没食子酸对水稻细菌性条斑病菌细胞结构的影响(英文)
2019-09-10张锡娇黎芳靖袁高庆魏昌英林纬黎起秦
张锡娇 黎芳靖 袁高庆 魏昌英 林纬 黎起秦


Abstract: Gallic acid (GA) is a phenolic compound, and presents various biological activities in plants. Our previous experiments demonstrated a relatively strong inhibitory effect of GA on Xanthomonas oryzae pv. oryzicola (Xoc). In order to elucidate the effects of GA on the cell structure and membrane permeability of the pathogen, morphological structures of bacteria treated with GA were observed by electron microscopy, the integrity and the permeabilities of membrane of Xoc were investigated by determining the release from cells of materials that absorb at 260 nm, and changes in the fluorescence of cells treated with the fluorescein diacetate(FDA) and lactate dehydrogenase(LDH) activity. After treating with 200 μg·mL-1of GA, many pits and irregular vesicles were found on the cell surface under electron microscopy, indicating that GA could damage the cell walls of Xoc. The electrical conductivity from Xoc suspensions 24 h after GA treatment was 135.48 μS·cm-1(that of control was 127.85 μS·cm-1), and the fluorescence intensity of Xoc suspensions 2 h after GA decreased by 58.10%, indicating that cells leaked electrolytes and cytosolic contents. Meanwhile, the acti-vities of LDH in bacterial suspensions treated with GA also increased, suggesting that GA could damage the structure of the bacterial cell membrane. In addition, the absorbance at 260 nm from Xoc suspensions was 1.004(that of control was 0.018), indicating that GA could negatively affect Xoc cell wall integrity. These results indicate that GA can not only alter the permeability of the cell membrane of Xoc, but also can impact the integrity of cell membrane.
Key words: Xanthomonas oryzae pv. oryzicola, gallic acid, cell structure, membrane permeability, bacteriostasis me-chanism
CLC number: Q945.8Document code: AArticle ID: 1000-3142(2019)12-1681-10
摘要: 沒食子酸(gallic acid, GA)是一种植物酚类化合物,具有多种生物活性。前期实验发现,GA对水稻细菌性条斑病菌(Xanthomonas oryzae pv. oryzicola, Xoc)具有较强的抑制作用。为了解该物质对Xoc的细胞结构和细胞膜的影响,该研究用电子显微镜观察GA对Xoc的形态结构的影响,通过测定GA处理后的Xoc培养液的电导率、紫外吸收物含量(260 nm的吸光值)、乳酸脱氢酶的活性以及菌体的二乙酸荧光素(fluorescein diacetate, FDA)的强度等,探讨了GA对Xoc细胞膜的完整性和通透性的影响。结果表明:经浓度为200 μg·mL-1的 GA处理后,Xoc的菌体形态结构发生改变,表面有明显的凹陷或不规则囊泡状突起,表明GA对Xoc细胞壁有损伤作用。200 μg·mL-1的GA处理24 h后,病菌培养液的电导率为135.48 μS·cm-1(对照处理为127.85 μS·cm-1)。GA处理2 h后,Xoc细胞荧光强度下降58.10%,说明病菌细胞内电解质外渗和细胞溶质发生渗漏;同时,乳酸脱氢酶的活性增加,表明菌体的细胞膜受到破坏。此外,GA处理24 h后,Xoc培养液在260 nm下的吸光值为1.004(对照处理为0.018),表明病菌细胞膜的完整性受到破坏。这表明GA不仅破坏Xoc的细胞膜通透性,而且还影响膜的完整性。
關键词: 水稻细菌性条斑病菌, 没食子酸, 细胞结构, 膜通透性,抑菌机制
Bacterial leaf streak of rice, which is caused by Xanthomonas oryzae pv. oryzicola (Xoc), is one of the important plant diseases in South China. This disease was first reported in Philippines in 1918 and was reported in China in 1953. Bacterial leaf streak of rice can reduce yield loss by 40%-60%, and the disease severely threatens the high and stable yield production of rice. Furthermore, the transportation of rice seeds during production tends to expand and aggravate this disease (Zhang et al., 2015). Because bacterial leaf streak-resistant rice cultivars are not available, bactericides are mainly used to control the disease. However, few bactericides, such as zinc thiazole and thiodiazole copper, are registered in China (Wei et al., 2007). Therefore, the development of new bactericides to manage this disease is desirable.
Gallic acid [GA: 3,4,5-trihydroxybenzoic acid (C6H2(OH)3COOH] is a phenolic compound in plants that exists as a free molecule and as a constituent of tannins (Masoud et al., 2012). Studies have demonstrated the antimicrobial activity of GA against Salmonella typhimurium (Nohynek et al., 2006), Escherichia coli (Chanwitheesuk et al., 2007), Bacillus subtilis (Zhang et al., 2013), Staphylococcus aureus and Candida albicans (Sarjit et al., 2015). GA may effectively inhibit the invasion ability of gastric cancer MGC-803 cells (Li et al., 2017). Our early work involving the screening of plants for antibacterial activity against Xoc showed that GA extracted from Sedum lineare significantly inhibited the growth of plant pathogens such as Xoc, X. oryzae pv. oryzae, X.campestris pv. pruni, X. axonopodis pv. citri, Ralstonia solanacearum, Pseudomonas syringae pv. glycinea, P. syringae pv. tomato and Pectobacterium carotovora subsp. carotovora in vitro.
Reports on the antibacterial mechanisms of GA exist. GA damages the cell membrane of Pseudomonas fluorescens, which induced the leakage of intracellular electrolytes and abundant molecular material (Lu et al., 2015). GA may potentially capture calcium ions from the calcium-binding proteins on the cell surfaces of Campylobacter by chelation, resulting in the loss of vital functions of those proteins and therefore cell death (Sarjit et al., 2015). GA inhibits the proliferation of human hepatocellular carcinoma SMMC-7721 cells by inhibiting the expression of Survivin mRNA (Li et al., 2014), inhibits the expression of MMP2 and MMP9 by regulating PI3K/AKT signaling pathway, and also effectively inhibits plankton SH FurnRi activity and biofilm formation by regulating the expression of MDOH gene and OPGH protein (Kang et al., 2018). GA plays an antibacterial role by destroying the membrane integrity of Aeromonas hydrophila and aeromonas cold blood (Lu et al., 2016). To our knowledge, no reports on the mechanisms of GA against Xoc have been published. In the present paper, we evaluated the minimum inhibitory concentration (MIC) of GA against Xoc and the effects of GA on the cell structure and membrane permeability of the pathogen. The results will provide a theoretical basis for further GA applications.
1Materials and Methods
1.1 Materials
GA was obtained from the Tianjin Kemiou Chemical Reagent Development Center. An assay kit for determining the activity of lactate dehydrogenase (LDH) was obtained from Suzhou Comin Biotechnology Co., Ltd., China. Methanol, ethyl acetate, glutaraldehyde, iodoacetic acid, sodium phosphate, propanedioic acid, phenol, fluorescein diacetate (FDA), 2,4-dinitrophenylhydrazine, trichloroacetic acid, sodium carboxymethyl cellulose, sodium polypectinate, sodium hydroxide and absolute alcohol were of the highest grade commercially available.
1.2 Bacterial strains and growth conditions
Xoc strain Xo-002 was isolated from an infected rice leaf in 2010 in Guangxi and was preserved at the Plant Pathology Research Institute of Guangxi University, Nanning, China. The strain was stored on nutrient agar (NA: 3 g of beef extract, 5 g of peptone, 10 g of dextrose, 17 g of agar, 1 000 mL of distilled water, pH 7.0) at -80 ℃ and was initially cultured on NA at 30 ℃ for 2 d, after which the strain was transferred into beef extract broth and shaken at 120 r·min-1 at 28 ℃ for 24 h (during the logarithmic phase).
1.3 Antimicrobial susceptibility tests
Minimum inhibitory concentration(MIC) was tested using a 2-fold serial broth dilution as described here. Xoc at the logarithmic growth phase were diluted in a 107 colony-forming units (CFU)·mL-1 suspension of beef extract. GA dissolved in 10% methanol was added to the bacterial suspension to a final GA concentration ranging from 3.125 to 400 μg·mL-1. The bacterial suspension with only 10% methanol served as the control. The suspension was then shaken at 120 r·min-1 at 30 ℃ for 24 h. The MIC was defined as the lowest concentration of antimicrobial agent at which the cell growth was not visible with the naked eye. Each treatment was replicated three times.
To determine the effects of initial bacterial density on the GA inhibition,Xoc at the logarithmic growth phase were diluted to 106 CFU·mL-1, 107 CFU ·mL-1 or 108 CFU·mL-1 in suspension of beef extract. GA was added to the bacterial suspension until final GA concentrations of MIC or 2 MIC were reached. The suspension was then shaken at 120 r·min-1 30 ℃ for 24 h. Each treatment was replicated for three times.
1.4 Electron microscopy
Xoc at the logarithmic growth phase were diluted with beef extract to 108 CFU·mL-1. GA (dissolved in 10% methanol) was added to the bacterial suspension to reach a final GA concentration of MIC. The bacterial suspension with only 10% methanol served as the control. After shaking at 120 r·min-1 and 28 ℃ for 6, 12 or 24 h, the suspension was centrifuged. The cells were washed three times with 0.2 mol·L-1 sodium phosphate buffer (PBS, pH 7.4) and then fixed with 2.5% glutaraldehyde in 0.2 mol·L-1 PBS. The samples were prepared for electron microscopy as previously described (Nakajima et al., 2003). The prepared samples were examined using an electron microscope (H-500, Hitachi, Japan)
1.5 Electrical conductivity assay
Pathogens were diluted with beef extract to 108 CFU·mL-1. GA was added to the bacterial suspension to reach final GA concentrations of 1/2 MIC, MIC or 2 MIC. The bacterial suspension with only 10% methanol served as the control. After shaking at 120 r·min-1 at 28 ℃ for 0, 1, 2, 4, 8 or 24 h, the suspension was centrifuged, after which the supernatant was diluted 20 times with water and measured for its electrical conductivity. Each treatment was replicated three times.
1.6 Cell membrane integrity assay
The cell membrane integrity of Xoc was evaluated by determining the release of materials that absorb at 260 nm. Bacteria at the logarithmic growth phase were washed three times with PBS and then diluted with PBS to 108 CFU·mL-1. GA was added to the bacterial suspension to reach final GA concentrations of 1/2 MIC, MIC or 2 MIC. The suspension with only 10% methanol served as the control. Each treatment was replicated three times. The suspension was shaken at 130 r·min-1 at 28 ℃ for 0, 2, 4, 8, 16 or 24 h, after which it was centrifuged at 4 000 g for 10 min. The release of mate-rials that absorb at 260 nm was monitored over time using a UV-Visible spectrometer (Shimadzu UV-1800; Shimadzu Corp, Kyoto, Japan)
1.7 Outer membrane (OM) permeabilization assay
The OM permeabilization activity of cell was determined using an FDA assay as described by Zeng et al.(2013). Xoc at the logarithmic growth phase were diluted with beef extract to a 108 CFU·mL-1. GA was added to the bacterial suspension to reach a final GA concentration of MIC. The suspension with only 10% methanol served as the control. After shaking at 130 r·min-1 at 28 ℃ for 2 h, the suspension was centrifuged. The cells were washed three times with PBS. FDA was then added to the bacterial suspension to reach a final FDA concentration of 0.25 μg·mL-1, and the suspension was incubated at room temperature for 10 min. Fluorescence excited at a wavelength of 460 nm and an emission wavelength of 680 nm was recorded using an RF-5301PC fluorescence spectrophotometer (Shimadzu, Japan). All experiments were replicated three times.
1.8 Determination of LDH activity in bacterial suspension
Xoc at the logarithmic growth phase were diluted with beef extract to 108 CFU·mL-1. GA was added to the bacterial suspension to reach a final GA concentration of 100, 200 or 400 μg·mL-1. The bacterial suspension with only 10% methanol served as the control. After being exposed to GA for 0, 2, 4, 8, 16 or 24 h, 2 mL of culture was centrifuged at 1 000 × g for 5 min and 4 ℃. The LDH activity in the supernatant was determined using an assay kit (Suzhou Comin Biotechnology Co. Ltd., China) according to the manufacturer’s instructions.
All experiments were replicated three times. The formation of 1 nmol of pyruvic acid upon the exposure of 1 mg of bacterial protein to extracellular matrix for 15 min was defined as 1 unit of activity in the reaction systems.
Protein concentration was determined in accordance with the coomassie brilliant blue staining method. A 3 mL coomassie brilliant blue color reagent was added to 0.05 mL of distilled water, 0.05 mL of standard protein solution and 0.05 mL of sample liquid, which represented a control tube, standard tube and testing tube, respectively, for 10 min. The tubes were then measured for their optical density at 595 nm (OD595) nm using a UV spectrophotometer, and the protein content was calculated as follows:
Protein content (mg·mL-1)=
Standard tube concentration×(test tube OD-standard tube)(Standard tube OD-Standard tube)
1.9 Statistical analysis
The data were subjected to the analysis of variance using SAS software (version 6.08, SAS Institute, Cary, NC). Mean comparisons were conducted using a least significant difference (Fisher’s LSD) test at P=0.05. The standard error and LSD results were recorded.
2Results and Analyses
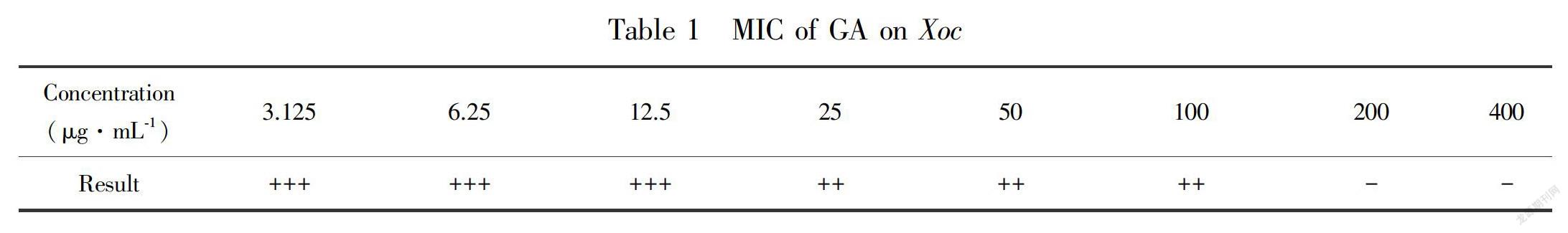
2.1 MIC of GA on Xoc
Xoc cultured for 24 h grew in nutrient agar (NA) containing 3.125 μg·mL-1 to 100 μg·mL-1 GA (Table 1). However, the culture solution was transparent in the NA supplemented with 200 μg·mL-1 GA, which indicates that the MIC of GA was 200 μg·mL-1.
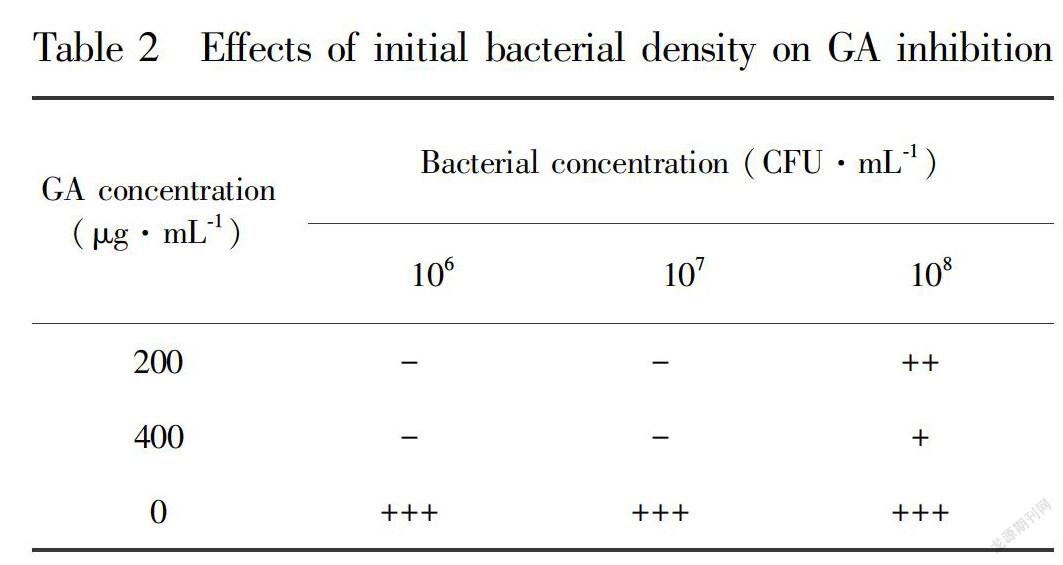
The effects of initial bacterial density on GA inhibition were shown in Table 2. Initial bacterial densities of 106 CFU·mL-1 and 107 CFU·mL-1 inhibited the growth of Xoc under conditions of 200 μg·mL-1 and 400 μg·mL-1 GA. However, an initial bacterial density of 108 CFU·mL-1 resulted in turbid culture media when treated with 200 μg·mL-1 and 400 μg·mL-1 GA, indicating Xoc growth.
2.2 Effects of GA on Xoc cell morphology
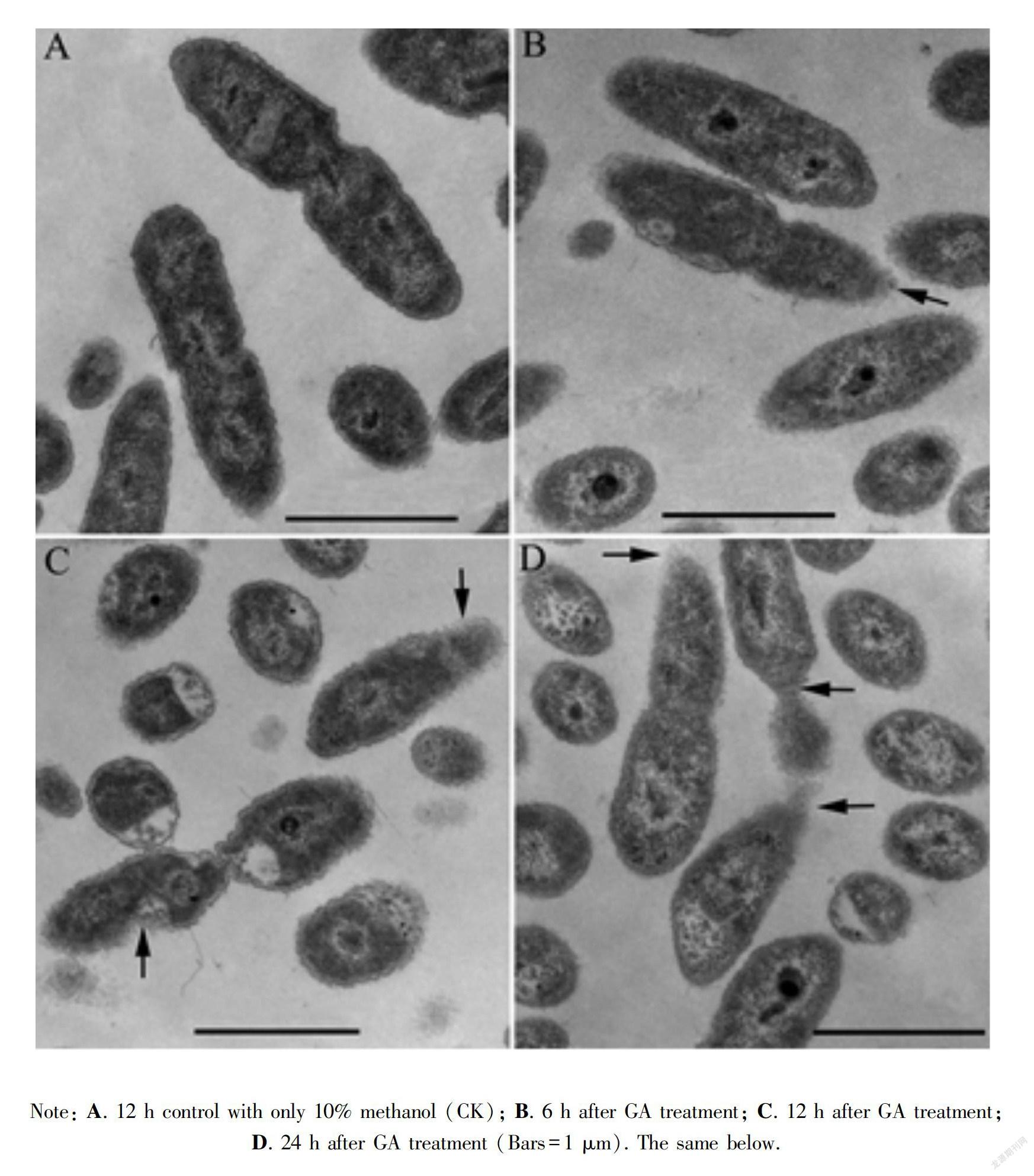
The effect of GA on the cellular structure of the Xoc was visualized using transmission electron microscopy (Fig.1). Control cells cultured for 12 h had a full shape and smooth surface; the cell membrane adhered to the cell wall, and the cytoplasm was homogeneous (Fig.1:A). However, GA-treated Xoc showed deformation and roughness of cell walls (Fig.1:B,C) after 6 h and 12 h, the cell outlines appeared blurry, the cell wall was loose after 24 h, and some cells had an irregular shape and no membrane or cell wall on one side (Fig.1:D).
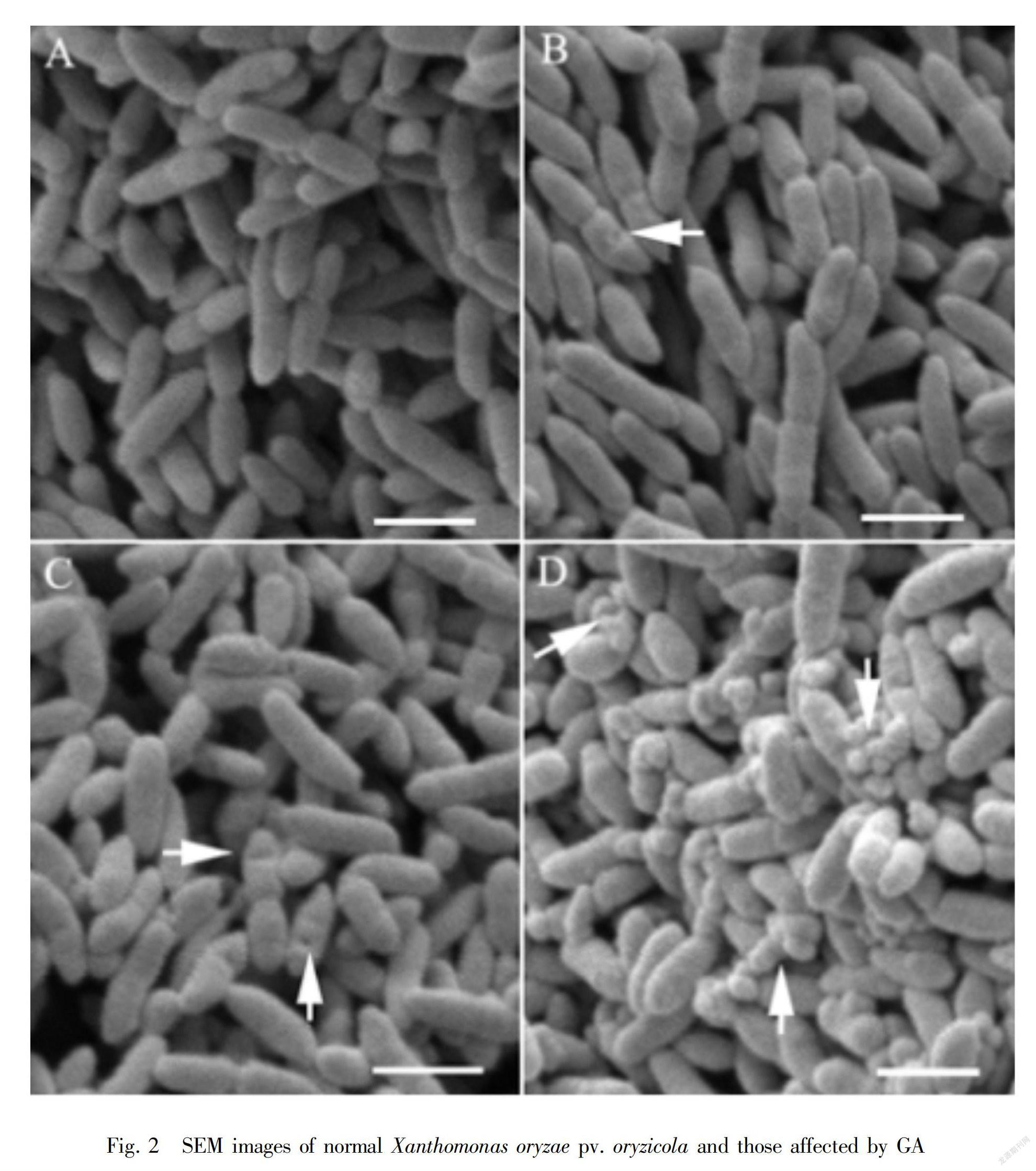
Scanning electron microscopy (SEM) showed that Xoc cells treated with GA at the MIC changed remarkably compared with control cells (Fig.2). The surface of control cells was smooth and cells were obvious after 12 h of culture (Fig.2:A). However, granules and holes were observed on the surface of GA-treated cells after 6 h and 12 h (Fig.2:B,C), and many pits and irregular vesicles were observed on the cell surface after 24 h (Fig.2:D). Therefore, GA could damage the cell wall of Xoc, and the effect was greater with the duration of treatment.
2.3 Effects of GA on the electrical conductivity of bacteria culture media
The effect of GA on the electrical conductivity of the culture solution was shown in Fig.3. Electrical conductivity increased with time. GA treatments at concentrations of 100 μg·mL-1, 200 μg·mL-1, and 400 μg·mL-1 and the control culture liquid resulted in electrical conductivities of 129.03, 132.80, 135.48 and 127.85 μS·cm-1, respectively, after 24 h of incubation. The results indicate that the higher the concentration of GA was, the more serious the leakage of electrolytes from the cell.
2.4 Effects of GA on the content of UV-absorbing compounds of bacterial culture media
When antimicrobial agents damage bacterial membranes, intracellular components, such as DNA and RNA, tend to leak. The release of DNA and RNA from cells can be detected by UV at 260 nm as an indication of membrane damage (Chen & Cooper, 2002). The content of UV-absorbing compounds from Xoc suspensions treated with GA was shown in Fig.4. GA treatment concentrations altered the absorbance: the higher the GA treatment concentration was, the larger the absorbance. Treatment with 100, 200 and 400 μg·mL-1 GA for 24 h resulted in absorbances of 0.195, 1.004 and 1.720, respectively, and the difference between GA treatment and the control (0.018) was statistically significant. These results indicate that GA can negatively affect Xoc cell wall integrity.
2.5 Effects of GA on the permeability of bacteria
FDA, which can enter the cell, is a nonuorescent compound that is hydrolyzed to uorescein and acetate Note: A. 12 h control with only 10% methanol (CK); B. 6 h after GA treatment; C. 12 h after GA treatment; D. 24 h after GA treatment (Bars=1 μm). The same below.
materials in the culture liquid of Xoc by nonspecic esterases. The intracellular retention of fluorescein depends on the integrity of the cell membrane (Prudêncio et al., 1998). The change in fluorescence intensity after GA (200 μg·mL-1) treatment for 2 h was presented in Fig.5. Stained cells displayed the most intense fluorescence at 510 nm. The fluorescence intensities of both 200 μg·mL-1 GA-treated and control cells were 53.06% and 91.33%, respectively. In addition, the fluorescence intensity of the treated cells decreased by 58.10%, and the cytosolic contents had leaked out from the cells.
2.6 Effects of GA on the activity of the LDH of bacteria
The bacterial endoenzyme LDH could leak from bacteria upon cell membrane damage. Therefore, the activity of LDH in the bacterial suspension reflects the change in permeability of the cell membrane (Hara & Yamakawa, 1995). The activities of LDH in the bacterial suspensions were shown in Fig.6. The activities of LDH in bacterial suspensions treated with GA (100, 200, 400 μg·mL-1) and the control were less than 1.5 U·mg-1 protein after treatment for 2 h; there was no significant difference between the GA treatment and the control. However, the enzymatic activities of GA-treated suspensions were higher than those of the control after 4 h. The enzymatic activities increased with GA dose and duration. The results suggest that GA can damage the structure of the bacterial cell membrane and cause the leakage of LDH enzymes.
3Discussion
A concentration of 200 μg·mL-1 GA completely inhibited cells at 107 CFU·mL-1; therefore, the MIC of GA on Xoc was 200 μg·mL-1 in these conditions. The antibacterial function of GA differed against different concentrations of bacteria. However, treatment with 200 and 400 μg·mL-1 GA inhibited the development and growth of the pathogenic bacteria at concentrations of 106 and 107 CFU·mL-1, but these GA treatments could not completely suppress the growth of bacteria at 108 CFU·mL-1.
The cell wall maintains the inherent morphology of cells and exchanges substances. When the cell wall of a bacterium has been disrupted by an antimicrobial substance, the cell permeability is altered, materials can leak from the cell (Bush, 2012). Electron micrographs showed that GA-treated Xoc displayed damaged cell walls that showed signs of pits and irregular vesicles on the outer surface. A similar finding was reported by Helander et al. (2001). Furthermore, GA treatment resulted in a higher culture liquid electrical conductivity than that of the control. Thus, cell wall damage was at least one mechanism by which Xoc was negatively affected by GA.
An essential function of the cell membrane is serving as a selectively permeable barrier. The exposure to high concentrations of antimicrobial agents can alter the permeability of bacterial cell membranes and hinder normal bacterial metabolism (Bush, 2012). Our experimental results showed that GA enhanced the conductivity of the culture medium, which led to cytoplasm leakage and resulted in the leakage of intracellular DNA and RNA. These phenomena were similar to those reported for Pseudomonas fluorescens treated with GA (Lu et al., 2015), suggesting that GA could negatively affect Xoc cell wall integrity. Furthermore, the fluorescence intensity of cells treated with GA decreased to 58.10%, indicating that the cytosolic contents had leaked out from cells. In addition, the activity of intracellular LDH in the bacterial suspension treated with GA was higher than that of control, suggesting that LDH diffused outside the cell.
Our early works have demonstrated that GA could control rice bacterial leaf streak, with control efficacy of 64.54% in field(Wang et al., 2018). GA derivatives, methyl gallate, had antibacteria activity against Ralstonia solanacearum, Pseudomonas syringae pv. lachrymans and Pectobacterium carotovora subsp. carotora(Yuan et al., 2012) , and could effectively reduce the incidence of tomato bacterial wilt in the field(Li et al., 2014). Meanwhile, four gallic acid derivatives have also antibacteria activity against Ahernaria mali, Physalospora piricola, Rhizoctonia solani and Phytophthora infestans(Tan et al., 2018). Therefore, GA and its derivatives have potential to be developed as a new pesticide to control plant disease.
In conclusion, the results suggest that GA can alter Xoc membrane permeability and disrupt membrane integrity. Increasing concentrations of GA resulted in more evident damage. The results showed that the cell membrane was a target for bacterial growth inhibition.
References:
BUSH K, 2012. Antimicrobial agents targeting bacterial cell walls and cell membranes [J]. Rev Scientifique Et Technique (International Office of Epizootics), 31(1): 43-56.
CHANWITHEESUK A, TEERAWUTGULRAG A, KILBURN JD, et al., 2007. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk [J]. Food Chem, 100(3): 1044-1048.
CHEN CZ, COOPER SL, 2002. Interactions between dendrimer biocides and bacterial membranes [J]. Biomaterials, 23(16): 3359-3368.
HARA S, YAMAKAWA M, 1995. Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori [J]. J Biol Chem, 270(50):29923-29927.
HELANDER IM, NURMIAHO-LASSILA EL, AHVENAINEN R, et al., 2001. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria [J]. Internat J Food Microbiol, 71(2-3):235-244.
KANG J, LIU L, LIU M, et al., 2018. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression [J]. Food Control, 94: 147-154.
LI MH, WANG MY, CHEN HB, et al., 2014. Study of gallic acid in inducing human hepatoma SMMC-7721 cells apoptosis and its mechanism [J]. Trad Chin Drug Res Clin Plarmacol, 25(2):117-121.
LU J, REN MR, CHEN R, et al., 2015. Antibacterial effects of gallic acid on Pseudomonas fluorescens [J]. Food Sci Technol, 40(6): 300-303.
LU J, WANG Z, REN M, et al., 2016. Antibacterial effect of gallic acid against Aeromonas hydrophila and Aeromonas sobria through damaging membrane integrity [J]. Curr Pharm Biotechnol, 17(13):1153-1158.
LI WH, CHENG HB, LI L, et al., 2017. Effect of gallic acid on invasion of gastric cancer cell line MGC-803 through PI3K/AKT signaling pathway [J]. Trad Chin Drug Res Clin Pharmacol, 28(1):23-27.
LI Y, FAN WW, YUAN GQ, et al., 2014. Antimicrobial pro-perties of methyl gallate and its field effect on tomato bacterial wilt [J]. Agrochemicals, 53(11): 845-848.
MASOUD MS, HAGAGG SS, ALI AE, et al., 2012. Synthesis and spectroscopic characterization of gallic acid and some of its azo complexes [J]. J Molec Struct, 1014(16): 17-25.
NAKAJIMA Y, ISHIBASHI J, YUKUHIRO F, et al., 2003. Antibacterial activity and mechanism of action of tick defensin against gram-positive bacteria [J]. Biochim Biophy Acta, 1624(1-3): 125-130.
NOHYNEK LJ, ALAKOMI HL, KAHKONEN MP,et al 2006. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens [J]. Nutrit Cancer, 54(1): 18-32.
PRUDNCIO C, SANSONETTY F, CRTE-REAL M, 1998. Flow cytometric assessment of cell structural and functional changes induced by acetic acid in the yeasts Zygosaccharomyces bailii and Saccharomyces cerevisiae [J]. Cytometry, 31(4): 307-313.
SARJIT A, WANG Y, DYKES GA, 2015. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions [J]. Food Microbiol, 46(3):227-233.
TAN P, LIU SY, ZHOU TP, et al., 2018. Synthesis of gallic acid derivatives and their antibacterial activities [J]. J Xihua Univ (Nat Sci Ed) , 37( 2) : 108-112.
WANG KH, WEI CY, XIE HT, et al., 2018. Gallic acid isolated from sedun linear and its control efficacy on rice baceterial leaf streak [J]. Guihaia, 38(1):119-127.
WEI FL, DAI JG, XU DQ, et al., 2007. Efficacy of the new creating pesticide znthiazole against bacteria disease [J]. Agrochemicals, 46(12):810-811.
YUAN GQ, LI QQ, YE YF, et al., 2014. Isolation of methyl gallate from Toxicodendron sylvestre and its effect on tomato bacterial wilt [J]. Plant Disease, 96:1143-1147.
ZENG ZL, ZHAO CY, LUO C, et al., 2013. Antibacterial function and mechanism of monolaurin and monocaprin [J]. Food Sci, 34(3): 70-74.
ZHANG RS, CHEN ZY, LIU YF, 2015. Advance in rice bacterial leaf streak researches [J]. Agric Sci Technol, 16(2): 298-305.
ZHANG YL, LI JK, LIU L, et al., 2013. Research progress in gallic acid from Galla chinensis [J]. Sci Technol Food Ind, 24(10): 1-5.
