Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
2018-01-19LiYunshengWenZhenhaoWeiZhenhaoYangFanZhuXuedong
Li Yunsheng; Wen Zhenhao; Wei Zhenhao; Yang Fan; Zhu Xuedong,2
(1. Engineering Research Center of Large Scale Reactor Engineering and Technology, East China University of Science and Technology, Shanghai 200237; 2. State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
1 Introduction
Para-xylene (PX), one of the most important chemical intermediates in petrochemical industry, is extensively used in the production of purified terephthalic acid (PTA). PTA is in turn used for producing polyester products, such as fibers,yarns and films. With the rapid growth of polyester industry,the conventional way to produce PX such as catalytic reforming and naphtha pyrolysis cannot meet the huge demand for PX due to the decline in crude oil resources.Finding new ways to increase the production of PX is of great commercial value[1-2]. With the improving production capacity of catalytic reforming and the increasing ethylene production capacity, benzene is gradually over-produced in China[3]. Meanwhile, the domestic methanol production capacity is also expanding dramatically, which is propped up by the development of coal chemical industry[4]. Therefore,the alkylation of benzene with methanol is a promising alternative to increase the production of PX.
Currently, the ZSM-5 zeolite showed good catalytic performance in the alkylation of benzene with methanol owing to its suitable acidity and shape selectivity characteristics. Fu[5]firstly investigated the catalytic performance of different zeolites in the alkylation of
benzene with methanol and the results showed that the Hβ zeolite exhibited lower benzene conversion and poor stability as compared with ZSM-5, while the selectivity of toluene and xylene (TX) could be significantly improved by impregnating ZSM-5 with MoO3and MgO. Gao, et al.[6]found the selectivity of TX could be increased to 90.7% via impregnating ZSM-5 with Co3O4and La2O3,but the conversion of benzene decreased. The selectivity of TX could increase to 92% by modifying Pt/ZSM-5 with HF[7]. However, the selectivity of PX in xylene did not increase although the selectivity of TX could be greatly improved by modification of the zeolite with different metals or nonmetal oxide. Few studies were focused on increasing the selectivity of PX in xylene, and it was discovered by Zhao, et al.[8]that the ZSM-5 zeolite modified by Si-P-Mg could improve the selectivity of PX in xylene by as high as 70%, but the conversion of benzene reduced to 20%.
Separating or removing ethylbenzene from the C8aromatics, an important byproduct in the alkylationof benzene with methanol, is characteristic of high energy cost because of the close boiling points between ethylbenzene and xylene. Hu, et al.[9-11]concentrated their research on decreasing the selectivity of ethylbenzene during the alkylation of benzene with methanol, and it was found that ZSM-5 modified by Pt and Pd could dramatically reduce the selectivity of ethylbenzene to 0.13% in a hydrogenation process, but the high price of Pt and Pd limits its industrial application.
The ZSM-22 zeolite with a TON topology structure was firstly synthesized by Dwyer, et al. in 1985. ZSM-22 contains one dimensional 10-member-ring channel system with a pore opening of 0.45 nm× 0.55 nm[12-13]. ZSM-22 can be used in the catalytic cracking, isomerization,alkylation, cyclization and aromatization reactions due to its special pore structure and excellent catalytic activity.It has been found that ZSM-22 showed good catalytic performance in the methanol-to-olefin and methanol-toaromatic reactions[14-16].
The ZSM-35 zeolite with a FER topology structure was fi rstly synthesized by Plank, et al. in an aqueous system in 1977[17]. ZSM-35 exhibits an intersecting channel system of 10-member-ring (0.42 nm× 0.54 nm) and 8-memberring (0.35 nm× 0.48 nm), and spherical cages (0.6 nm× 0.7 nm) are formed at the channel’s intersections[18]. ZSM-35 has been mainly used in the alkylation and isomerization reactions owing to its excellent shape selectivity characteristics and hydrothermal stability[19-20].
In this paper, the ZSM-22 and ZSM-35 zeolites were firstly synthesized via hydrothermal crystallization, and then a comparative study on the catalytic performance of ZSM-22, ZSM-35 and industrial ZSM-5 in the alkylation of benzene with methanol was performed to figure out the difference of their catalytic performance.
2 Experimental
2.1 Catalyst preparation
The ZSM-22 zeolite was synthesized under the hydrothermal condition following the procedure proposed by Chen, et al.[21]A solution was prepared by mixing KOH,Al2(SO4)3·18H2O and deionized water under stirring. Then 1,6-diaminohexane (DAH) was dissolved in the solution under stirring, followed by stirring for 2 h. Silica gel(containing 40% of SiO2) was added to the obtained clear solution under stirring. The resulting gel, having a molar composition of 60SiO2: Al2O3: 9KOH: 27DAH: 3600H2O was stirred for another 2 h. After aging at ambient temperature for 12 h, the gel was transferred into a Te flonlined steel autoclave and was rotated at a speed of 20 r/min for 48 h at 160 °C. After crystallization, the autoclave was quenched and the crystals were filtrated and washed with deionized water. The obtained samples were dried and then calcined at 550 °C for 12 h to remove the template. The calcined samples were ion exchanged with 1 mol/L NH4Cl three times and then calcined to yield the final product.
Then ZSM-35 zeolite was synthesized by referring to the synthetic method proposed by Zhang, et al.[22]Al2(SO4)3·18H2O was added into the solution composed of deionized water, NaOH and pyrrolidine (PY) under stirring.After stirring for 2 h, the silica gel (containing 40% of SiO2)was added into the solution and was again stirred for another 2 h. The resulting gel with a molar composition of 60SiO2:Al2O3: 10NaOH: 20PY: 3600H2O was subject to aging for 12 h at the ambient temperature and was then transferred into a Teflon-lined steel autoclave and was subject to crystallization at 160 °C for 72 h. The autoclave was quenched after completion of crystallization process, with the crystals being filtrated and washed with deionized water.The obtained samples were dried and then calcined at 550 °C for 12 h to remove the template. The calcined samples were ion exchanged with 1 mol/L NH4Cl three times and then calcined to yield the final product. The ZSM-5 zeolite was purchased from the Catalyst Plant of Nankai University(Tianjin, China), with the SiO2/Al2O3ratio being equal to 60.All samples were extruded with the promoter γ-Al2O3, then they were crushed and the particles with diameters in the range of 0.38-0.83 mm were screened. Finally, the screened catalysts were calcined at 550 °C for 6 h.
2.2 Catalyst characterization
X-ray diffraction (XRD) analysis was conducted by a Rigaku D/max 2550VB/PC powder diffractometer with CuKα (λ=0.154 06 nm) radiation source at 40 kV and 40 mA, operating at a scanning rate of 3(°)/min and a scanning diffraction angle ranging from 3° to 50°. Scanning electron micrographic (SEM) images were obtained on a NOVA Nano SEM450 microscope, with the samples being pretreated by platinum at the Q150R S sputter coater to enhance the effect of imaging. The N2adsorption-desorption isotherms were obtained at 77 K on a Micromeritics ASAP 2 020 analyzer, while the specific surface areas were deduced from the Brunauer-Emmett-Teller (BET) method and the micropore volumes were calculated by the t-plot method. The ammonia temperature programmed desorption(NH3-TPD) analysis was conducted by a Micromeritics ChemiSorb 2720 chemisorption instrument. A sample of 100 mg was evacuated at 550 °C for 1 h in He fl ow, and then was cooled to 150 °C and saturated with 5% NH3-He before fl ushing with He to remove the physisorbed NH3. The signal was quantified by a flame ionization detector (FID) at a heating rate of 10 °C/min.
The SiO2/Al2O3molar ratios were acquired by a Shimadzu XRF-1800 spectrometer at 40 V and 95 mA, operating at a scanning rate of 8(°)/min and a scan step length of 0.1°.The temperature programmed oxidation (TPO) in a quartz tube loaded with 100 mg of samples was carried out by a Micromeritics ChemiSorb 2720 chemisorption instrument.The samples were evacuated at 550 °C for 1 h in He fl ow, and then the samples were cooled to 250 °C for 30 min, followed by heating the samples to 800 °C in a gas fl ow consisting of 10% of O2in He gas at a heating rate of 10 °C/min.
Thermogravimetric analysis was recorded on a TherMax700 instrument. About 30 mg of the spent catalyst was preheated to 100 °C for 1 h in air fl ow, and then the temperature was increased linearly from 100 °C to 800 °C.The Raman spectra were acquired by using a Renishaw inVia Reflex instrument with a 514-nm laser excitation source. The exposure time was kept at 8 seconds to avoid oxidation. The scanning wavelength was in the range from 1 200 cm-1to 1 700 cm-1.
2.3 Catalyst evaluation
2.5 g of catalyst were mixed with an equal volume of inert quartz sand which was located in the central part of a fixedbed stainless steel reactor, 600 mm in length and 10 mm in diameter. A thermocouple was positioned in the center of the catalyst bed to monitor the temperature. The catalyst was reduced in situ with a temperature ramp from ambient temperature to 460 °C and maintained for 2 h in N2fl ow. Then a mixture of benzene and methanol was fed into the reactor with a N2flow rate of 50 mL/min (under conditions covering a B/M molar ratio of 1:1, a WHSV of 3.0 h-1, a temperature of 460 °C, and a pressure of 0.2 MPa). The products were cooled down and separated into liquid and gas effluent.The composition of liquid phase was analyzed on a GC6820 gas chromatograph, while the composition of gas effluent was determined online by a GC9160 gas chromatograph. The conversion of benzene, and the selectivity of TX, para-xylene in xylene, ethylbenzene,and C9+aromatics were used to evaluate the catalytic performance, and they are denoted as C(B), S(TX), S(PX),S(E), S(C9+), respectively, which are defined as follows:
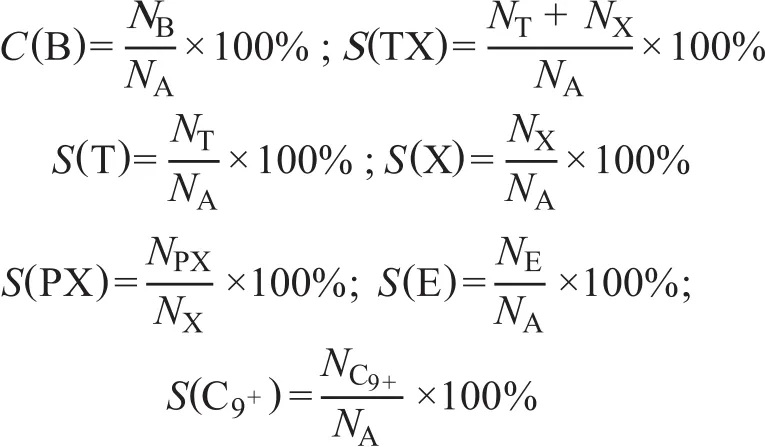
Nirepresents the molar quantity of different composition in the ef fl uent, and NArepresents the aromatics in the ef fl uent.
3 Results and Discussion
3.1 Catalyst characterization
The XRD patterns of the synthesized ZSM-22 and ZSM-35 zeolites and the industrial ZSM-5 zeolite are shown in Figure 1. Industrial ZSM-5 exhibited the typical MFI topology characteristics with peaks identified at 2θ of 7.9°, 8.8°, 23.1°, 23.9°, 24.3° and 45.2°[23]. The synthesized ZSM-22 showed the standard characteristic patterns with peaks observed at 2θ of 8.2°, 20.4°, 24.3°,24.6° and 25.7°[12], which corresponded to the structure of TON topology. The synthesized ZSM-35 exhibited characteristics of the FER topology, with the apparent diffraction peaks observed at 2θ of 9.4°, 25.1° and 25.6°[18]. No impure crystal phase was observed in these three samples and they all had high crystallinity.
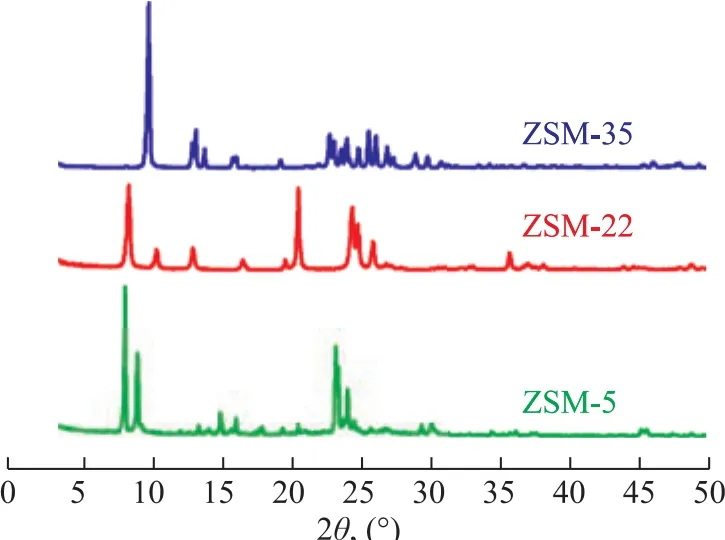
Figure 1 XRD patterns of ZSM-5, ZSM-22 and ZSM-35 samples

Figure 2 SEM images of ZSM-5, ZSM-22, and ZSM-35
The SEM images in Figure 2 show the crystal morphology of industrial ZSM-5, and the synthesized ZSM-22 and ZSM-35. The crystals of industrial ZSM-5 are hexagonalshaped crystal rods with a size of about 2 μm, and some fragments and defections can be seen on the surface.The synthesized ZSM-22 exhibited the morphology of needle-like aggregates, and the crystal length of individual needles varied from 0.5 μm to 1 μm. This morphology can contribute to molecular diffusion to inhibit coke formation.The synthesized ZSM-35 showed a laminar shape with some debris on the surface and the crystal is about 2 μm in length. All samples had good crystallinity.
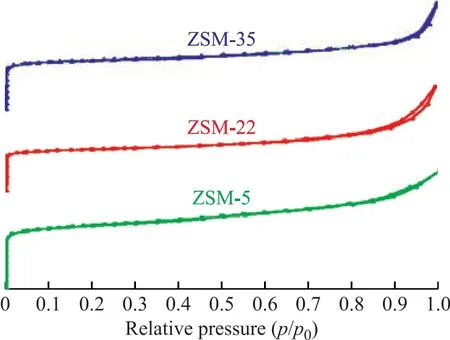
Figure 3 N2 adsorption-desorption isotherms of ZSM-5,ZSM-22 and ZSM-35
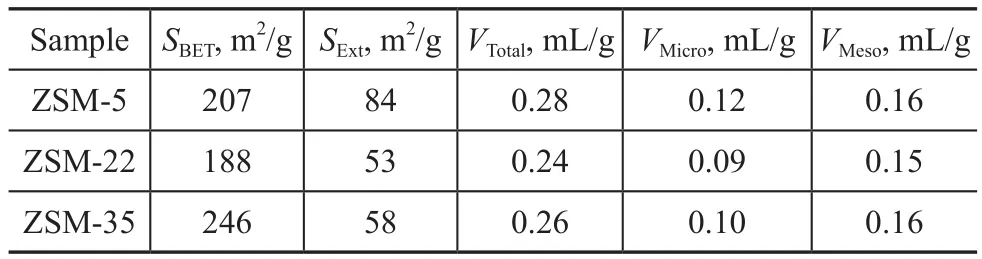
Table 1 Textural properties of ZSM-5, ZSM-22 and ZSM-35
The N2adsorption-desorption isotherms and textural properties of ZSM-5, ZSM-22 and ZSM-35 are displayed in Figure 3 and Table 1, respectively. As shown in Figure 3, all samples exhibited the characteristic features of type-I isotherm, in which the adsorption volume increased rapidly under low relative pressure and no obvious hysteresis loop associated with the micropore adsorption was observed.According to Table 1, ZSM-22 and ZSM-35 have similar external specific surface area and micropore volume, and both of which are slightly lower than those of ZSM-5.
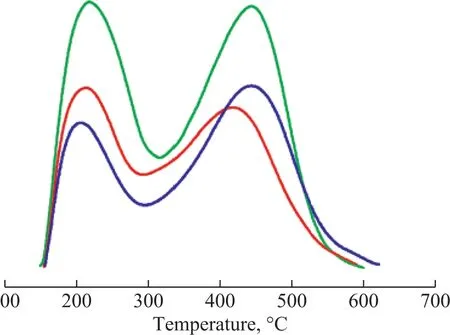
Figure 4 NH3-TPD pro fi les of ZSM-5, ZSM-22 and ZSM-35— —ZSM-35;— —ZSM-22;— —ZSM-5
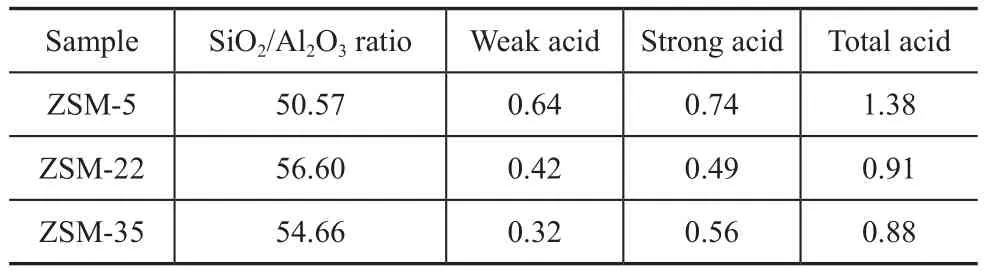
Table 2 Acid quantity and of ZSM-5, ZSM-22 and ZSM-35 mmol/g
The NH3-TPD pro files, SiO2/Al2O3ratio and acid quantity of ZSM-5, ZSM-22 and ZSM-35 are exhibited in Figure 4 and Table 2. Two peaks of NH3desorption were presented in the NH3-TPD pro files of the three samples representing the weak and strong acid sites, respectively. It can be seen that the weak and the strong acid sites of ZSM-22 are both weaker than those of ZSM-5, and the weak acid sites of ZSM-35 are weaker than those of ZSM-5. Compared with ZSM-35, ZSM-22 has much more weak acid amount, less strong acid amount and similar total acid amount. The acid amount of ZSM-22 and ZSM-35 are both less than that of ZSM-5, which is attributed to their higher SiO2/Al2O3ratios. Wen, et al.[24]found that the weak acid was favorable to benzene alkylation with methanol. The existence of weaker acidity may help to improve the selectivity of TX and reduce the selectivity of ethylbenzene.
3.2 Catalytic tests
Table 3 and Table 4 show the catalytic performance of ZSM-5, ZSM-22 and ZSM-35 in alkylation of benzene with methanol, the results are the average value obtained during the stable reaction period (4 h to 12 h). Compared with ZSM-5, the selectivity of TX and PX over ZSM-22 and ZSM-35 was both higher, while the selectivity of ethylbenzene and C9+ aromatics was both lower. ZSM-22 and ZSM-35 exhibited good catalytic performance. The straight 10-member-ring channel system of ZSM-22 is conducive to the diffusion of methanol and other products, so the side reaction of methanol to ethylene is inhibited[25]. It has been shown that coke species could be formed near the pore mouths in the MTO reaction[26].The mechanism of alkylation of benzene with methanol is similar to the mechanism of MTO[27], so it can be deduced that coke species formed near the pore mouth of ZSM-22 could narrow the pore size, and increase the selectivity of TX and the selectivity of PX during the activation stage of benzene alkylation with methanol. ZSM-35 contains two straight orthogonal channels and cages in the intersections[18]. During the activation stage of alkylation of benzene with methanol, coke species formed in the cages narrowed the channels, so the selectivity of TX and the selectivity of PX were improved. Meanwhile, the straight channels have better diffusion ability so that the side reaction of methanol to ethylene is suppressed, and the ethylbenzene species formed in benzene alkylation are decreased. Because of the close boiling points between ethylbenzene and xylene, the separation between them is quite difficult coupled with high energy consumption.The reduction of ethylbenzene content in the effluent is propitious to the industrial application of benzene alkylation with methanol.
All three samples were deactivated and the conversion of benzene decreased to about 10% after having been subjected to alkylation reaction for 24 h. The cokedeposited on the catalysts is characterized by TPO, TG and Raman spectrometry.
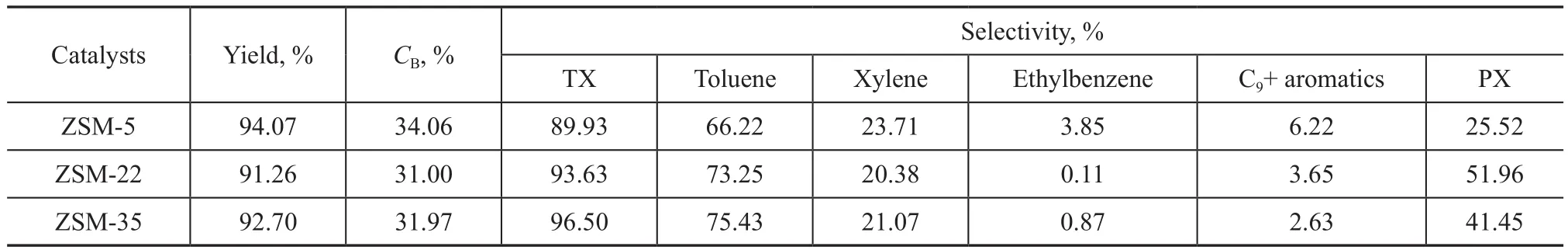
Table 3 Catalytic performance of three different catalyst samples in the alkylation of benzene with methanol (liquid phase)
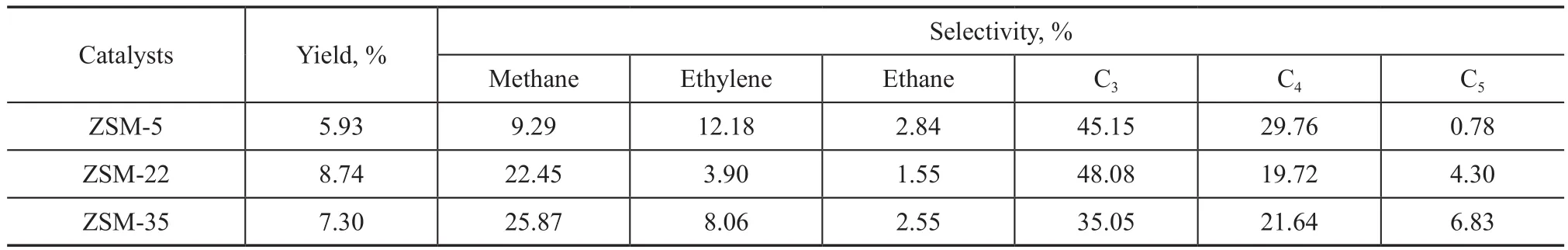
Table 4 Catalytic performance of three different catalyst samples in the alkylation of benzene with methanol (gas phase)
Figure 5 and Table 5 present the TPO profiles related to combustion of coke deposited on the deactivated catalysts. Castano, et al.[28-29]found that the different combustion peaks corresponded to various coke location.Only one peak at about 515 °C appeared for ZSM-22,indicating to the uniform coke deposition in the pore mouth. ZSM-5 and ZSM-35 both had a combustion peak identified approximately at 535 °C, which might be related to the same coke deposit location. Kim, et al.[30]found that coke deposition of ZSM-5 mainly occurred in the channels, so coke deposition of ZSM-35 also occurred in the channels. Furthermore, a combustion peak was observed approximately at 635 °C over ZSM-35, which might correspond to coke deposition in the cages. The increased selectivity for TX and PX over ZSM-22 and ZSM-35 was attributed to their different coke deposit locations as compared to ZSM-5. Table 5 shows the areas of combustion peaks of three spent catalysts. When the relative area of combustion peak of ZSM-5 is labeled as 100%, it can be calculated that the relative area of combustion peaks of ZSM-22 and ZSM-35 is 84% and 85%, respectively.The TG curves of deactivated catalysts are shown in Figure 6. The first weight loss below 250 °C is mainly caused by the physical desorption of reactants and products. The second weight loss is observed between 400 °C and 750 °C, and the weightlessness rate of ZSM-5, ZSM-22 and ZSM-35 is 18.16%, 14.31%and 15.36%, respectively. The weight loss per hour is defined as the average rate of coke deposition on the catalyst. The average rate of coke deposition for ZSM-5,ZSM-22 and ZSM-35 is 0.76%, 0.60% and 0.64% per hour, respectively. It is clear that ZSM-22 and ZSM-35 have better stability than ZMS-5. This phenomenon is attributed to their lower acidity and good diffusion ability of straight channels.
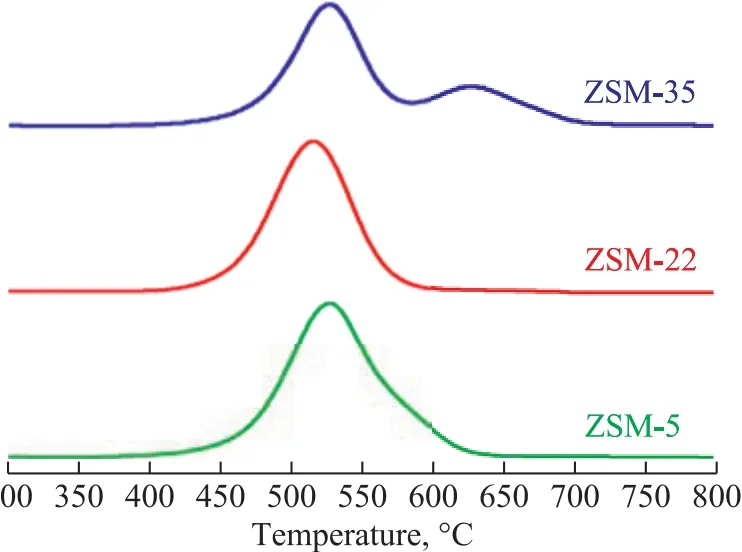
Figure 5 TPO profiles of coke combustion peak of deactivated ZSM-5, ZSM-22 and ZSM-35
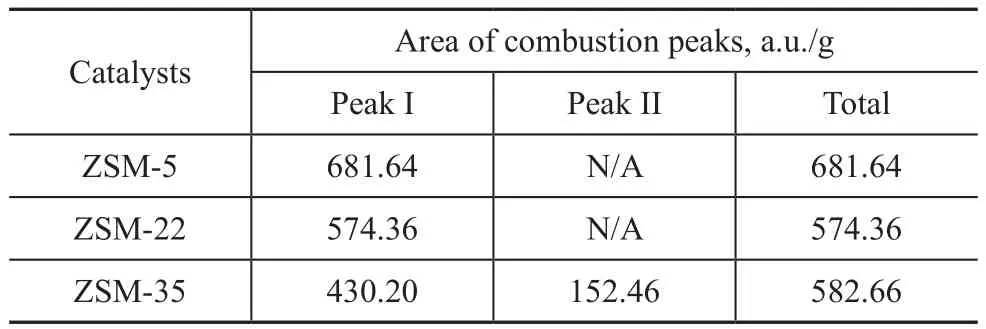
Table 5 The areas of combustion peaks of deactivated ZSM-5, ZSM-22 and ZSM-35
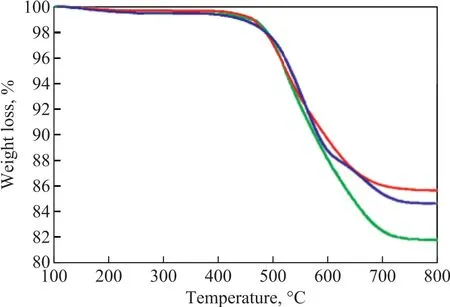
Figure 6 TG curves of the deactivated catalysts— —ZSM-35;— —ZSM-22;— —ZSM-5
Figure 7 exhibits the Raman spectra corresponding to the deactivated ZSM-5, ZSM-22 and ZSM-35. The spectra have been deconvoluted into 5 Lorentzian peaks.ThevC-Hpeak at 1 300 cm-1was assigned to the C-H vibration[31]. The D1 peak at 1 380 cm-1and the D3 peak at 1 430—1 500 cm-1were attributed to the vibrational mode of not-well-structured aromatics and structural defects of aromatic clusters, respectively[32-33]. The G peak at 1 540—1 570 cm-1was assigned to the in-plane bondstretching vibration of well-structured aromatics[34-35]and the D2 peak at 1 605 cm-1was caused by disordered aromatic structures[36]. Table 6 shows the intensity of the Raman bands corresponding to the deactivated catalysts after the normalization process. The high ratio ofID1/IGcorresponded to the low degree of graphitization. ZSM-22 and ZSM-35 both had a lower degree of graphitization than ZSM-5 due to their proper acidity and good diffusion characteristics.
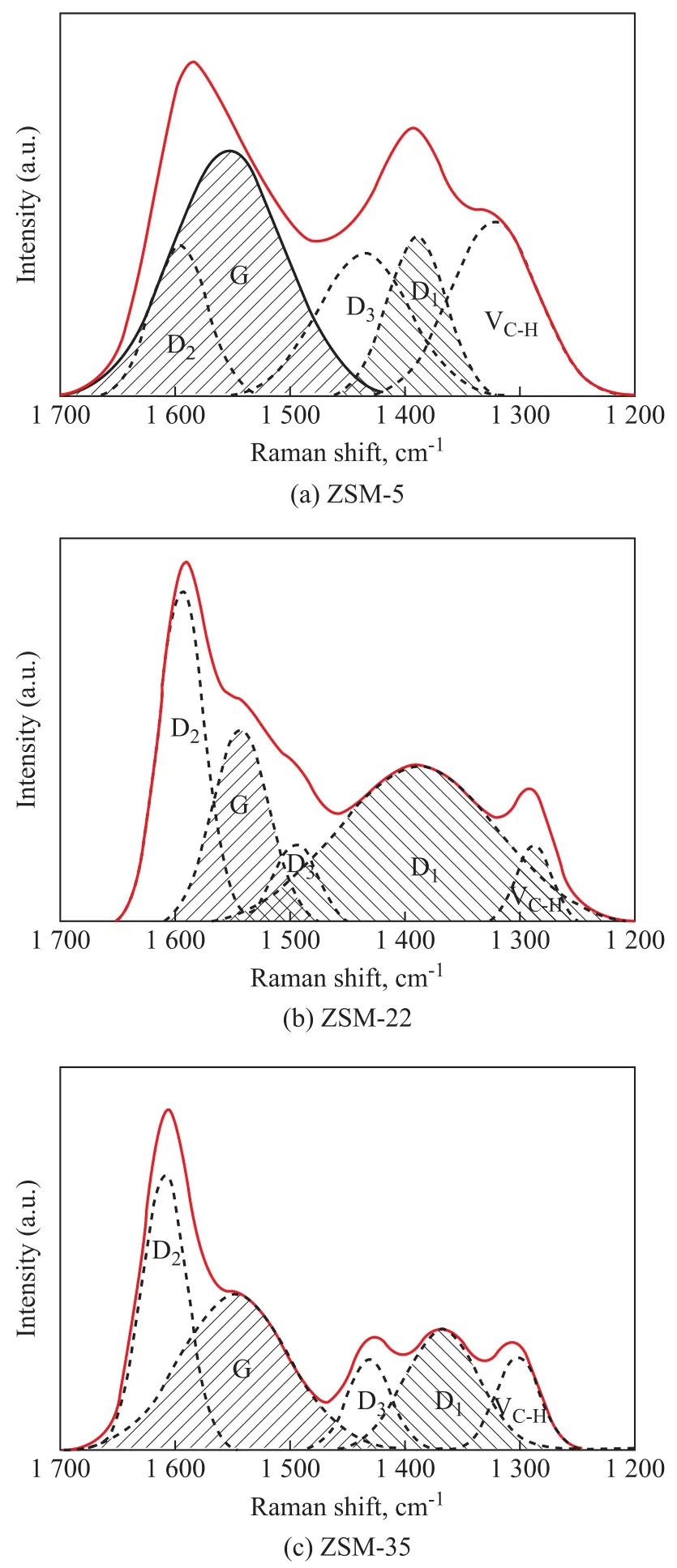
Figure 7 Raman spectra of the deactivated catalysts ((a)ZSM-5, (b) ZSM-22, (c) ZSM-35)
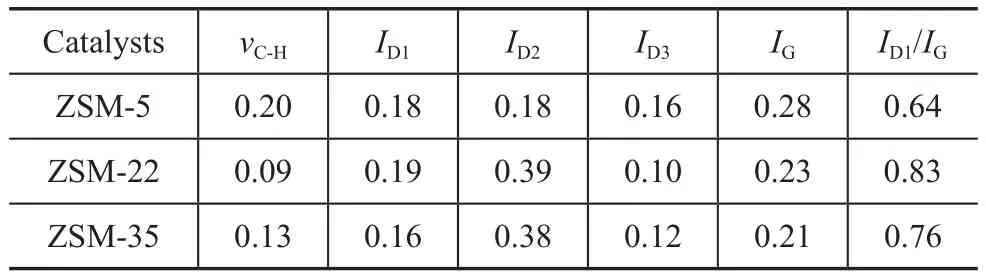
Table 6 Intensity of the Raman bands corresponding to the deactivated catalysts after normalization process
4 Conclusions
ZSM-22 and ZSM-35 synthesized by the hydrothermal crystallization method showed similar characteristics in external specific surface area, microporous volume and acidity. In the alkylation of benzene with methanol,the two samples exhibited different coke location, coke deposition rate and degree of graphitization.
Compared with the industrial ZSM-5, the selectivity of TX and PX was higher for the alkylation of benzene with methanol over ZSM-22 and ZSM-35, which was attributed to their different coke deposition during the activation stage. Both the coke formed near the pore mouth of ZSM-22 and the coke formed in the cages of ZSM-35 narrowed the channels, which improved the selectivity of TX and the selectivity of PX.
In the alkylation of benzene with methanol, the selectivity of ethylbenzene and C9+aromatics over ZSM-22 and ZSM-35 was lower than those over ZSM-5. On the one hand, this fact was attributed to the lower acidity of ZSM-22 and ZSM-35 in comparison with that of ZSM-5,which inhibited the formation of heavy aromatics. On the other hand, the straight channels of ZSM-22 and ZSM-35 exhibited better diffusibility than sinusoidal channels of ZSM-5, and the rapid diffusion of reactants and products also inhibited the coke deposition.
Acknowledgement: We gratefully acknowledge the financial support from the National Natural Science Foundation of China(No. 2177061270).
[1]Lee S M, Choi W J , Hwang K, et al. Effect of catalyst concentration and reaction time on one-step synthesized hypercrosslinked polyxylene[J]. Macromolecular Research,2014, 22(5): 481-486
[2]Pflug Kai. Challenges and opportunities for commodity chemicals in China[J]. China Chemical Reporter, 2016,27(21): 15-16
[3]Wang X, Xu J, Qi G, et al. Alkylation of benzene with methane over ZnZSM-5 zeolites studied with solid-state NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2013, 117(8): 4018-4023
[4]Li Min. Methanol Market in China in 2015[J]. China Chemical Reporter, 2016, 27(9): 16-17
[5]Fu Peng. Preparation and catalytic properties of catalysts for shape-selective alkylation of benzene with methanol for toluene andp-xylene[D]. Shanghai East China University of Science and Technology, 2010
[6]Gao Ke, Li Shuzhen, Wang Lei, et al. Study of the alkylation of benzene with methanol for the selective formation of toluene and xylene over Co3O4–La2O3/ZSM-5[J]. RSC Advances, 2015, 5(56): 45098-45105
[7]Liu Si, Wen Zhenhao, Yang Daqiang, et al. Application of HF modified Pt/ZSM-5 catalyst for benzene methylation with methanol[J]. Chinese Journal of Applied Chemistry,2016, 33(5): 571-576
[8]Zhao Bo, Liu Min, Tan Wei, et al. The effect of Si-PMg modification on alkylation of benzene with methanol over nano-scale HZSM-5 zeolite[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2013, 29(4): 605-611 (IN Chinese)
[9]Hu Hualei, Zhang Qunfeng, Cen Jie, et al. Catalytic activity of Pt modified hierarchical ZSM-5 catalysts in benzene alkylation with methanol[J]. Catalysis Letters,2014, 145(2): 715-722
[10]Hu Hualei, Zhang Qunfeng, Cen Jie, et al. High suppression of the formation of ethylbenzene in benzene alkylation with methanol over ZSM-5 catalyst modified by platinum[J]. Catalysis Communications, 2014, 57: 129-133
[11]Hu Hualei, Lyu Jinghui, Cen Jie, et al. Promoting effects of MgO and Pd modification on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol[J]. RSC Advance, 2015, 5(77):63044-63049
[12]Kokotailo G T, Schlenker J L, Dwyer F G, et al. The framework topology of ZSM-22: A high silica zeolite[J].Zeolites. 1985, 5(6): 349-351
[13]Anas Karrar Jamil, Oki Muraza. Facile control of nanosized ZSM-22 crystals using dynamic crystallization technique[J]. Microporous and Mesoporous Materials,2016, 227: 16-22
[14]Chen Lei, Lu Peng, Yuan Yangyang, et al. Hydrothermal synthesis of nanosized ZSM-22 and their use in the catalytic conversion of methanol[J]. Chinese Journal of Catalysis, 2016, 37(8): 1381-1388
[15]Dyballa M, Becker P, Trefz D, et al. Parameters in fl uencing the selectivity to propene in the MTO conversion on 10-ring zeolites: directly synthesized zeolites ZSM-5, ZSM-11, and ZSM-22[J]. Applied Catalysis A: General, 2016,510: 233-243
[16]Dyballa M, Obenaus U, Rosenberger M, et al. Postsynthetic improvement of H-ZSM-22 zeolites for the methanol-to-olefin conversion[J]. Microporous and Mesoporous Materials, 2016, 233: 26-30
[17]Plank C J, Rosinski E J, Rubin M K. Crystalline zeolite and method of preparing same: The United States,US4016245[P]. 1977, 4: 5
[18]Chen X, Todorova T, Vimont A, et al. In situ and postsynthesis control of physicochemical properties of FER-type crystals[J]. Microporous and Mesoporous Materials,2014, 200: 334-342
[19]Houzvicka J, Ponec V. Skeletal isomerisation of n-butene on phosphorus containing catalysts[J]. Applied Catalysis A:General, 1996, 145(1): 95-109
[20]Byggningsbacka R, Kumar N, Lindfors L E. Comparative study of the catalytic properties of ZSM-22 and ZSM-35/ferrierite zeolites in the skeletal isomerization of 1-butene[J]. Journal of Catalysis, 1998, 178(2): 611-620
[21]Chen L, Lu P, Yuan Y, et al. Hydrothermal synthesis of nanosized ZSM-22 and their use in the catalytic conversion of methanol[J]. Chinese Journal of Catalysis, 2016, 37(8):1381-1388
[22]Zhang M, Chen Y, Wang L, et al. Shape selectivity in hydroisomerization of hexadecane over Pt supported on 10-ring zeolites: ZSM-22, ZSM-23, ZSM-35, and ZSM-48[J]. Industrial & Engineering Chemistry Research, 2016,55(21): 6069-6078
[23]Moliner M, González J, Portilla M T, et al. A new aluminosilicate molecular sieve with a system of pores between those of ZSM-5 and beta zeolite[J]. Journal of the American Chemical Society, 2011, 133(24): 9497-9505
[24]Wen Zhenhao. Catalysts and reaction mechanism investigation for benzene methylation with methanol[D].Shanghai: East China University of Science and Technology, 2016
[25]Adebajo M O, Long M A. The contribution of the methanol-to-aromatics reaction to benzene methylation over ZSM-5 catalysts[J]. Catalysis Communications, 2003,4(2): 71-76
[26]Wei F F, Cui Z M, Meng X J, et al. Origin of the low ole fi n production over HZSM-22 and HZSM-23 zeolites: external acid sites and pore mouth catalysis[J]. ACS Catalysis,2014, 4(2): 529-534
[27]Wen Zhenhao, Yang Daqiang, Zhu Xuedong. Alkylation of benzene and methanol and research progress of its catalysts[J]. Modern Chemical Industry, 2017, 37(1): 41-44
[28]Castano P, Elordi G, Ibanez M, et al. Pathways of coke formation on an MFI catalyst during the cracking of waste polyolefins[J]. Catalysis Science & Technology, 2012,2(3): 504-508
[29]Ibáñez M, Valle B, Bilbao J, et al. Effect of operating conditions on the coke nature and HZSM-5 catalysts deactivation in the transformation of crude bio-oil into hydrocarbons[J]. Catalysis Today, 2012, 195(1): 106-113
[30]Kim J, Choi M, Ryoo R. Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanolto-hydrocarbon conversion process[J]. Journal of Catalysis,2010, 269(1): 219-228
[31]Park J W, Seo G. IR study on methanol-to-olefin reaction over zeolites with different pore structures and acidities[J].Applied Catalysis A: General, 2009, 356(2): 180-188
[32]Guichard B, Roy-Auberger M, Devers E, et al.Characterization of aged hydrotreating catalysts. Part I: Coke depositions, study on the chemical nature and environment[J]. Applied Catalysis A: General, 2009,367(1): 1-8
[33]Castaño P, Elordi G, Olazar M, et al. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene[J]. Applied Catalysis B:Environmental, 2011, 104(1): 91-100
[34]Vogelaar B M, van Langeveld A D, Eijsbouts S, et al.Analysis of coke deposition pro files in commercial spent hydroprocessing catalysts using Raman spectroscopy[J].Fuel, 2007, 86(7): 1122-1129.
[35]Robertson J. Diamond-like amorphous carbon[J]. Materials Science and Engineering: Reports, 2002, 37(4): 129-281
[36]Wragg D S, Johnsen R E, Balasundaram M, et al. SAPO-34 methanol-to-olefin catalysts under working conditions:A combined in situ powder X-ray diffraction, mass spectrometry and Raman study[J]. Journal of Catalysis,2009, 268(2): 290-296
杂志排行
中国炼油与石油化工的其它文章
- Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
- Research Progress in Catalytic Cracking Reaction of Tetralin and Decalin
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance
