抑制水稻细菌性条斑病菌的没食子酸分离及其对水稻细菌性条斑病的防治作用
2018-05-30汪锴豪魏昌英谢慧婷袁高庆林纬范腕腕黎起秦
汪锴豪 魏昌英 谢慧婷 袁高庆 林纬 范腕腕 黎起秦


摘 要: 通過液—液萃取、硅胶和凝胶柱层析法,从佛甲草(Sedum lineare)分离出一种可以抑制水稻细菌性条斑病菌(Xanthomonas oryzae pv. oryzicola, Xoc)生长的单体化合物,经质谱分析,确定该化合物为没食子酸(gallic acid, GA)。在30 mg·mL-1浓度下,GA能抑制一些植物病原细菌如桃细菌性穿孔病菌(X. campestris pv. pruni)、水稻细菌性条斑病菌(X. oryzae pv. oryzicola)、水稻白叶枯病菌(X. oryzae pv. oryzae)、柑橘溃疡病菌(X. axonopodis pv. citri)、大豆细菌性斑点病菌(Pseudomonas syringae pv. glycinea)、番茄细菌性斑点病菌(P. syringae pv. tomato)和胡萝卜软腐果胶杆菌( Pectobacterium carotovora subsp. carotovora)的生长;GA还对11种植物病原真菌如烟草疫霉(Phytophthora nicotianae)、指状青霉(Penicillium digitatum)、滇刺枣褐腐病菌(Streptobotrys streptothrix)、瓜果腐霉(Pythium aphanidermatum)、芒果拟盘多毛孢(Pestalotiopsis mangiferae)、新月弯孢霉(Curvularia lunata)、立枯丝核菌(Rhizoctonia solani)、(Fusarium oxysporum f. sp. niverum)、西瓜专化型尖孢镰刀菌(F. oxysporum f. sp. nicotianae)、番茄灰霉病菌(Botrytis cinerea)和齐整小核菌(Sclerotium rolfsii)的生长具有一定的抑制作用。在300 mg·mL-1浓度下,GA对水稻细菌性条斑病的田间防治效果达到64.62%。该研究结果表明没食子酸具有开发成为一种防治水稻细菌性条斑病的杀菌剂的潜力。
关键词: 抑菌物质, 提取和纯化, 佛甲草, 没食子酸, 水稻细菌性条斑病, 防治效果
Rice bacterial leaf streak, caused by Xanthomonas oryzae pv. oryzicola (Xoc), is one of the major rice diseases in the rice planting areas of tropical and subtropical zones. In China, the disease was firstly spotted in Pearl River Delta in China, and prevailed in Guangdong, Guangxi, Hainan, Sichuan and Zhejiang in 1950s -1960s (Fang et al, 1957). This disease could result in 15%-20% loss of production, even 40%-60%, and is the quarantined disease in China (Raymundo & Briones, 1995; Nino-Liu et al, 2006). In the past few years, because breeding for resistance did not resulted in useful varieties yet, the disease brought great loss to the safe production of rice (Levy et al, 1991; He et al, 2012). Application of bactericide is the major strategy for control of this disease but, at present, few bactericides, such as Zn-thiazole and thiodiazole-copper etc., are registered in China (Wei et al, 2007; Zhu et al, 2010). It is desirable to develop a new bactericide to manage this disease. Sedum lineare is a perennial herb in the genus Sedum, family Crassulaceae. The plant lives in the condition of drought and coldness, and is easy to grow. Therefore, it is mainly used for roof greening (Feng et al, 2010; Liu et al, 2012; Lu et al, 2015). It has been found some medical applications of the extracts of Sedum lineare (Niu et al, 2014; Rouhier & Jacquot, 2005; Zhou et al, 2013). In the early works on screening of plants for antibacterial activity against Xoc, we found that the methanol extracts of S. lineare could significantly inhibit the growth of Xoc in vitro.
At present, there are no report on the using antimicrobial substances of S. lineare to control plant diseases. The objective of this study was to isolate and identify the compound responsible for the antibacterial activity against Xoc from S. lineare, and to evaluate the effects of selected compounds for control of rice bacterial leaf streak.
1 Materials and Methods
1.1 Pathogen and culture conditions
Xoc XD1109 was used in the screening of prospective plant constituents and greenhouse experiments. Other strains, listed in tables 1 and 2, were used as determining the antibiotic spectrum of gallic acid. All strain used in this study were provided by the Plant Pathology Research Institute, College of Agriculture, Guangxi University, China.
Strains of pathogenic bacterium were cultured initially on nutrient agar (NA: beef extract 3 g, peptone 8 g, agar 18 g, distilled water 1 000 mL) for 24-48 h and then transferred into nutrient agar for suspension cultivation (28 ℃, 130 r·min-1) for 24 h. Strains of pathogenic fungi were cultured on potato sucrose agar, (PSA: potato 200 g, sucrose 20 g, agar 15 g, distilled water 1 000 mL) at 28 ℃ for 7-15 d.
1.2 Preparation of methanol extract of S. lineare
The samples of S. lineare, collected from Guangxi University, Nanning, Guangxi, China in 2012, were dried in an oven under the temperature of 50 ℃, then grounded into powder using 40-mesh screen (the diameter of the holes was 0.37 mm). The powder (200 g) was soaked in methanol of volume eight times of the powder at room temperature for 100 h. Methanol solution was evaporated under reduced pressure in water of permanent 55-65 ℃ with a rotary evaporator to obtain crude extract.
1.3 Isolation of anti-bacterial substance
According to the different polarities of the components, solvent extraction method described by Yao & Wen(2011) was used to isolate anti-bacterial substance in methanol extract of S. lineare. The methanol extract of S. lineare was suspended in water (1∶2 by volume) and extracted four times with petroleum ether. The petroleum ether extract was evaporated to remove the solvent under reduced pressure in water of permanent 55-65 ℃ with a rotary evaporator, and the remaining aqueous layer was successively extracted with chloroform, ethyl acetate, and n-butanol. Each extract was concentrated to dryness by a rotary evaporator and tested its antibacterial activity against Xoc in vitro.
1.4 Purification of anti-bacterial substance
The crude extract of anti-bacterial substance obtained from solvent extract was subjected to silica gel column GF254 (Qingdao Haiyang Chemical Industry Factory) chromatography. A total 20 g of dried crude extract was eluted with chloroform and methanol. The proportions of their volumes were 40∶1, 20∶1, 10∶1, 5∶1, 2∶1, 1∶1 by volume, and finally with methanol. Eluant was collected into fractions of 400 mL, and then was evaporated under reduced pressure in water of permanent 55-65 ℃ with a rotary evaporator.
Thin layer chromatography (TLC) was used to determine their purity of fractions. The fractions, showing the same TLC profiles, were combined and tested their antibacterial activity in vitro by the agar well diffusion method.
The fractions, which had antibacterial activity against Xoc, were dissolved in methanol and filtered by a membrane with holes (0.22 μm in diameter), and then purified by Sephadex LH-20 (Pharmacia, USA) column (100 cm × 0.8 cm). 0.55 g of sample (20 mg·mL-1) was loaded into the column and eluted with methanol. Eluant was collected into fractions of 5 mL. The antibacterial activities of all fractions was tested in vitro by the agar well diffusion method.
1.5 Structure identification
Nuclear magnetic resonance (NMR) method (Ibargoitia et al, 2014; Kwang-Hyun et al, 2014) was used to determine the structure of the monomeric compound isolated from S. lineare, which had antibacterial activity against Xoc. Mass spectrum was recorded by electrospray ionization mass spectrometry. NMR spectra were recorded on a superconducting flourier transform NMR spectrometer (INOAVA, 600 MHz; Varian, America) at 600 MHz for 1H and at 150 MHz for 13C, with TMS as an internal standard.
1.6 Determination of in vitro anti-bacterial activity
Antibacterial activity of extracts from S. lineare was tested by the agar well diffusion method described by Yang et al (2010). The nutrient agar medium containing Xoc (108 cfu·mL-1) was poured into petri plates and allowed to set. A 7 mm cork borer was used to bore holes on the medium. About 70 μL of the extract solution was introduced into the well. The extracts of S. lineare were dissolved with 10% methanol. The wells loaded with the same concentration of solvent served as controls and 0.1 mg·mL-1 streptomycin as positive control. Each treatment consisted of three replicates. Inhibitory diameter of the well in each treatment was measured at 48 h after incubation at 28 ℃.
1.7 Determination of in vitro antifungal activity
Antifungal activity of gallic acid (GA) was tested by a mycelia radial growth inhibition test (Yuan et al, 2010). The test strains were cultured on potato sucrose agar at 28 ℃ for 7 d. A mycelium disc (5 mm in diameter) of the test strains, which was cut with cork borer from the periphery of young cultures, was placed in the center of potato sucrose agar medium plates containing GA at concentration 30 mg·mL-1. GA was dissolved with methanol. Control plates were treated with methanol at a concentration of 0.1% (vol·vol-1). Each treatment consisted of three replicates. The plates were incubated at 28 ℃ for 7-15 d. Growth inhibition of the treatment were calculated using the following formula:
Growth inhibition = 100 × (C-T) / C, where C is the colony diameter of the control and T is the colony diameter of the GA-treated plate.
1.8 Determination of disease control efficacy
Potted experiments: The rice cultivar “Boyou 680” was cultivated in a 20-cm-diameter pots (10 plants per pot) in a greenhouse at (30 ± 5) ℃. The suspension (108 cfu·mL-1) of Xoc was sprayed on rice leaf at tillering stage (10 mL per plant). Gallic acid at the concentrations of 200, 300 and 400 mg·L-1 was sprayed on rice leaves (10 mL per plant) 24 h after Xoc inoculation. Meanwhile, water (control) and 20% thiodiazole-copper suspension concentrate at the concentration of 570 mg·L-1 were sprayed on rice leaf (10 mL per plant) 24 h after Xoc inoculation. Each treatment consisted of four replicates and each replicates contained 30 plants. The disease severity was investigated 15 d after pathogen inoculation.
The disease severity was divided into six ratings (Luo et al, 2011): 0 = no symptom, 1 = only a small spot of lesion and less than 1% the leaf area infected, 3 = scattered short steaks of lesions and 1%-5% the leaf area infected, 5 = plenty of lesions on the leaf and 6%-25% leaf area infected, 7 = 26%-50% leaf area infected and 9 = more than 51% leaf area infected. The disease index(DI )and control efficacy were calculated using the following formulae:
DI = ∑ (the value of each level × the number of leaves that are at this level of infection)/(the total number of the leaves tested × 9) × 100
Control efficacy (%)=(DI of the reference control-DI of the pesticide group)/DI of the reference control × 100
Field experiments: Rice cultivar Boyou 680 was also planted in a field located in the suburb of Nanning City, Guangxi, China in 2015. The experimental field was known to be naturally infested with Xoc. The field was divided into twelve plots and each plot had an area of 20 m2. GA (300 mg·L-1), 20% thiodiazole-copper suspension concentrate(570 mg·L-1) and water (control) were sprayed on rice leaf in the early occurrence of disease. The volume of water delivered was 750 L·hm-2. The application of the bactericide was performed three times at a 10-day-interval. Each treatment consisted of four replicates. No other bactericides were sprayed to the experimental field. Fertilizers were used in accordance with technical standard of agricultural production. The disease severity was recorded on the first day the bactericide was performed and in 10 days after the last application of bactericide. Effect of bactericide on rice bacterial leaf streak was evaluated based on more than 150 rice plants collected randomly from each plot. The disease severity and DI described earlier were separately investigated. The field control efficacy of bactericide was evaluated using the following formula:
Field control efficacy (%) = [1 - (DI of control at the 1st application × DI of treatment 10 d after the last application)/(DI of treatment at the 1st application × DI of control 10 d after the last application)] × 100
1.9 Statistical analysis
Data were subjected to analysis of variance using SAS software (version 6.08; SAS Institute,Cary, NC). Mean comparisons were conducted using a least significant difference (Fishers LSD) test at P=0.05. Standard error and LSD results were recorded.
2 Results and Analysis
2.1 Isolation and purification of an antibacterial compound from methanol extract of S. lineare
The inhibitory effect of solvent extract from methanol extract of S. lineare on Xoc varied with solvents. Ethyl acetate extract showed the most effective antibacterial activity against Xoc in the test, with an inhibitory diameter of 32.00 mm, followed by N-butanol extract, with an inhibitory diameters of 18.67 mm. Inhibitory diameters of petroleum ether layer, chloroform layer and distilled water layer were 12.67, 15.00 and 16.00 mm, respectively. Therefore, the antibacterial substance of methanol extract of S. lineare was mainly in ethyl acetate layer.
The ethyl acetate extract was further separated by silica gel column chromatography. A total 123 fractions were obtained. These fractions were merged into eighteen fractions according to the test of TLC. The fractions of F2 to F9, which had antibacterial activity against Xoc , were combined and then purified through a silica gel column again. A total 74 fractions were obtained, and were merged into nine fractions according to the test of TLC. Fraction of F1 to F5, which showed antibacterial activity against Xoc, was further separated through a gel filtration chromatography using methanol. The white crystals, which was obtained from 100% methanol and tentatively called Compound A, showed inhibitory activity on the growth of Xoc.
2.2 Structure identification of monomeric compound
Compound A was white acicular crystal, easily dissolvable in methanol, and had a formula of C7H6O5 as determined from the electrospray ionization mass spectrometry, the Rf value in TCL plate was the same as that of gallic acid. The spectral data of the Compound A were as follows: 13C NMR (Fig. 1) (pyridine, 150.95 MHz): δ 170.0 (-COOH), 147.9 (C-3, 5), 140.8 (C-4), 122.8 (C-1), 110.9 (C-2, 6); 1H NMR (Fig. 2) (pyridine, 600.24 MHz): δ 7.22 (2H, s, H-2, 6). According to the references (Ahmed et al, 2003; Eldahshan, 2011; Sushma et al, 2013), the Compound A was identified as 3,4,5- Trihydroxybenzoic acid, namely gallic acid, the chemical structure of the Compound A was shown in Fig. 3.
2.3 Antibiotic spectrum of GA
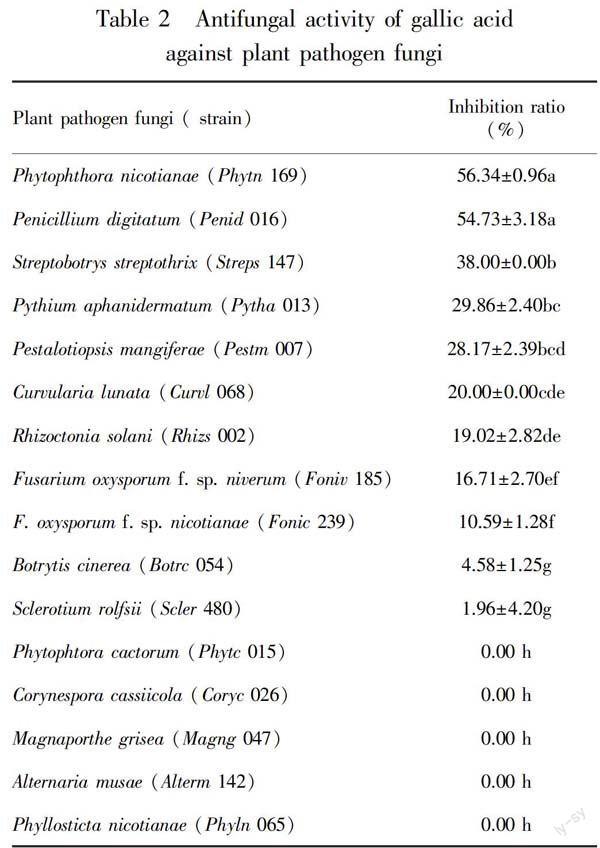
The growth of all test plant-pathogenic bacteria was inhibited by GA atconcentration 30 mg·mL-1. The test bacteria,including X.campestris pv. pruni, X. oryzae pv. oryzicola, X. oryzae pv. oryzae and X. axonopodis pv. citri, were most strongly inhibited by GA, with inhibition zones width of 25.33, 24.00, 22.33 and 20.67 mm, respectively (Table 1). Pseudomonas syringae pv. glycinea and P. syringae pv. tomato showed the least sensitive to GA, with inhibition zones width of 11.00 mm and 8.68 mm ,respectively (Table 1). At concentration of 30 mg·mL-1, GA could also weakly inhibit the growth of eleven plant-pathogenic fungi, including Phytophthora nicotianae, Penicillium digitatum, Streptobotrys streptothrix, Pythium aphanidermatum, Pestalotiopsis mangiferae, Curvularia lunata, Rhizoctonia solani, Fusarium oxysporum f. sp. niverum, F. oxysporum f. sp. nicotianae, Botrytis cinerea and Sclerotium rolfsii (Table 2). Growth inhibition exceeding 50% by GA was only observed in Phytophthora nicotianae and Penicillium digitatum. The growth of Botrytis cinerea and Sclerotium rolfsii was slightly inhibited by GA, with growth inhibition 4.58% and 1.96%, respectively. But GA could not inhibit the growth of Phytophtora cactorum, Corynespora cassiicola, Magnaporthe grisea, Alternaria musae and Phyllosticta nicotianae.
2.4 Control efficacy of gallic acid on the disease
Potted experiment:The results (Table 3) in a greenhouse experiment showed that gallic acid could significantly reduce disease index of rice bacterial leaf streak relative to the water control group. Gallic acid at 200, 300 and 400 mg·L-1 provided 63.55%, 71.38% and 77.39% control efficacy respectively. The control efficacy of gallic acid at 200 mg·L-1 was lower than that of thiodiazole-copper at 570 mg·L-1, but the control efficacy of gallic acid at 400 mg·L-1 was significantly higher than that of thiodiazole-copper at 570 mg·L-1. Therefore, gallic acid at concentration 300 mg·L-1 was used for field experiment.
Field experiment: Gallic acid could effectively reduce disease index of rice bacterial leaf streak relative to the water control in field. The control efficacies of GA (300 mg·L-1) and 20% thiodiazole-copper suspension concentrate (570 mg·L-1) on rice bacterial leaf streak were 64.62% and 63.37% respectively (Table 4). The control efficacies of GA were similar to that of thiodiazole-copperal (1994) found that GA not only shows the antioxidant activity, but also has stability, so it is widely used in the clinical experiment and food industry. Tumors, treated with GA, showed cytotoxic activity against cancer cells, without harming normal cells(Bajpai & Patil, 2008). GA can be used to produce pyrogallic acid as a fresh agent to preserve food (Cheng et al, 1994). GA can be also used in food packaging, because a bio-based multilayer packaging film with GA as the oxygen scavenger (Pant et al, 2017). At present, there are no report on application of GA to control plant diseases. The result of this research showed that gallic acid could significantly decrease the incidence of rice bacterial leaf streak at concentration of 300 mg·L-1 in field, the control efficacy of GA at 300 mg·L-1 on rice bacterial leaf streak in field was similar to that of 20% thiodiazole-copper suspension concentrate (570 mg·L-1). Therefore, gallic acid has the potential to be further developed as a bactericide against rice bacterial leaf streak.
Sedum lineare is mainly used for roof greening. Feng et al (2010) found that S. lineare presented a simple but practical energy balance model, which could dissipate 99.1% of the total heat gain of an extensive green roof. S. lineare have better temperature reduction effects than purple/red leafed plants and have temperature reduction effectiveness (Liu et al, 2012). The extracts of S. lineare can be used in medicine and related applications. Liao et al (2011) found that S. lineare has obvious anti-inflammatory effect. δ-Amyrone, a compound from S. lineare, is a bioactive agent which possesses anti-inflammatory effects (Niu et al, 2014). The ethyl acetate extracts, n-butyl alcohol extracts and total flavone extracts of S. lineare have the antitumor activity (Chen et al, 2011). We found that GA form S. lineare could control the rice bacterial leaf streak. Therefore, S. lineare presents widely used in many fields.
Reference:
AHMED AG, MOHAMMED FL, MASETAKE N, 2003. Antibacterial polyphenol from Erodium glaucophyllum [J]. Verlag der Zeitschrift für Naturforschung, 58:670-674.
BAJPAI B, PATIL S, 2008. A new approach to microbial production of gallic acid [J]. Braz J Microbiol, 39(4): 708-711.
CHEN YJ, LIN QX, WAN DR,et al, 2011, Study on the anti-tumor activities of the different extract fractions and total flavones of the three Sedum plant drug [J]. J Minzu Univ Chin, 20(2): 88-92.
CHENG CQ, WANG YB, LU GA, 1994. Study on new tecnnology to prepare pyrogallol [J]. Chem Ind For Prod, 4(13): 15-18.
ELDAHSHAN OA, 2011. Isolation and structure elucidation of phenolic compounds of Carob leaves grown in Egypt [J]. Curr Res J Biol Sci, 3:52-55.
FANG CT, REN HC, CHEN TY, et al, 1957. Acomparison of the rice bacterial leaf blight organism with the bacterial leaf streak organisms of rice and Leersia hexandra swartz [J]. Acta Phytopathol Sin, 2:99-124.
FENG C, MENG QL, ZHANG YF, 2010. Theoretical and experimental analysis of the energy balance of extensive green roofs [J]. Energ Bldg, 42:959-965.
HE WA, HUANG DH, LI RB,et al, 2012. Identification of a resistance gene bls1 to bacterial leaf streak in wild rice Oryza rufipogon Griff [J]. J Integr Agr, 11:962-969.
IBARGOITIA ML, SOPELANA P, GUILLIN MD, 2014. 1H nuclear magnetic resonance monitoring of the degradation of margarines of varied compositions when heated to high temperature [J]. Food Chem, 165:119-128.
KWANG-HYUN K, ADITYA R, VEENA S, 2014. Analysis of thermal degradation kinetics and carbon structure changes of co-pyrolysis between macadamia nut shell and PET using thermogravimetric analysis and 13C solid state nuclear magnetic resonance [J]. Energ Conv Manage, 86:154-164.
LEVY M, ROMAO J, MARCHETTI MA,et al, 1991. DNA fingerprinting with a dispersed repeated sequence resolves pathotype diversity in the rice blast fungus [J]. Plant Cell, 3:95-102.
LIAO YH, WU LZ, CHENG GZ, et al, 2011. Anti-inflammatory effect of Sedum lineare in mice [J]. Chin J Exp Trad Med Form, 17(3): 142-144.
LIU TC, SHYU GS, FANG WT, et al, 2012. Drought tolerance and thermal effect measurements for plants suitable for extensive green roof planting in humid subtropical climates [J]. Energ Bldg, 47:180-188.
LU J, YUAN JG, YANG JZ, et al, 2015. Effect of substrate depth on initial growth and drought tolerance of Sedum lineare in extensive green roof system [J]. Ecol Eng, 74: 408-414.
LUO ZY, YANG XJ, ZHU MX, et al, 2011. High efficacy control of rice bacterial leaf streak [J]. Hubei Plant Prot, 124:37-38.
MA Y, ZHANG JZ, CONG JB, et al, 1994. Studies of antioxidation mechanism of gallic acid and its derivatives [J]. Chin Sci Bull, 39(2): 149-153.
NINO-LIU DO, RONALD PC, BOGDANOVE AJ, 2006. Xanthomonas oryzae pathovars: model pathogens of a model crop [J]. Mol Plant Pathol, 7:303-324.
NIU XF, YAO H, LI WF,et al, 2014. δ-Amyrone, a specific inhibitor of cyclooxygenase-2, exhibits anti-inflammatory effects in vitro and in vivo of mice [J]. Int Immun pharmacol, 21:112-118.
PANT AF, SFNGERLAUB S, MaLLER K, 2017. Gallic acid as an oxygen scavenger in bio-based multilayer packaging films [J]. Materials, 10(5), 489.
RAYMUNDO AK, BRIONES JRA, 1995. Genetic diversity in Xanthomonas oryzae pv. oryzicola [J]. Int Rice Res New, 20:3-5.
ROUHIER N, JACQUOT JP, 2005. The plant multigenic family of thiol peroxidases [J]. Free Radical Biol Med, 38,1413-1421.
SUSHMA P, RITA D, ANUPAMA K, 2013. Quantum chemical density functional theory studies on the molecular structure and vibrational spectra of Gallic acid imprinted polymers [J]. Spectrochim Acta Pt A-Mol Biol, 116:562-573.
WEI FL, DAI JG, XU DQ, et al, 2007. Efficacy of the new creating pesticide zn-thiazole against bacteria disease [J]. Agrochemicals, 46(12):810-811.
YANG DM, ZHU XY, WANG QS,et al, 2010. Antibacterial activity and stability of extracts from Potentilla ansterina L [J]. Food Sci, 31:127-130.
YAO XJ,WEN JM, 2011. Extraction and separation of natural organic matter [J]. Technol & Dev Chem Indust: 26-28,54.
YUAN GQ, LI QQ, WANG J, et al, 2010. Screening of plants for antimicrobial activity to phytopathogenic bacteria [J]. Guangxi Agric Sci, 36(4):184-187.
ZHOU Q, LIAN LF, WU LZ, et al, 2013. Antitumor effect and immune mechanism of Sedum lineare on tumor-bearing mice [J]. Pharmacol Clin Chin Mat Med, 29:83-85.
ZHU K, DUAN GF, ZHANG JP, et al, 2010. Inhibition of rice bacterial leaf stripe by filtrate and crude toxins of helminthosporium gramineum rabenhf. sp. echinochlone [J]. Chin Agr Sci Bull, 26(8):240-242.
