A pH Model for Calculating the pH Value of Mixed–Acid–Base Equilibria of Overhead Condensing Systems in Crude Distillation
2018-01-19WangHaiboLiYunChengGuangxuWuWeiChenXuanZhangYaohengLiXinyun
Wang Haibo; Li Yun; Cheng Guangxu; Wu Wei; Chen Xuan; Zhang Yaoheng; Li Xinyun
(1. School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049;2. College of Mechanical and Electrical Engineering, Beijing University of Chemical Technology, Beijing 100029;3. Research Institute of Lanzhou Petrochemical Corporation, Lanzhou 730060)
1 Introduction
Crude distillation plays an important role at refineries since it can affect the economic benefits and energy consumption[1]. However, corrosion is a common problem in the overhead condensing system of crude distillation unit. The corrosive species are HCl, H2S, organic acids and CO2. The emphasis on HCl control has a significant effect on corrosion control of the tower overhead systems in crude distillation. The primary control of chlorides is required during desalting. Since the desalting efficiency cannot reach 100%, the chlorides always exist in the system. However, when the crude is thermally treated for fractionation, these salts (NaCl, MgCl2and CaCl2)will hydrolyze to form HCl vapor. The HCl vapor would dissolve in the condensed water, resulting in a particularly severe attack to the local environment because the pH value of the water can be as low as pH = 1[2]. The low pH value of the condensate will certainly increase the corrosion rates of carbon steel and alloy steel. The pH value of the condensed water is normally adjusted to a nearly neutral point by neutralizing with amines. The advantage of amines used as the neutralizing agent can increase the corrosion inhibition efficiency at a small dosage. However, the online monitoring of pH value of the stream in overhead condensing system of crude distillation is practically impossible.
The pH model was used to calculate the relationship of the pH value, the temperature and the appropriate amount of neutralizers that could help to avoid corrosion issues for the overhead condensing systems of crude distillation unit. The protonation constants involving these neutralizing amines were incorporated in the pH model. A comparative study on the corrosion inhibition performances of five neutralizing amines(N,N-dimethylethanolamine, 3-methoxypropylamine,morpholine, ethylenediamine and triethylamine)for carbon steel was performed under the overhead condensing conditions of crude distillation unit by using weight loss measurements. The principle objective of this study was to investigate the effect of the selected amineson the corrosion rate in typical oil field environments and their relationship with the change in pH value.
2 Experimental
Table 1 shows the chemical structure of the amines used in this study. Carbon steel, having a chemical composition covering 0.20% of C, 0.28% of Si, 0.55% of Mn, 0.25%of Cu, 0.022% of S, 0.01% of P, 0.25% of Cr, 0.30% of Ni, 0.01% of V, with the remainder consisting of iron, was used in this study. The mass loss experiments were carried out at 90 °C for 72 h using 150 mg/L of HCl in admixture with different concentrations of neutralizing amines. The pH value of the solutions was measured using a pH meter.The corrosion ratevcorr(g/(m2·h)) and the inhibition efficiencyηwere calculated as follows:

whereS(m2) is the total surface area,m0(g) andm1(g) are the weights before and after exposure to the test solution,respectively,t(h) is the immersion time,v0(g/(m2·h))andv(g/(m2·h)) are the corrosion rates without and with addition of the neutralizing amines, respectively.
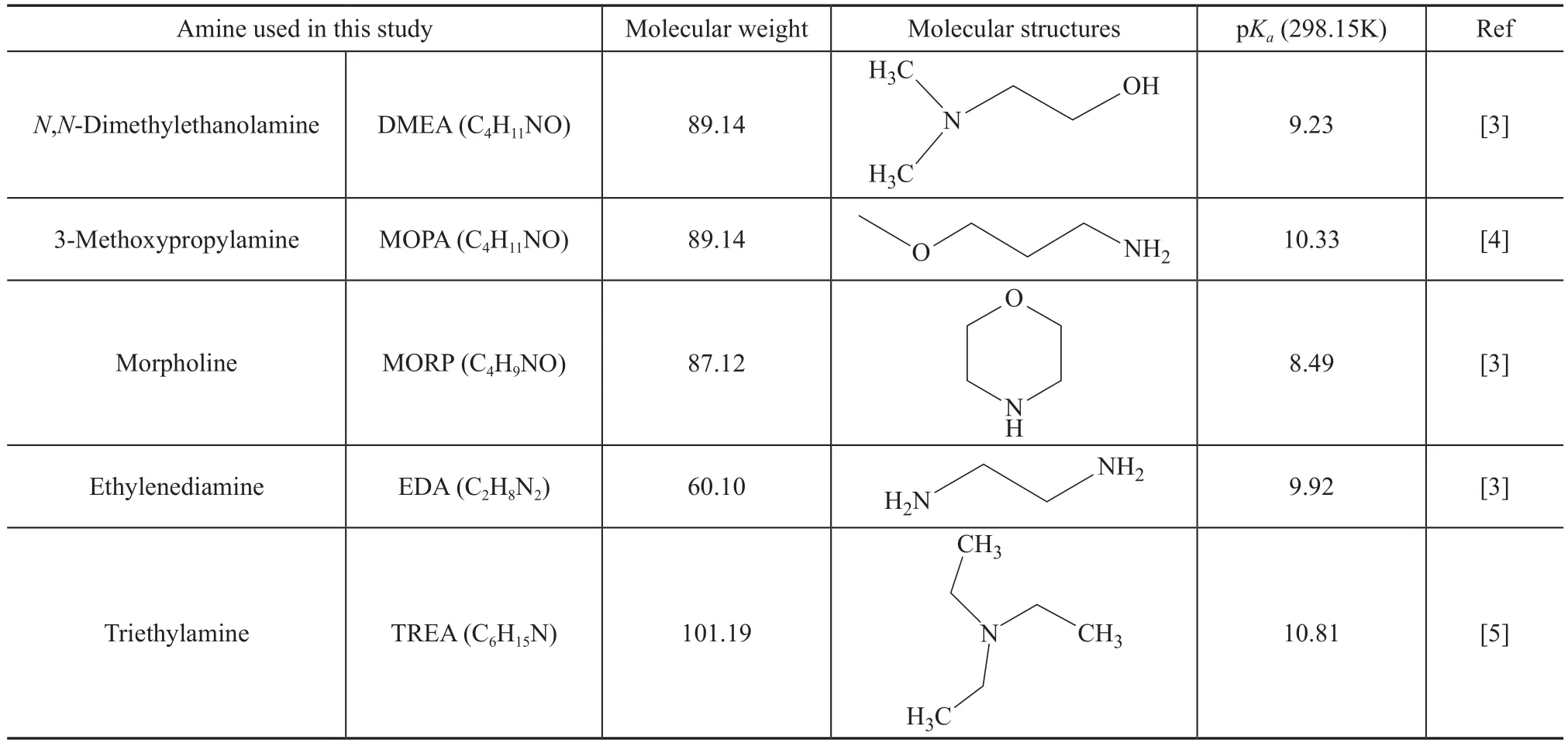
Table 1 Name, chemical structure and the pKa values of the amine
3 The pH Model
Figure 1 shows a simplified diagram of the overhead condensing system of crude distillation and the process for the chemical equilibrium reactions of the system.The concentration of HCl was predicted by measuring the Cl-content of the overhead accumulator drum. HCl was assumed to be completely dissociated, and the dissociation of water was neglected.
3.1 Acid–base equilibria
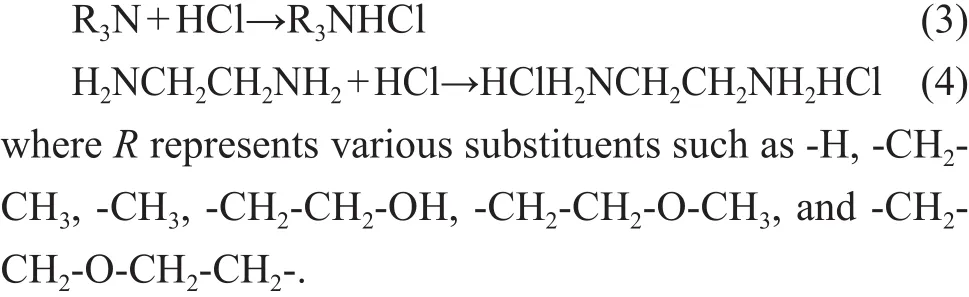
The main chemical reactions involved in amines-HCl-H2O system are shown in Eq. 3 for DMEA, MOPA,MORP, and TREA. However, the behavior of EDA is quite different according to Eq. 4.
3.2 Dissociation equilibria
HCl may undergo the following hydrolysis reaction:

These amines may undergo the hydrolysis reaction as described by Eq. 6.
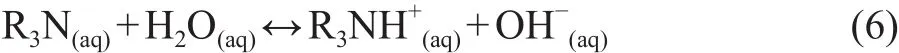
The base strength of the amine is described by the acidionization constant (Ka) of the ammonium ion.
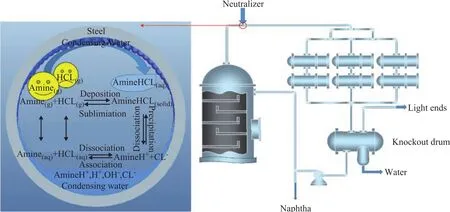
Figure 1 A simplified diagram of the overhead condensing system of crude distillation

3.3 The pH model
At a given temperature, if [HCl]>m[Amine](m = 1 for DMEA, MOPA, MORP, and TREA; m = 2 for EDA), the pH values are calculated from Eq. 10. By considering the acid–base equilibrium reactions, one amine molecule of DMEA, MOPA, MORP, or TREA can react upon one HCl molecule; however, one EDA molecule can react upon two HCl molecules.

In contrast, when [HCl]< m[Amine], the hydrolysis reaction of organic amines plays an important role in controlling the solution pH value. The pH values are calculated by using Eq. 11 (n = 1 for the DMEA, MOPA,MORP, or TREA; n = 1/2 for EDA). The predicted model was shown to represent the solution pH value depending on the pKavalues of amines, and the concentration of HCl and amines.
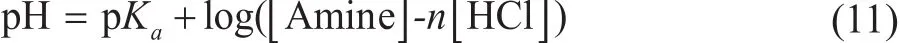
where [Amine]is the concentration of amines (mol/L)and [HCl]is the concentration of HCl (mol/L).
4 Results and Discussion
4.1 Comparison of computational and experimental results
The acid-ionization constant is one of the main factors in the choice of a chemical solution for the removal of the acid gas. The pKavalues of the neutralizing amines at 298.15 K are listed in Table 1. The predicted pH values are determined by Eqs. 10 and 11. Figure 2 shows the effect of different concentrations of neutralizing amines on the predicted pH values at 298.15 K. The Cl-concentration in the overhead accumulator drum was 150 mg/L. When the concentration of neutralizing amines was 200 mg/L, the equilibrium pH value with EDA (10.02) was significantly higher than that of other amines (Figure 2). Two nitrogen atoms of one EDA molecule can react upon HCl molecules;therefore, EDA had higher basicity than other amines,requiring less amount of neutralizing agent.
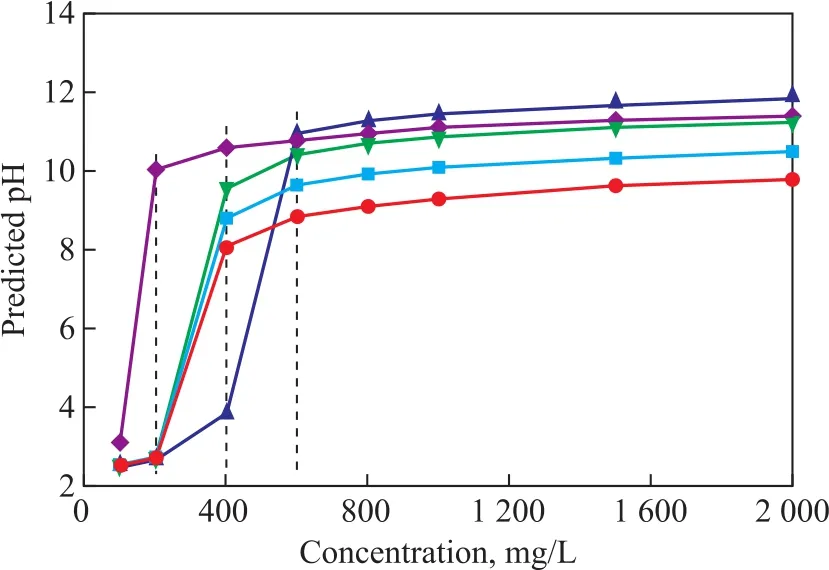
Figure 2 Effect of different concentration of neutralizing amines on the predicted pH values at 298.15 K■—DMEA; ▲—TREA; ●—MORP; ▼—MOPA; ◆—EDA
Figure 3 shows the comparison of the predicted and experimental pH values of five amine samples with different concentrations (100, 200, 400, 600, 800, 1 000,1 500, and 2 000 mg/L amine) for neutralizing the 150 mg/L HCl at 298.15 K. The predicted pH values had a good fit to the experimental values. The deviation of most pH values, which were predicted by the model, from the experimentally measured pH values was within ± 5%.
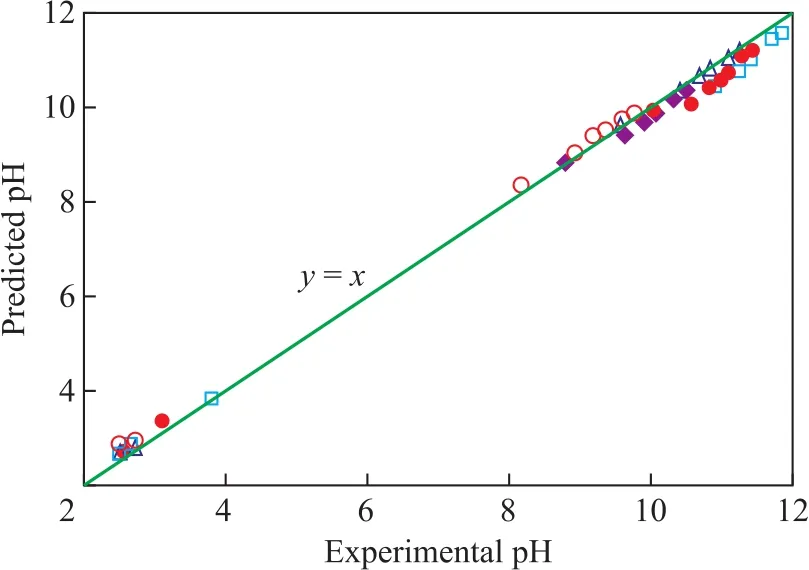
Figure 3 Comparison of the predicted and experimental pH values of fi ve amines○—MORP; □—TREA; ●—EDA; △—MOPA; ◆—DEMA
4.2 Effect of temperature on pH value
The temperature is one of the operating conditions of crude distillation; therefore, it is important to study the relationship between temperature and pH value at the unmeasured point of the overhead system. TheKavalues of DMEA,TREA, MORP, MOPA, and the first and second dissociation constants of EDA at different temperatures were obtained from the literature[6–9]. Figures 4 and 5 show that the pKavalues tend to decrease with an increasing temperature;therefore, the base strength of the amines also decreases with an increasing temperature. The pKaof amine samplesvs.1/Tfollows a linear relationship (Eqs. 12–17).
The trend of the linear slope of pKavaluesvs.1/Tdecreases in the following order: MOPA>EDA>TREA>MORP>DMEA. A small slope, as observed for DMEA,indicated that the change in the standard enthalpy with temperature is small, suggesting a lower heat requirement in the regeneration process. Judging from the kinetics point of view, DMEA is a good substitute for the other amines,because it can react faster. A linear relationship between the pH value and temperature can be derived (Eq. 18).


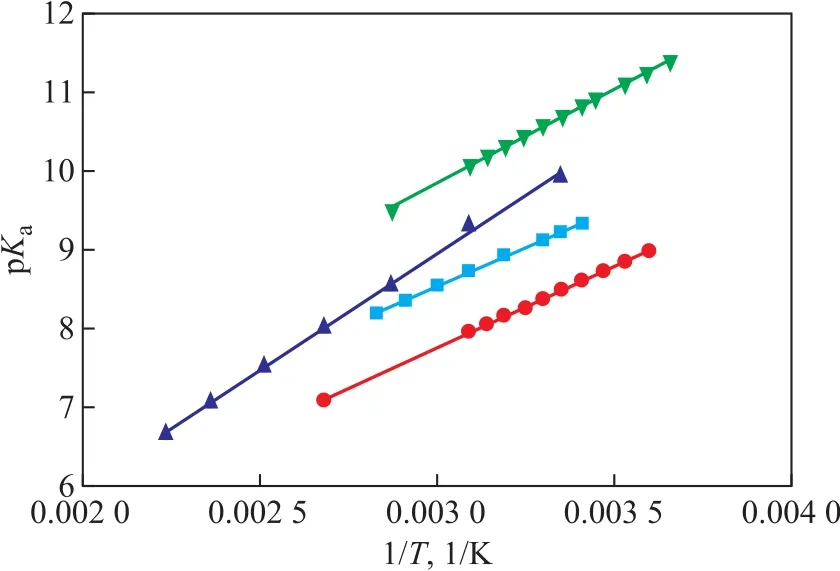
Figure 4 The pKa values of DMEA, MORP, MOPA, and TREA at different temperatures■—DMEA; ▲—MOPA; ▼—TREA; ●—MORP
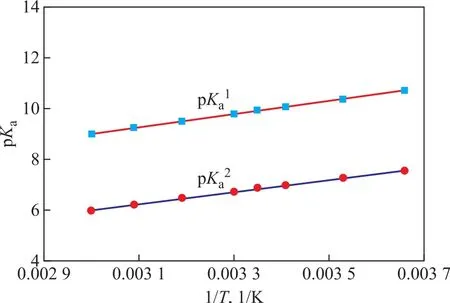
Figure 5 The first and second protonation constants of EDA in the temperature range of 273–333 K
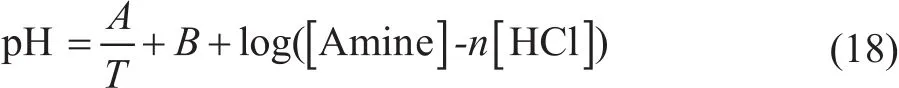
whereAandBare constants;Tis the temperature in K;n= 1 for DMEA, MOPA, MORP, and TREA;n=1/2 for EDA.
4.3 Applications of the pH model
One example of the application of the pH prediction model is shown in Table 2, which presents the efficiency of neutralizing amines for inhibiting the corrosion of overhead condensing system in the crude distillation unit.The corrosion rate of carbon steel in 150 mg/L HCl in the absence of neutralizing amines was 0.645 6 g/(m-2·h)obtained during the blank experiment. The analysis of these results clearly showed that the weight loss decreased while the inhibition efficiency increased with an increasing neutralizing amines concentration. EDA showed the maximum inhibition efficiency than other amines because of its high reactivity and high absorption rates, followed by DMEA. One hydroxyl group was attached to the DMEA molecule, which could increase the water solubility of DMEA[10], thus reducing the corrosion rates. The inhibition efficiency of TREA was the least, because it had the highest molecular weight, and also the highest activation energy. Besides, the inhibition efficiency of MORP was the minimum, which was attributed to the steric hindrance effect of cyclic structure. The use of EDA for corrosion inhibition is more economical, and DMEA as a neutralizing amine is a better choice because of its better solubility. The trend of the corrosion inhibition efficiency decreases in the following order: EDA>DMEA>TREA>MOPA>MORP.
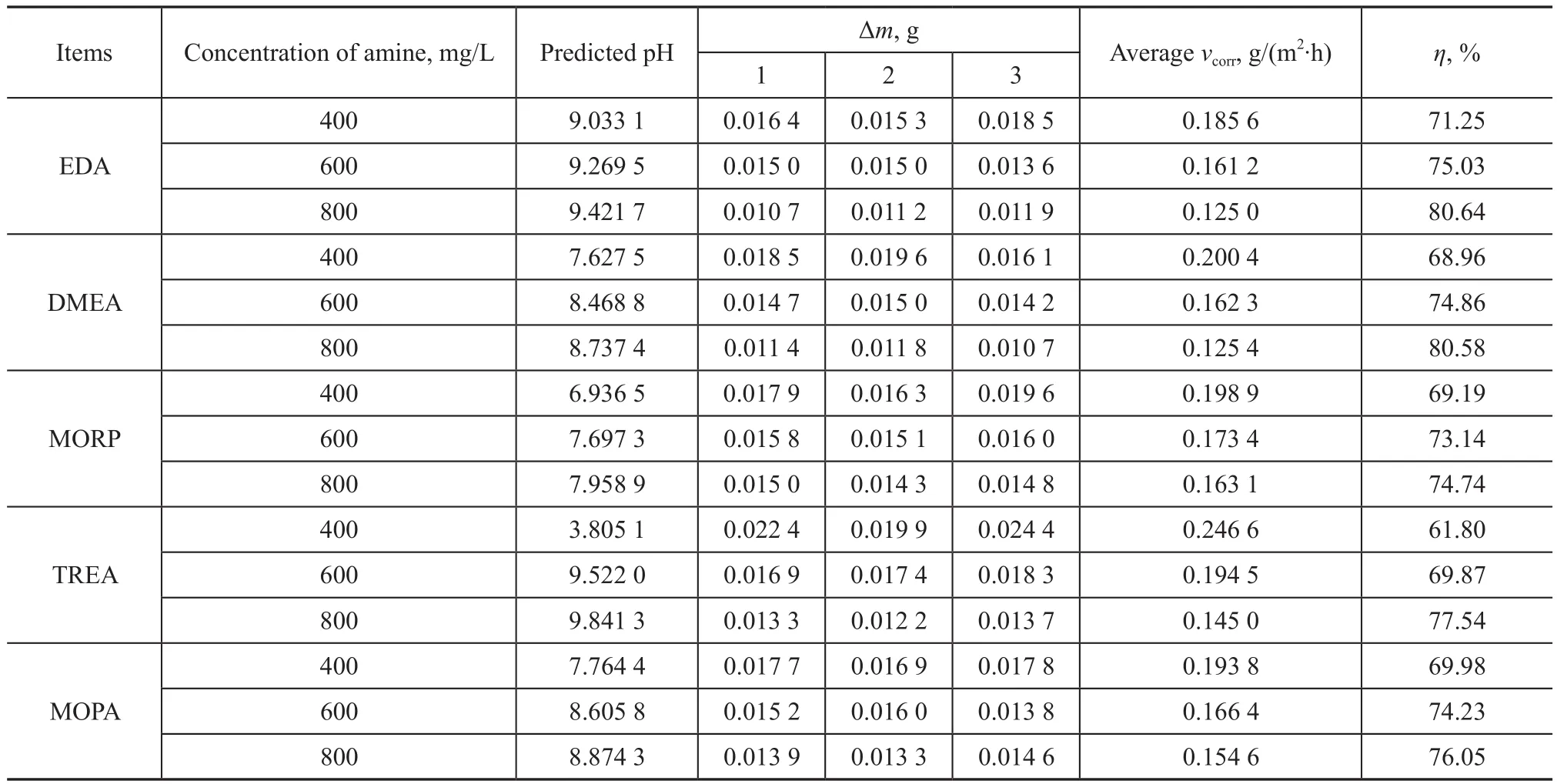
Table 2 Weight loss data for carbon steel protected by different concentrations of amine samples at 90 °C
4 Conclusions
A pH predictive model was proposed to calculate the pH value in a mixed-acid-base equilibrium system of the overhead condensing system. The predicted pH values of various neutralizing amines (DMEA, MOPA, MORP,EDA and TREA) were found to have a good fit to the experimental data. The effect of temperature on the pH value of these amine samples was investigated, and the relationship between the pH valuevs. temperature, the concentration of HCl, and the concentration of amines was established asThe pH model reflected the effect of temperature and pH at the unmeasured point of the overhead system. The trend of the corrosion inhibition efficiency decreased in the following order: EDA>DMEA>TREA>MOPA>MORP.We believe that the implementation of this pH predictive model would result in lower corrosion risk and enhance the improvements in the run length of the overhead condensing system of the crude distillation unit.
[1]Luo Y, Wang L, Wang H, Yuan X. Simultaneous optimization of heat-integrated crude oil distillation systems [J]. Chinese J Chem Eng, 2015, 23 (9): 1518-1522
[2]Chambers B, Srinivasan S, Yap K M, et al. Corrosion in crude distillation unit overhead operations: a comprehensive review [C]// NACE International,Corrosion 2011, Parper No. 11360
[3]Silva E F, Svendsen H F. Computational chemistry study of reactions, equilibrium and kinetics of chemical CO2absorption [J]. Int J Greenhouse Gas Con, 2007, 1 (2): 151-157
[4]Kumbhar A G, Narasimhan S V, Mathur P K. Copperamine speciation: An electrochemical investigation of the selection of volatile amines for steam generator water [J].Anal Chim Acta, 1994, 294 (294): 103-111
[5]Little R J, Van Swaaij W P M, Versteeg G F. Kinetics of carbon dioxide with tertiary amines in aqueous solution [J].AIChE J, 1990, 36 (11): 1633-1640
[6]Hamborg E S, Versteeg G F. Dissociation constants and thermodynamic properties of amines and alkanolamines from 293 to 353 K [J]. J Chem Eng Data, 2009, 54 (4):1318-1328
[7]Balakrishnan P V. Liquid-vapor distribution of amines and acid ionization constants of their ammonium salts in aqueous systems at high temperature [J]. J Solution Chem,1988, 17 (9): 825-840
[8]Hamborg E S, Versteeg G F. Dissociation constants and thermodynamic properties of amines and alkanolamines from 293 to 353 K [J]. Energy Procedia, 2009, 1 (1):1213-1218
[9]Ridley M K, Xiao C, Palmer D A, et al. Thermodynamic properties of the ionization of morpholine as a function of temperature and ionic strength [J]. J Chem Eng Data, 2000,45 (3): 502-507
[10]Li J, Henni A, Tontiwachwuthikul P. Reaction kinetics of CO2in aqueous ethylenediamine, ethyl ethanolamine, and diethyl monoethanolamine solutions in the temperature range of 298−313 K: Using the stopped- fl ow technique [J].Ind Eng Chem Res, 2007, 46(13): 4426-4434
杂志排行
中国炼油与石油化工的其它文章
- Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
- Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance
