oxides phase between manganese and the added transition metals(Cu and Cr)[20].
Complicated Ce 3d XPS spectra are presented in Fig.3(b). XPS peaks denoted as u‴,u″,u and V‴,V″,V are attributed to Ce4+species while u′and v′are attributed to Ce3+.From these peaks,it can be known that Ce4+and Ce3+are the two main patterns for cerium on the surface of the catalysts.As shown in Fig. 3(b),Ce3+species on the surface of the catalyst seem to increase with the addition of Fe or Cu,but this phenomenon does not appear with the addition of V.

Table 1 Physical properties of the fresh catalysts
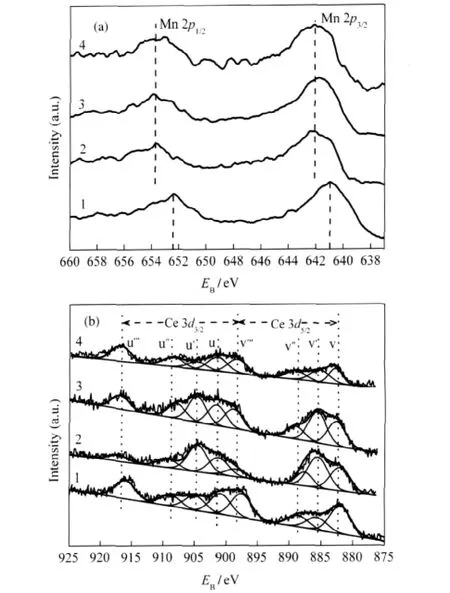
Fig.3 Mn 2p(a)and Ce 3d(b)XPS spectra for various catalysts(1)MnOx-CeOx/ACF,(2)Cu-MnOx-CeOx/ACF,(3)Fe-MnOx-CeOx/ACF, (4)V-MnOx-CeOx/ACF
2.4 Results of catalytic activities
The activities of the catalysts based on MnOx-CeOx/ACF for SCR of NO with NH3were measured in the fixed-bed flue reactor.Fig.4 illustrates the effect of reaction temperature on the NO conversion over various catalysts in the absence of SO2.For all the catalysts,the NO conversion increases with the increasing temperature.MnOx-CeOx/ACF exhibits high NO conversion of nearly 90%at 190℃.For M-MnOx-CeOx/ACF(M=Fe,Cu,or V), the results indicate that the addition of Fe,Cu,or V into thecatalysts does not change the activities of the catalysts significantly.

Fig.4 Effect of reaction temperature on the NO conversion in the absence of SO2(a)MnOx-CeOx/ACF,(b)Fe-MnOx-CeOx/ACF,(c)Cu-MnOx-CeOx/ACF, (d)V-MnOx-CeOx/ACF
Fig.5 shows the effect of SO2on NO conversion over the catalysts based on MnOx-CeOx/ACF at 150℃.The SCR performance of the catalysts in the presence of SO2was carried out as follows:The SCR of NO with NH3over all the catalysts in the absence of SO2was stabilized for 1 h at first,and then 0.01% SO2was added into SCR gas conditions till 11 h.From 11 to 13 h,SO2was out and the catalysts were under SCR conditions in the absence of SO2.From 13 to 16 h,the pure N2were turned on instead of the reaction gases,and the catalysts were heated in N2at 350℃.From 16 to 19 h,the activities of the catalysts after above thermal treatment were carried out under SCR conditions in the absence of SO2.
As shown in Fig.5,the NO conversion for MnOx-CeOx/ACF decreases sharply upon 0.01%SO2addition into the feed gases. Cu-MnOx-CeOx/ACF and V-MnOx-CeOx/ACF are also found to be deactivated seriously by SO2.For Fe-MnOx-CeOx/ACF,the NO conversion remains at nearly 75%in the first 6 h in the presence of SO2but then decreases as other catalysts.For all the catalysts,the NO conversion decreases to approximately 20%in the presence of SO2within 11 h.After cutting off the SO2supply,the original SCR activities of all above catalysts are not restored to any extent in 2 h,showing that the deactivation occurs for all catalysts in the presence of SO2.Ce doped Mn/TiO2[10]and Fe modified MnOx-CeO2catalyst[21]exhibited SO2tolerance in a short time under low-temperature SCR conditions at 150℃. However,Fe-MnOx-CeOx/ACF does not exhibit well SO2tolerance in a long time.
Ammonium salts,such as ammonium sulfates and sulfites,can form under low-temperature SCR conditions with NH3as reductant in the presence of SO2.These ammonium salts can deposit on the catalyst surface and deactivate the catalysts significantly when the reaction temperature is below 300℃.These ammonium salts have been reported to decompose at temperature below 350℃[22].As shown in Fig.5,the NO conversion for all deactivated catalysts after thermal treatment is partially restored from 16 to 19 h,showing that ammonium salts formed in the SCR reaction make some contributions to the deactivation of the catalysts.However,there must be some reasons for the unrecovered activity of the catalysts.The catalyst deactivation phenomena are discussed in the followings.
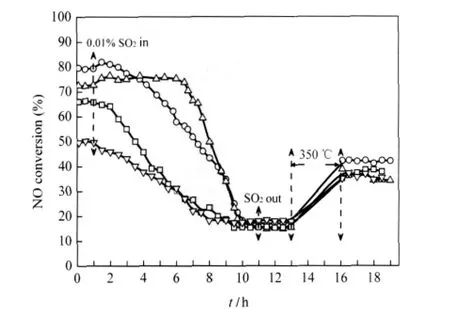
Fig.5 Effect of SO2on NO conversion over various catalystsT=150℃;(○)MnOx-CeOx/ACF,(△)Fe-MnOx-CeOx/ACF, (□)Cu-MnOx-CeOx/ACF,(▽)V-MnOx-CeOx/ACF
The physical properties of the deactivated catalysts are shown in Table 2.The catalysts with superscript“S”indicate the deactivated catalysts before the thermal treatment in N2at 350℃for 3 h,i.e.,MnOx-CeOx/ACFS.The catalysts with superscript“T”denote the above deactivated catalysts after thermal treatment in N2at 350℃ for 3 h.For example,MnOx-CeOx/ACFTcorresponds to MnOx-CeOx/ACFSafter the thermal treatment in N2at 350℃for 3 h.
Compared with the physical properties of the fresh catalysts as shown in Table 1,the surface area and pore volume of corresponding deactivated catalysts before thermal treatment are reduced apparently as shown in Table 2.Such catalysts have been under low-temperature SCR conditions in the presence of SO2for 10 h as shown in Fig.5,so the decrease of surface area and pore volume can be attributed to the deposition of ammonium salts on the surface of the catalysts.Huang et al.[23]also indicated that the ammonium salts formed in the NO reduction with NH3decreased the catalyst surface area and blocked the pore volume.Compared with the fresh catalysts,the decreased values(Δ)of surface area value for deactivated catalysts are in the order of MnOx-CeOx/ACFS(Δ=268.8 m2·g-1)>Fe-MnOx-CeOx/ACFS(Δ=206.28 m2·g-1)>Cu-MnOx-CeOx/ACFS(Δ=193.96 m2·g-1)>V-MnOx-CeOx/ACFS(Δ=188.26 m2·g-1)and pore volume in the same order of MnOx-CeOx/ACFS>Fe-MnOx-CeOx/ACFS>Cu-MnOx-CeOx/ACFS>V-MnOx-CeOx/ACFS.
It is found from Table 2 that there is obvious difference in physical characteristics for the deactivated catalysts before and after thermal treatment.The surface area and pore volume of the deactivated catalysts increase obviously after thermal treatment. For MnOx-CeOx/ACFT,the surface area increases from 16.9 to 285 m2·g-1and the pore volume increases from 0.050 to 0.15 cm3· g-1compared with MnOx-CeOx/ACFSafter thermal treatment.This change in physical properties can be due to the thermal decomposition of ammonium salts formed under low-temperature SCR conditions in the presence of SO2.
It is interesting to note that the pore structures of the fresh catalysts mainly compose of micropores as shown in Table 1.Asshown in Table 2,the micropores of the corresponding deactivated catalysts disappear before thermal treatment but dominate again after thermal treatment.It can be concluded that the deposition of ammonium salts prefers to micropores.
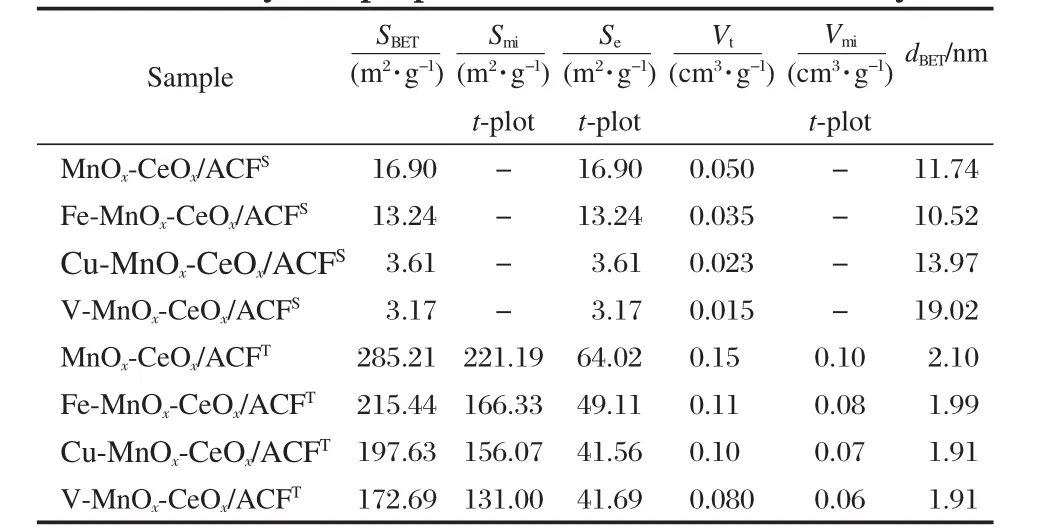
Table 2 Physical properties of the deactivated catalysts
The N2adsorption-desorption isothermals and pore size distribution of the deactivated catalysts before and after thermal treatment are shown in Fig.6.For the deactivated catalysts before thermal treatment,the shapes of the isothermals indicate that there are few pores in the catalysts,which results in a low adsorption capacity.Especially at the relative pressure below 0.2, the pore volume is hardly filled.The deactivated catalysts after thermal treatment exhibit isothermals with high nitrogen uptakes atalowrelativepressure(p/p0)and a remarkably enhanced uptake of nitrogen at the end(p/p0=1.0).As shown in Fig.2 and Fig.6(a), the isothermals of the deactivated catalysts after thermal treatment show features similar to those of the fresh catalysts.The pore size distribution of these catalysts is showed in Fig.6(b). For all deactivated catalysts before thermal treatment,the deposition of ammonium salts blocks all the micropores and most mesopores.While for all the deactivated catalysts after thermal treatment,the obvious pore structure over the entire pore width range recovers and the physical properties,such as surface area (SBET),total volume(Vt)and average pore diameter(dBET),are close to those of corresponding fresh catalysts as shown in Table 1, which indicates that the ammonium salts blocking the pores of the catalysts decompose completely after the thermal treatment in nitrogen at 350℃for 3 h.
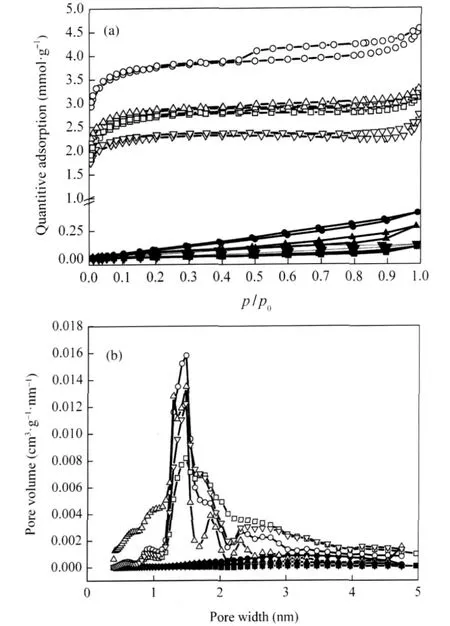
Fig.6 N2adsorption-desorption isotherms(a)and DFT differential pore size distribution of various catalysts(b)(●)MnOx-CeOx/ACFS,(○)MnOx-CeOx/ACFT,(▲)Fe-MnOx-CeOx/ACFS, (△)Fe-MnOx-CeOx/ACFT,(■)Cu-MnOx-CeOx/ACFS,(□)Cu-MnOx-CeOx/ACFT, (▼)V-MnOx-CeOx/ACFS,(▽)V-MnOx-CeOx/ACFT
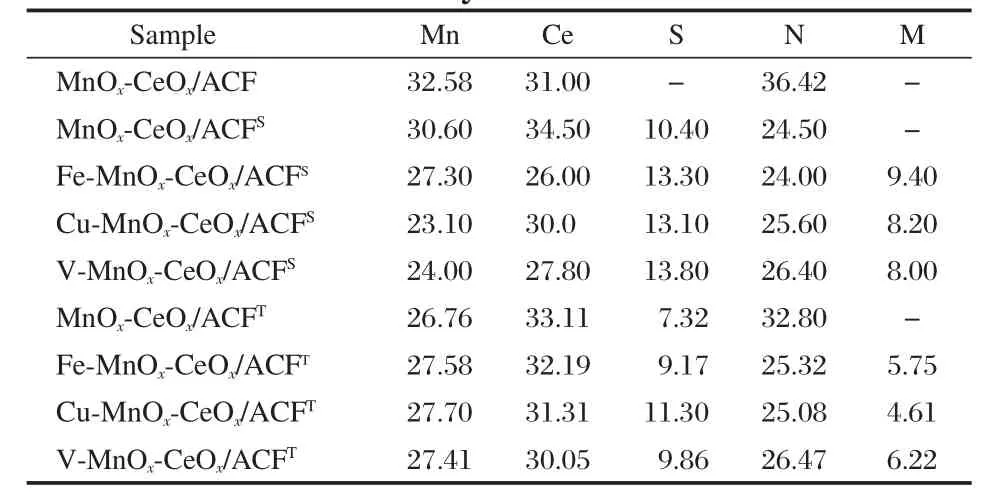
Table 3 Atomic fraction(%)of the catalysts determined by XPS
In order to further investigate the catalyst deactivation by SO2, XPS was also used to analyze the sulfur element existed in the deactivated catalysts before and after thermal treatment.The sulfur concentrations in the catalysts are summarized in Table 3. The fresh catalyst of MnOx-CeOx/ACF,used as reference material, is analyzed and no sulfur element is detected.For MnOx-CeOx/ ACFS,the catalysts have been under SCR conditions including 0.01%SO2for 10 h and the sulfur contents in the catalysts are as high as 10.4%.Although the thermal treatment makes the ammonium salts decompose completely as shown in Table 2,corresponding MnOx-CeOx/ACFTstill has a sulfur content of 7.32%. M-MnOx-CeOx/ACFT(M=Fe,Cu,or V)also has a high sulfur content after the thermal treatment,i.e.,9.17%in Fe-MnOx-CeOx/ACFT,11.30%in Cu-MnOx-CeOx/ACFT,and 9.86%in VMnOx-CeOx/ACFT.Therefore,the much higher sulfur contents in deactivated catalysts after thermal treatment than that in MnOx-CeOx/ACF indicate that the active phase of the catalysts can be sulfated by SO2under the low-temperature SCR conditions.In addition,Fe-MnOx-CeOx/ACFT,Cu-MnOx-CeOx/ACFT,and VMnOx-CeOx/ACFTall have higher sulfur contents than MnOx-CeOx/ACFT.
Fig.7 shows the XPS spectra of S 2p for the deactivated catalysts after thermal treatment.The single peak of S 2p is found to be located at 167.5 eV for MnOx-CeOx/ACFT.This adsorption peak around 167.5 eV indicates the coexistence of S4+and S6+on the surface of MnOx-CeOx/ACFT[24].The S 2p peaks for M-MnOx-CeOx/ACFT(M=Fe,Cu,or V)shift to higher binding energies, nearly 168.9 eV,which indicates much more concentration of S6+in M-MnOx-CeOx/ACFT.As shown in Fig.3(a),the oxidation state of manganese increases with the addition of Fe,Cu,or V. Mn3+and Mn4+coexist in M-MnOx-CeOx/ACF(M=Fe,Cu,or V). The higher the oxidation state of manganese is,the more easily S6+might form during low-temperature SCR process.So much more S4+is found for MnOx-CeOx/ACFTand much more S6+is found for M-MnOx-CeOx/ACFT(M=Fe,Cu,or V).
FT-IR spectra of the deactivated catalysts after thermal treatment are shown in Fig.8.The spectra of MnOx-CeOx/ACFT exhibit adsorption peaks corresponding to the stretching vibration of Mn—O[25](605 cm-1)and C—O(1392 cm-1)which indicate the oxygenated groups on the surface of the catalysts.The adsorption peak centered at 837 cm-1is assigned to the stretching vibration of S—O.M-MnOx-CeOx/ACFT(M=Fe,Cu,or V)shows almost the same spectra of MnOx-CeOx/ACFT.The adsorption peak centered at 500 cm-1is assigned to outplane blending vibration of SO2-3which indicates sulfites in the deactivated catalysts after thermal treatment.The characteristic adsorptions in the frequency range 1000-1420 cm-1have been reported to be the infrared active normal mode of SO2-4groups in the catalysts.The band centered at 1115 cm-1can be assigned to manganese sulfates and the broad band in 1000-1200 cm-1have also been reported to correspond to Ce(SO4)2formation[26].It is verified that the reactions between metals and SO2take place under low-temperature SCR conditions.
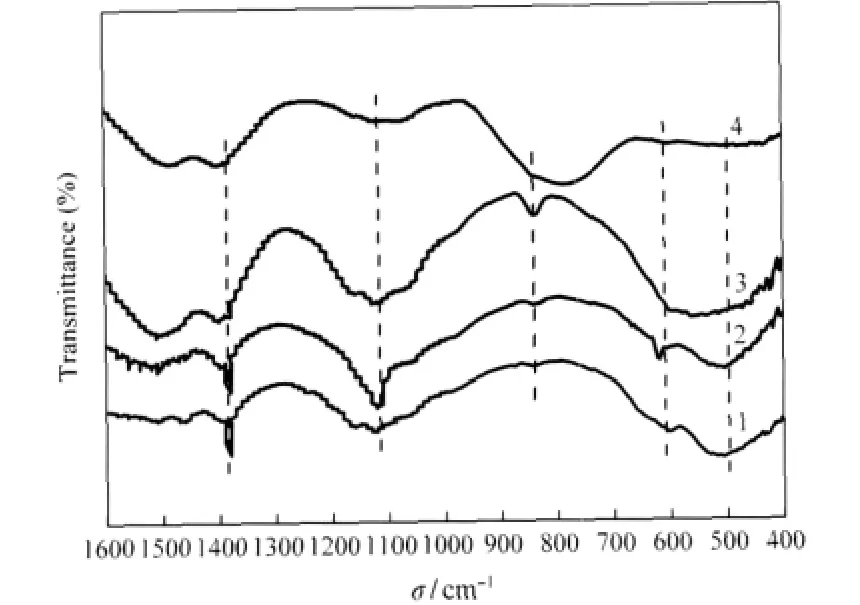
Fig.8 FT-IR spectra of various catalysts(1)MnOx-CeOx/ACFT,(2)Cu-MnOx-CeOx/ACFT,(3)Fe-MnOx-CeOx/ACFT, (4)V-MnOx-CeOx/ACFT

Fig.9 DTG curves of the thermal decomposition for various catalysts in N2(●)MnOx-CeOx/ACF,(○)MnOx-CeOx/ACFT,(△)Fe-MnOx-CeOx/ACFT, (□)Cu-MnOx-CeOx/ACFT,(▽)V-MnOx-CeOx/ACFT
The thermal behavior of MnOx-CeOx/ACF and the deactivated catalysts after thermal treatment was evaluated(Fig.9).The first mass loss in the temperature range 50-300℃is matched to the dehydration of the catalysts.Zhu et al.[27]studied the decomposition of ammonium bisulfate depositing on the activated carbon and indicated that the decomposition temperature of ammonium bisulfate on the activated carbon started at 250℃and peaked at 325℃.As shown in Fig.9,M-MnOx-CeOx/ACFT(M=Fe,Cu,or V)shows DTG curves(the rate of mass loss plotted against temperature)similar to that of MnOx-CeOx/ACF in the temperature range 200-400℃.It means that there are no ammonium salts in the catalysts after the thermal treatment,which is consistent with the results from N2adsorption analysis.Compared with MnOx-CeOx/ACF,the thermal decomposition of M-MnOx-CeOx/ACFT(M=Fe,Cu,or V)takes place in two stages in 400-1000℃,as predicted by DTG curves.The mass loss in 400-1000℃can be attributed to the decomposition of metallic sulfites and sulfates, whicharedescribed as follows:MSO3→MOx+SO2,MSO4→MOx+ SO3(M=Fe,Cu,V,Mn,or Ce).The first stage starts from 400-650℃with maximum rate at 550℃,which corresponds to the decom-position of metallic sulfites and some metallic sulfates, such as Fe2(SO4)3(about 480℃),CuSO4(about 600℃).The second stage degradation temperature is in the range of 650-1000℃,which can be due to the decomposition of metallic sulfates.MnSO4[9]and Ce(SO4)2[28]have been reported to decompose at 750℃and 840℃,respectively.Moreover,in two stages of 400-650℃and 650-1000℃,the mass loss of MnOx-CeOx/ ACFTis 5.85%and 11.4%,respectively which is less than that of M-MnOx-CeOx/ACFT(M=Fe,Cu,or V).It means that the added Fe,Cu,or V can be sulfated by SO2to form metallic sulfites and sulfates in the SCR reaction and the thermal degradation of such sulfated metals increases the mass loss of M-MnOx-CeOx/ACFT. Therefore,it can be concluded that the active components of the catalysts are sulfated by SO2in low-temperature SCR process and corresponding metallic sulfates and sulfites are formed.
According to the analysis from Table 3 and Fig.9,the sulfur contents in metallic salts and ammonia salts on the surface of deactivated catalysts are demonstrated in Table 4.It is clear that the formation of metallic sulfates and sulfites,and ammoniumsalts are both reasons for the deactivation of the catalysts.Much sulfur on the surface of the deactivated catalysts is in the form of metallic sulfate and sulfite,and metallic sulfates seem to be more than metallic sulfites.About 70.4%of sulfur element on the surface of the deactivated MnOx-CeOx/ACF is in the form of metallic sulfates and sulfites,and 68.9%on the deactivated Fe-MnOx-CeOx/ACF,86.3%on Cu-MnOx-CeOx/ACF,71.4%on VMnOx-CeOx/ACF.
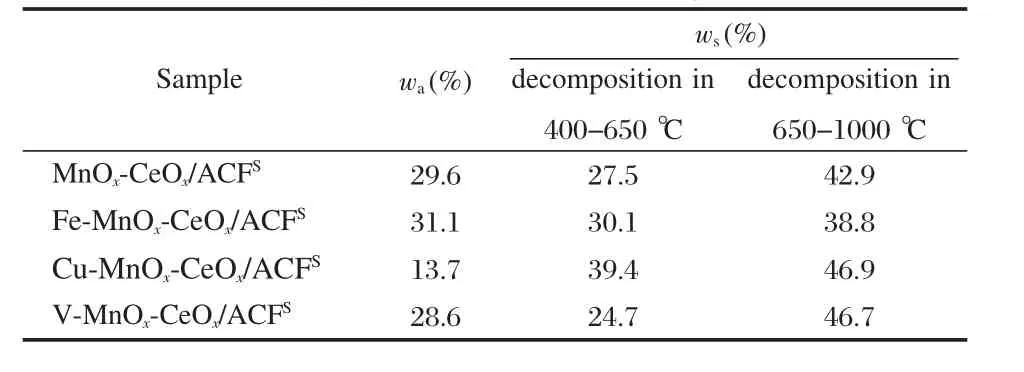
Table 4 Sulfur contents(ws)from metallic and ammonia salts(wa)in deactivated catalysts
3 Conclusions
The modification by Fe,Cu,or V increased the oxidation state of manganese in MnOx-CeOx/ACF but decreased the surface area and pore volume.The catalytic results indicated that the addition of Fe,Cu,or V into the catalysts did not change the activities of the catalysts significantly.Under low-temperature SCR conditions,low concentrations of SO2in the feed gases deactivated MnOx-CeOx/ACF catalyst seriously.According to the results from BET,XPS,FT-IR,and TGA,it could be found that the catalyst deactivation in the presence of SO2was twofold: one was the ammonium salts covering on the surfaces of the catalysts and the other was metallic sulfates and sulfites formed by the reaction between SO2and metal oxides.With the addition of Fe,Cu,or V,it seemed that more metallic sulfates and sulfites formed.The sulfur element on the surface of the deactivated catalysts was mainly in the form of metallic sulfates and sulfites, with 70.4%on deactivated MnOx-CeOx/ACF,68.9%on deactivated Fe-MnOx-CeOx/ACF,86.3%on Cu-MnOx-CeOx/ACF, and 71.4%on V-MnOx-CeOx/ACF.Fe addition into MnOx-CeOx/ ACF enhanced the catalyst resistance to SO2positioning to some extent.
1 Qi,G.S.;Yang,R.T.J.Catal.,2003,217:434
2 Casapu,M.;Kröcher,O.;Elsener,M.Appl.Catal.B,2009,88: 413
3 Tang,X.L.;Hao,J.M.;Yi,H.H.;Li,J.H.Catal.Today,2007, 126:406
4 Wu,Z.B.;Jin,R.B.;Liu,Y.;Wang,H.Q.Catal.Commun.,2008, 9:2217
5 Yoshikawa,M.;Yasutake,A.;Mochida,I.Appl.Catal.A,1998, 173:239
6 Marbán,G.;Fuertes,A.B.Appl.Catal.B,2001,34:55
7 Marbán,G.;Antuña,R.;Fuertes,A.B.Appl.Catal.B,2003,41, 323
8 Kijlstra,W.S.;Biervliet,M.;Poels,E.K.;Bliek,A.Appl.Catal.B, 1998,16:327
9 Park,T.S.;Jeong,S.K.;Hong,S.H.;Hong,S.C.Ind.Eng.Chem. Res.,2001,40:4491
10 Wu,Z.B.;Jin,R.B.;Wang,H.Q.;Liu,Y.Catal.Commun.,2009, 10:935
11 Marbán,G.;Fuertes,A.B.Catal.Lett.,2002,84:13
12 Zhu,Z.P.;Liu,Z.Y.;Liu,S.J.;Niu,H.X.Appl.Catal.B,1999, 23:L229
13 Valdés-Solís,T.;Marbán,G.;Fuertes,A.B.Appl.Catal.B,2003, 46:261
14 Huang,B.C.;Huang,R.;Jin,D.J.;Ye,D.Q.Catal.Today,2007, 126:279
15 Hsu,L.Y.;Teng,H.Appl.Catal.B,2001,35:21
16 Peña,D.A.;Uphade,B.S.;Reddy,E.P.;Smirniotis,P.G.J.Phys. Chem.B,2004,108:9927
17 Kapteijn,F.;van Vanlangeveld,A.D.;Moulijn,J.A.;Andreini,A.; Vuurman,M.A.;Turek,A.M.;Jehng,J.M.;Wachs,I.E.J.Catal., 1994,150:94
18 Chang,L.H.;Sasirekha,N.;Chen,Y.W.;Wang,W.J.Ind.Eng. Chem.Res.,2006,45:4927
19 Wu,Z.B.;Jiang,B.Q.;Liu,Y.Appl.Catal.B,2008,79:347
20 Sreekanth,P.M.;Peña,D.A.;Smirniotis,P.G.Ind.Eng.Chem. Res.,2006,45:6444
21 Qi,G.S.;Yang,R.T.;Chang,R.Appl.Catal.B,2004,51:93
22 Nam,I.S.;Eldridge,J.W.;Kittrell,J.R.Ind.Eng.Chem.Prod. Res.Dev.,1986,25:192
23 Huang,Z.G.;Zhu,Z.P.;Liu,Z.Y.Appl.Catal.B,2002,39:361
24 Román,E.;de Segovia,J.L.;Martín-Gago,J.A.;Comtet,G.; Hellner,L.Vacuum,1997,48:597
25 Alonso,L.;Palacios,J.M.Energy Fuels,2002,16:1550
26 Peralta,M.A.;Milt,V.G.;Cornaglia,L.M.;Querini,C.A. J.Catal.,2006,242:118
27 Zhu,Z.P.;Niu,H.X.;Liu,Z.Y.;Liu,S.J.J.Catal.,2000,195: 268
28 Casari,B.M.;Langer,V.J.Solid State Chem.,2007,180:1616
April 12,2010;Revised:August 2,2010;Published on Web:September 28,2010.
Deactivation of MnOx-CeOx/ACF Catalysts for Low-Temperature NH3-SCR in the Presence of SO2
SHEN Bo-Xiong*LIU Ting
(College of Environmental Science and Engineering,Nankai University,Tianjin 300071,P.R.China)
The low-temperature selective catalytic reduction of NO with NH3(NH3-SCR)over MnOx-CeOx/ACF (activated carbon fiber)modified by Fe,Cu,or V was investigated in the presence of SO2.The results revealed that the SO2tolerance of MnOx-CeOx/ACF was not enhanced after Cu and V modification.The addition of Fe to MnOx-CeOx/ ACF enhanced its resistance to SO2poisoning over the first 6 h in the presence of SO2,but then deactivated quickly. The samples were analyzed by N2adsorption,X-ray photoelectron spectroscopy(XPS),Fourier transform infrared(FTIR)spectroscopy,and thermogravimetric analysis(TGA).We found that catalyst surface coverage by ammonium salts as wellasmetallicsulfatesandsulfitesformedbythereactionbetweenSO2andmetaloxides in the catalysts was responsible for catalyst deactivation in the presence of SO2.Sulfur on the surface of the deactivated catalysts was mainly in the form of metallic sulfates and sulfites with 70.4%,68.9%,86.3%,and 71.4%(w)on deactivated MnOx-CeOx/ACF,Fe-MnOx-CeOx/ACF,Cu-MnOx-CeOx/ACF,and V-MnOx-CeOx/ACF,respectively.A valuable contribution towards understanding the deactivation of catalysts based on MnOx-CeOx/ACF for the SCR in the presence of SO2was made.
Low-temperature selective catalytic reduction;MnOx-CeOx/ACF;NO;SO2
*Corresponding author.Email:shenbx@nankai.edu.cn;Tel:+86-22-23503219
The project was supported by the National Natural Science Foundation of China(0976050),Program for New Century Excellent Talents in University, China(07-0457)and National Key Technology Research and Development Program of Tianjin,China(09ZCKFSH01900).
国家自然科学基金(50976050),新世纪优秀人才支持计划(07-0457)和天津市科技支撑计划项目(09ZCKFSH01900)资助
O643
