六元瓜环和Keggin型杂多酸自组装及催化合成乙酸乙酯
2023-09-15曾俐玲李晓迪方兰王沙张朝张云黔
曾俐玲 李晓迪 方兰 王沙 张朝 张云黔
Synthesis of Ethyl Acetate Catalyzed by Self-assembled Hybrids Derived from Cucurbit[6]uril and Keggin-type Heteropolyacids
ZENG Liling, LI Xiaodi, FANG Lan, WANG Sha, ZHANG Chao, ZHANG Yunqian*
(College of Chemistry and Chemical Engineering, Guizhou University, Guiyang 550025, China)
Abstract:
Heteropolyacids (HPAs) are a class of multifunctional material with excellent catalytic properties in esterification reaction. Because of their good solubility, HPAs are difficult to separate from the product and hard to recycle and reuse when used as a homogeneous catalyst. In this paper, a series of insoluble Q[6]-HPAs catalysts are prepared by self-assembly of cucurbit[6]uril (Q[6]) and Keggin-type heteropolyacids, which have the characteristics of stable properties, large specific surface area, high recovery rate and recyclability. The heterogeneous Q[6]-HPAs catalysts exhibit high catalytic activity and recycling efficiency in the synthesis of ethyl acetate (EA), which have the potential application prospects.
Key words:
cucurbit[6]uril (Q[6]); Keggin-type heteropolyacids; supramolecular self-assembly; catalysts; ethyl acetate(EA)
CLC number:O641.3
Document code:A
Heteropolyacids (HPAs)[1]are a class of polymetallic oxyacids that comprise heteroatoms (such as P, Si, Ge etc.) and transition metal atoms (such as Mo, W, V etc.) and adopt certain structures based on oxygen coordination. HPAs with the Keggin-type structure are widely used as acid catalysts due to their novel structure and very strong Br?nsted acidity. Silicotungstic acid (H4SiW12O40, STA), phosphotungstic acid (H3PW12O40, PTA), and phosphomolybdic acid (H3PMo12O40, PMA) are readily available and most frequently used as acid catalysts[2]. The total acidity of these HPAs decrease in the order PTA>STA>PMA[3]. However, the main disadvantages of HPAs as catalysts are their relatively low surface areas (1-10 m2/g) and the difficulty in recovery from the reaction mixture[4-5]. Combining HPAs with a suitable carrier is expected to circumvent the abovementioned problems. As a result, many traditional HPAs have been immobilized on SiO2, Al2O3, TiO2, active carbon, anion-exchange resin[6-7], clay[8], ZrO[9]2and Nb2O[10]5.
Cucurbit[n]urils[11](Q[n]s) are a family of molecular container hosts possessing a rigid hydrophobic cavity and two identical carbonyl-fringed portals. They have attracted much attention in supramolecular chemistry because of their superior molecular recognition properties in aqueous media[12-16]. In particular, the electrostatically positive outer surface of Q[n]s can provide a driving force, the so-called the outer-surface interaction of Q[n]s, which is capable of generating numerous novel Q[n]s-based supramolecular assemblies[17-18]. K?gerler and co-workers investigated interactions of the [H2O@V18O42]12-anion with Q[6]and Q[8] molecules, respectively, and characterized two novel hybrid porous complexes[19]. Cao and colleagues prepared a series of Q[n]s-HPAs self-assembly hybrids, and investigated their structural characteristics, modes of interaction and functional properties[20-22]. These results indicate that the driving force of Q[n]s and HPAs self-assembly is also derived from the outer surface interaction of Q[n]s.Ethyl acetate (EA) is commercially produced by the esterification of acetic acid with ethanol catalyzed by mineral acids. Mineral acids, such as sulfuric and phosphoric acids, are conventionally used as catalysts in industrial esterification reactions. Although these mineral acid catalysts have many advantages, such as high catalytic activity and selectivity, they are corrosive, toxic, and difficult to separate from reaction solutions[23-24]. In our previous work, cucurbit[6]uril-silicotungstic acid (Q[6]-STA) and tetramethyl cucurbit[6]uril-phosphomolybdic acid (TMeQ[6]-PMA) catalysts showed good catalytic activity in esterification reactions[25-26]. These results motivated us to further investigate Q[6]-HPAs catalysts for esterification of acetic acid with ethanol. In the present work, a series of Q[6]-HPAs self-assembled hybrids have been prepared and examined as catalysts for the synthesis of EA.
1 Experimental section
1.1 Materials
Reagent grade silicotungstic acid hydrate (H4SiW12O40·xH2O; STA), phosphotungstic acid hydrate (H3PW12O40·xH2O; PTA), phosphomoly-bdic acid hydrate (H3PMo12O40·xH2O; PMA), ethyl acetate, ethanol, and acetic acid were purchased from Shanghai Aladdin Biochem Technology Co. Ltd. They were used as received without further purification. Q[6] was synthesized according to the literature[27], and Q[6]-HPAs self-assembled hybrids were prepared according to the literature[26].
1.2 Synthesis of ethyl acetate
The catalytic activity of Q[6]-HPAs self-assembly hybrids was investigated for the synthesis of EA. The esterification reaction was conducted at 353 K in a three-necked flask (50 mL) fitted with a reflux condenser, containing 0.1-0.5 g (optimized amount 0.3 g) of freshly activated Q[6]-HPAs catalysts (dried at 353 K for 3 h in an oven), 0.12 mol of acetic acid, and 0.12-0.48 mol(optimized amount, 0.24 mol) of ethanol. The Q[6]-HPAs catalyst was recovered by filtration, washed with water, and reactivated for the next experiments. The reaction products were collected from the flask. The composition of the reaction mixture and yield of EA was analyzed on an Agilent 6820 gas chromatograph equipped with a flame-ionization detector (FID) and a capillary column (KB-FFAP, 30 m×0.31 mm×0.25 μm), with nitrogen as a carrier gas and a programmable temperature range from 313 K to 453 K. The temperatures of the column oven and the FID were set at 473 K and 523 K, respectively.
2 Results and discussion
2.1 Interaction between Q[6] and HPAs
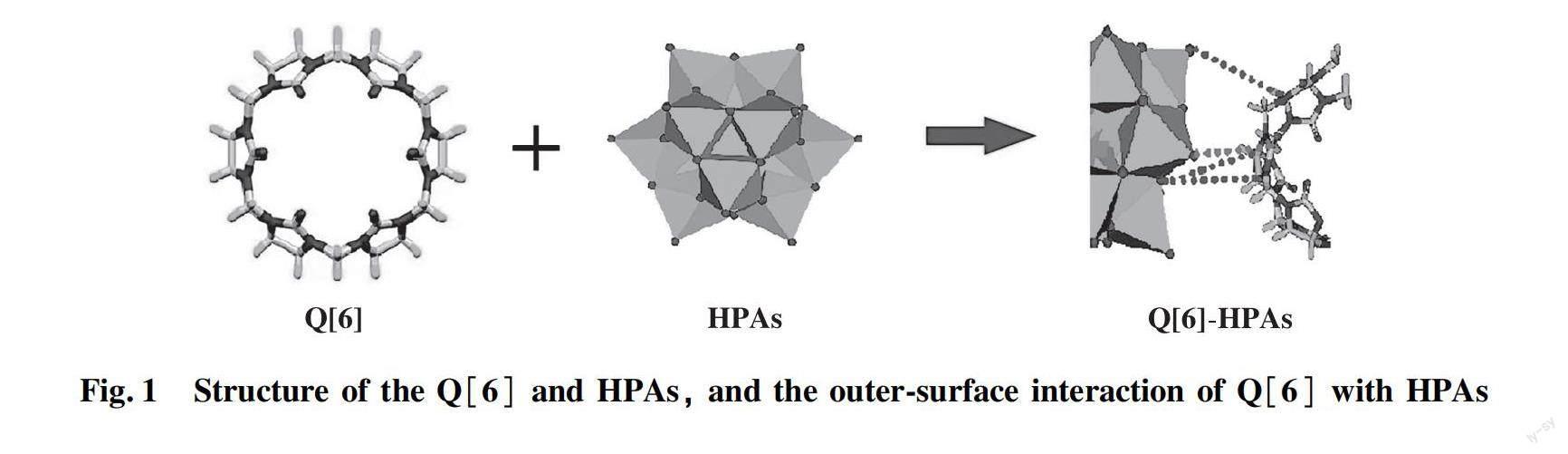
In our previous work, the Q[6]-HPAs self-assembled hybrids were prepared by simple dropwise addition of HPAs to an acidic aqueous solution of Q[6] to formed insoluble compounds[26]. The structure of the Q[6] and HPAs, and the outer-surface interaction of Q[6] with HPAs are shown in Fig.1.
Fourier-transform infrared (FTIR) spectroscopy provides an insight into the interaction of Q[6] with HPAs. When compared to the spectra obtained for the two pure compounds (Q[6] and HPAs), the characteristic IR bands of Q[6] and HPAs can still be observed in the Q[6]-HPAs self-assembled hybrids (Fig.2). In particular, the bands due to the portal carbonyl groups showed no obvious changes, whereas the bands due to the bridging -CH2- showed different shapes, suggesting the Q[6] molecules formed interacts with HPAs anions, and without changed their initial structure. The XRD patterns of Q[6]-HPAs was completely different form that of a simple mixture of Q[6] and HPAs (Fig.3), indicating that a new phase was formed between Q[6] and HPAs in solution, that is, Q[6] molecules formed interactions with HPAs anions.
FTIR spectra and XRD showed that Q[6] molecules interacts with HPAs anions in a solution, whereby the driving force is derived from the outer-surface interaction of the Q[6]. That is, the electrostatic interaction between the positive outer-surface of Q[6] and HPAs anion, including the ion dipole interaction between the oxygen atom protruding from HPAs anion and the carbonyl carbon atom at the port of Q[6], and the C-H…O hydrogen bonding interaction between the oxygen atom protruding or bridging oxygen atom from HPAs anion and methine or methylene of the Q[6] (Fig.1).
Scanning electron microscope results showed that Q[6]-HPAs self-assembled hybrids were cubic particles of about 200-400 nm with uniform dispersion and rough surface which was conducive to increasing the contact area between catalysts and reactants. Fig.4 is the SEM images of Q[6]-STA and the morphology of Q[6]-PTA and Q[6]-PMA is similar to that of Q[6]-STA. EDS spectrum confirmed that the composition of the sample was consistent with the Q[6] and HPAs. The elemental analysis results showed that the Q[6]∶HPAs stoichio-metry was 2∶1 and the empirical chemical formula was C72H72N48O64XM12·n H2O (X=P, Si and M=W, Mo).
The TG and DTA experimental results suggested that the presence of Q[6] increased the thermal stability of HPAs[26]. N2gas adsorption-desorption isotherms measurements were applied to characterize the porosities of the Q[6]-HPAs self-assembled hybrids. The pore sizes and the surfaces conditions of the three Q[6]-HPAs are shown in Tab.1. Evidently, the surface area of the Q[6]-HPAs was greater than that of the HPAs[4-5]. NH3-TPD and py-FTIR experimental results showed that Q[6]-HPAs exhibited strong Br?nsted acidity[25].
2.2 Catalytic activity of Q[6]-HPAs
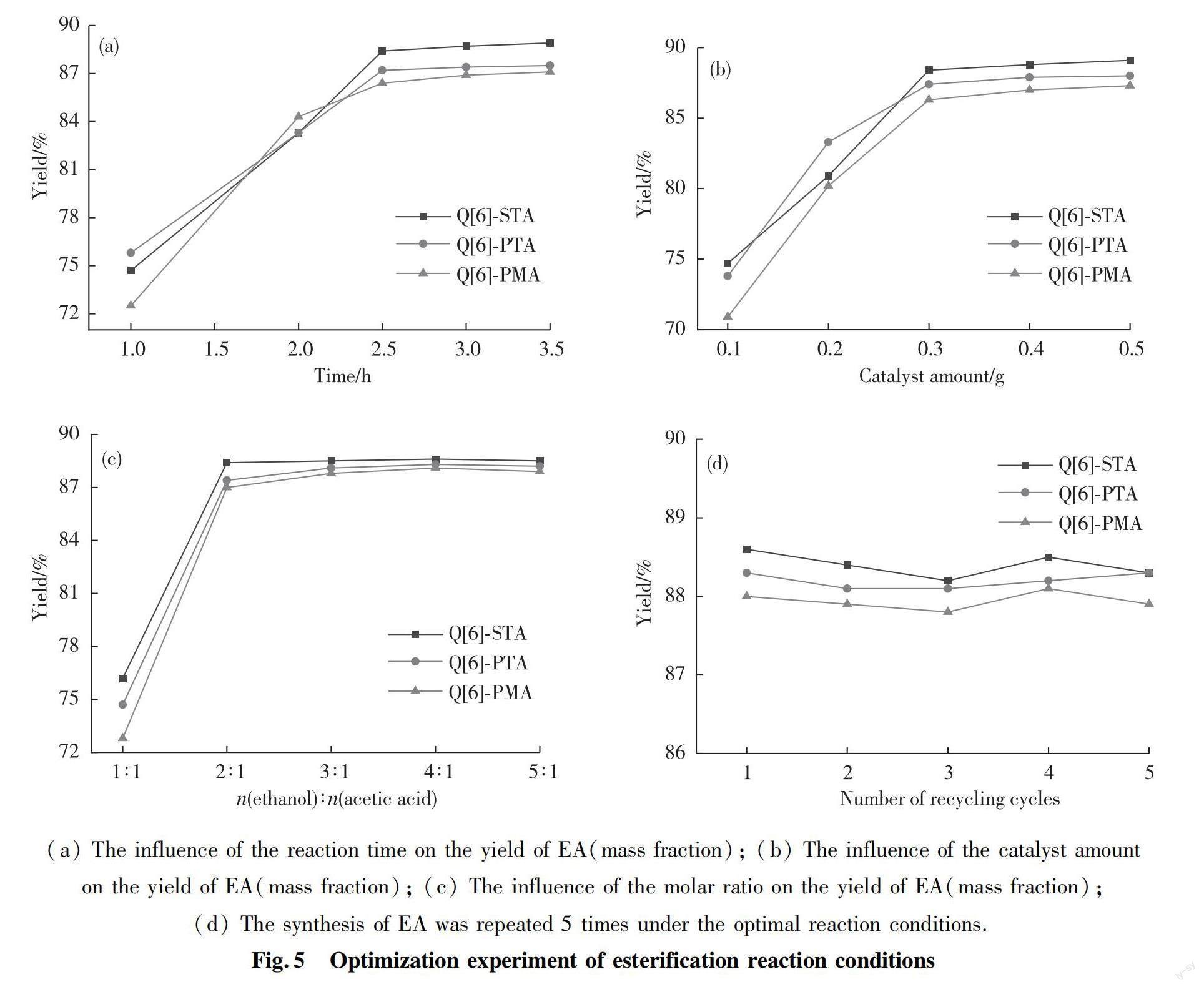
In order to examine the catalytic activities of the Q[6]-HPAs self-assembled hybrids, the effects of reaction time, catalyst amount and molar ratio of acetic acid to ethanol on the synthesis of EA were investigated. The amount of fixed catalyst was 0.3 g, 0.12 mol acetic acid and the molar ratio of ethanol to acetic acid was 3∶1, and the reaction temperature was 353 K(reflux temperature). The effect of reaction time on the yield of EA was investigated. The experimental results revealed that the yield of EA increased with the prolongation of reaction time. The yield of EA (mass fraction, %) leveled off at above 88% for Q[6]-STA after 2.5 h, above 87% for Q[6]-PTA and above 86% for Q[6]-PMA (Tab.2 and Fig.5(a)).
When the molar ratio of ethanol to acetic acid was 3∶1, the reaction time was 2.5 h, and the reaction temperature was 353 K, the influence of catalyst amount on the yield of EA was investigated. The obtained data revealed that the yield of EA increased with the amount of catalyst. The appropriate catalyst loading corresponding to a reasonable yield was 0.3 g for all three Q[6]-HPAs (Tab.3 and Fig.5(b)).
In Tab.4 are the yields of EA under the condition of Q[6]-HPAs catalyst 0.3 g, reaction time 2.5 h, reaction temperature 353 K. When the optimal molar ratio of ethanol to acetic acid was identified as 3∶1, the yields were 88.5%, 88.1% and 87.8% for the Q[6]-STA, Q[6]-PTA, and Q[6]-PMA, respectively (Fig.5(c)).
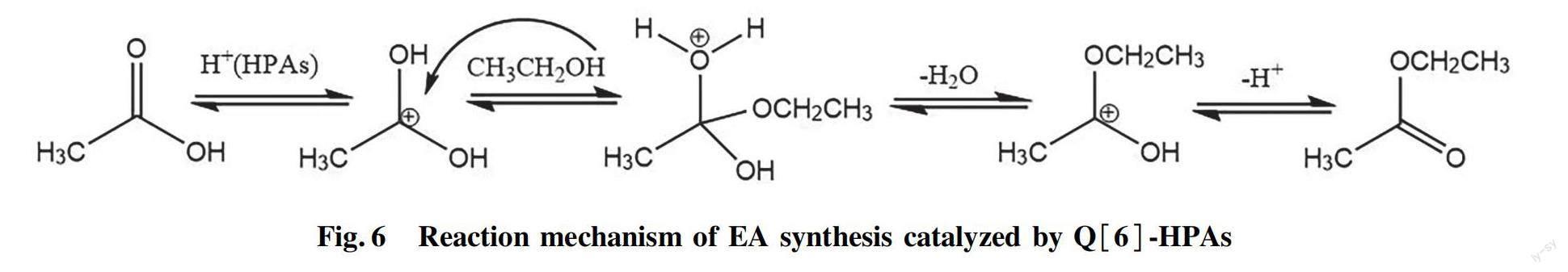
Q[6] has no catalytic activity in esterifcation reaction, and its role is to formed a stable solid catalyst through the self-assembly interaction of Q[6] and HPAs. The active species in Q[6]-HPAs catalysts are HPAs. Comparative experiments revealed the low effciency of unassembled HPAs, and the catalytic performance of Q[6]-HPAs is better than that of unassembled HPAs[25]. Q[n]s-HPAs self-assembled systems often have the characteristics of porous structure[18,28]. In addition, the morphology of Q[6]-HPAs nanoparticles also increases its surface area and active sites on the catalyst surface. The greater the specifc surface area of the catalyst, the more dispersed the active species, and the larger the number of active sites. The high porosity of the catalyst makes it easy to adsorb the reactants, thus enhancing its catalytic performance. The catalytic activities of the Q[6]-HPAs in the EA synthesis reaction decreased in the order Q[6]-STA>Q[6]-PTA>Q[6]-PMA. This is different from the acidity order of HPA, which may be related to the different BET surface area of Q[6]-HPAs. Since HPAs are Br?nsted acids[2], we speculate that the aforementioned reaction can be explained by acid-catalyzed Fischer esterifcation mechanism[29]. When the carbonyl oxygen atom of carboxylic group binds with a proton of HPAs, the density of positive charge on carbonyl carbon atom increases. The nucleophillic attack of the alcohol on the protonated carbonyl group gives a tetrahedral intermediate bearing two hydroxyl groups, hich undergoes a water and proton elimination process to produce the ester (Fig.6).
2.3 Recyclability of the Q[6]-HPAs catalysts.
We mentioned above that the Q[6]-HPAs catalysts can be easily prepared in high yields and show high stability because they are insoluble in either acidic aqueous solutions or organic solvents. As esterification reaction catalysts, they are characterized by high catalytic activities compared to their classical counterparts, and may be easily isolated from reaction media due to their insolubility. An additional important advantage of these Q[6]-HPAs catalysts is their stability with respect to leaching of the acid sites during the process of the esterification; thus, the catalysts can be collected by simple filtration after completion of the reaction. The catalysts can be regenerated by washing with water several times followed by drying at 373 K in an vacuum oven. Thereafter, they can be used again in esterification reactions. Tab.5 and Fig.5(d) shows the results of five consecutive runs under optimal conditions of ethanol to acetic acid molar ratio of 2∶1, 0.3 g catalyst, 353 K and 2.5 h for the Q[6]-STA, Q[6]-PTA, Q[6]-PMA catalysts, respectively. The normalized yields suggest that all three catalysts retained their original activities.
3 Conclusion
In this work, three Q[6]-HPAs catalysts showed good catalytic activities in the esterification reaction of acetic acid with ethanol. Their insolubility makes it easy to recover them from reaction mixture. The optimal reaction conditions are reflux temperature 353 K, catalyst amount 0.3 g, alcohol-acid ratio 3∶1 and reaction time 2.5 h. Furthermore, all the three Q[6]-HPAs catalysts showed high catalytic activity and the yield of EA was above 86% under the optimal reaction conditions. A reusability study showed the catalysts remain stable and active, suggesting that these inexpensive Q[6]-HPAs supramolecular self-assembly catalysts may potentially be applied in industry.
References:
[1]WANG E B, HU C W, XU L. Introduce in polyacid chemistry[M]. Beijing: Chemical Industry Press, 1997.
[2] HABER J, PAMIN K, MATACHOWSKI L, et al. Catalytic performance of the dodecatungstophosphoric acid on different supports[J]. Applied Catalysis A: General, 2003, 256: 141-152.
[3] KOZHEVNIKOV I V. Catalysts by heteropoly acids and multicomponent polyoxometalates in Liquid-phase reactions[J]. Chemical Reviews, 1998, 98: 171-198.
[4] SAWANT D P, VINU A, JUSTUS J, et al. Catalytic performances of silicotungstic acid/zirconia supported SBA-15 in an esterification of benzyl alcohol with acetic acid[J]. Journal of Molecular Catalysis A: Chemical, 2007, 276: 150-157.
[5] TIMOFEEEVA M N, MATROSOVA M M, RESHETENKO T V, et al. Filamentous carbons as a support for heteropoly acid[J]. Journal of Molecular Catalysis A: Chemical, 2004, 211: 131-137.
[6] WU Y, YE X K, YANG X G, et al. Heterogenization of Heteropolyacids: a general discussion on the preparation of supported acid catalysts[J]. Industrial & Engineering Chemistry Research, 1996, 35: 2546-2560.
[7] ROSA M L, MANUEL O, JOSE L G, et al. TiO2-supported heteropoly acid catalysts for dehydration of methanol to dimethyl ether: relevance of dispersion and support interaction[J]. Catalysis Science & Technology, 2015, 5: 484-491.
[8] SIDDHARTHA K B, DIPAK K D. Activated clay supported heteropoly acid catalysts for esterification of acetic acid with butanol[J]. Applied Clay Science, 2011, 53: 347-352.
[9] HERNANDEZ-CORTEZA J G, MANRIQUEZB M, LARTUNDO-ROJASC L. Study of acid-base properties of supported heteropoly acids in the reactions of secondary alcohols dehydration[J]. Catalysis Today, 2014, 220/222: 32-38.
[10]AN S, SONG D Y, SUN Y N, et al. Design of highly ordered mesoporous Nb2O5-based hybrid catalysts bifunctionalized by the Keggin-type heteropoly acid and phenyl-bridged organosilica moieties for the synthesis of methyl levulinate[J]. Microporous and Mesoporous Materials, 2016, 226: 396-405.
[11]FREEMAN W A, MOCK L, SHIH N Y. Cucurbituril [J]. Journal of the American Chemical Society, 1981, 103: 7367-7368.
[12]KIM K, SELVAPALAM N, KO Y H, et al. Functionalized cucurbiturils and their applications[J]. Chemical Society Reviews, 2007, 36: 267-279.
[13]ISAACS L. Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers[J]. Accounts of Chemical Research, 2014, 47: 2052-2062.
[14]ASSAF K I, NAU W M. Cucurbiturils: from synthesis to high-affinity binding and catalysis[J]. Chemical Society Reviews, 2015, 44: 394-418.
[15]NI X L, XIAO X, CONG H, et al. Cucurbit[n]uril-based coordination chemistry: from simple coordination complexes to novel poly-dimensional coordination polymers[J]. Chemical Society Reviews, 2013, 42: 9480-9508.
[16]TAO Z, ZHU Q J. Recognition and response of metal cations by cucurbit[n]urils-based host-guest probes[J]. Journal of Guizhou University (Natural Sciences), 2018, 35(4): 1-7.
[17]NI X L, XIAO X, CONG H, et al. Self-Assemblies based on the “outer-surface interactions” of cucurbit[n]urils: new opportunities for supramolecular architectures and materials[J]. Accounts of Chemical Research, 2014, 47: 1386-1395.
[18]CHEN L X, HUANG Y, GAO R H, et al. Cucurbit[n]uril-based supramolecular frameworks assembled through outer surface interaction and their functional propertiies[J]. Journal of Guizhou University(Natural Sciences), 2020, 37(1): 31-40.
[19]FANG X K, KGERLER P, ISAACS L, et al. Cucurbit[n]uril-Polyoxoanion hybrids[J]. Journal of the American Chemical Society, 2009, 131: 432-433.
[20]LIN J X, LU J, YANG X, et al. Construction of train-like supramolecular structures from decamethylcucurbit[5]uril and Iso- or Hetero- Keggin-type polyoxotungstates[J]. Crystal Growth & Design, 2010, 10: 1966-1970.
[21]CAO M, LIN J X, LU J. et al. Development of a polyoxometallate-based photocatalyst assembled with cucurbit[6]uril via hydrogen bonds for azo dyes degradation[J]. Journal of Hazardous Materials, 2011, 186: 948-951.
[22]LU J, LIN J X, ZHAO X L, et al. Photochromic hybrid materials of cucurbituril and polyoxometalates as photocatalysts under visible light[J]. Chemical Communications, 2012, 48: 669-671.
[23]PETERS T A, BENES N E, HOLMENT A, et al. Comparison of commercial solid acid catalysts for the esterification of acetic acid with butanol[J]. Appl Catal A: General, 2006, 297: 182-188.
[24]DIAS J A, CALIMAN E, DIAS S C L, et al. Preparation and characterization of supported H3PW12O40on silica gel: a potential catalyst for green chemistry processes[J]. Catal Today, 2003, 85: 39-48.
[25]LI S, XIA W, ZHANG Y Q, et al. Self-assembled tetramethyl cucurbit[6]uril-polyoxometalate nanocubes as efficient and recyclable catalysts for the preparation of propyl gallate[J]. New Journal Chemistry, 2020, 44: 11895-11900.
[26]XIA W, NIE Y M, LEI N. et al. A recyclable cucurbit[6]uril-supported silicotungstic acid catalyst used in the esterification reaction[J]. Inorganica Chimica Acta, 2021, 523: 120418.1-120418.7.
[27]LUO X Q, XUE S F, ZHU Q J, et al. A new method for synthesis and separate of a new family cage compounds- cucurbit[n=5-8]urils[J]. Journal of Guizhou University (Natural Sciences), 2003, 20(2): 184-186.
[28]XIA X, GE W W, CHEN H Y, et al. Porous supramolecular assemblies and functional properties of perhydroxylated cucurbit[6]uril and polyoxometallates[J].New Journal of Chemistry, 2019, 43: 10297-10304.
[29]WANG J T, WANG Y M, ZHANG B S, et al. Organic chemistry: 2th vol. [M]. 3rd ed., Tianjin: Nankai University Press, 2009: 516-517.
(責任编辑:周晓南)
六元瓜环和Keggin型杂多酸自组装及催化合成乙酸乙酯
曾俐玲,李晓迪,方兰,王沙,张朝,张云黔*
(贵州大学 化学与化工学院,贵州 贵阳 550025)
摘 要:杂多酸(HPAs)是一类用途广泛的多功能材料,在酯化反应中具有优异的催化剂性能。由于HPAs良好的溶解性,作为均相催化剂使用时,不易与产物分离、难以回收和重复利用。利用六元瓜环(Q[6])与 Keggin型杂多酸的自组装作用,制备了系列难溶性Q[6]-HPAs催化剂。该类催化剂具有性质稳定、比表面积大、回收率高,可循环利用等特点,在乙酸乙酯(EA)合成中表现出较高的催化活性和循环利用率,具有潜在的应用前景。
关键词:六元瓜环 (Q[6]);Keggin型杂多酸;超分子自组装;催化剂;乙酸乙酯 (EA)
