Degradation of Nitrobenzene Wastewater via Iron/Carbon Micro-electrolysis Enhanced by Ultrasound Coupled with Hydrogen Peroxide
2018-01-19QinYuejiaoYuLishengLuoShuaiJiaoWeizhouLiuYouzhi
Qin Yuejiao; Yu Lisheng; Luo Shuai; Jiao Weizhou; Liu Youzhi
(1. Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering, North University of China,Taiyuan 030051; 2. Department of Civil and Environmental Engineering, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, USA)
1 Introduction
Nitrobenzene (NB) is mainly originated from chemical process, petrochemical, pharmaceutical, explosive and dyestuff industries[1], which has been included in the list of “World’s Toxic Organic Pollutants Under Priority Environmental Control”[2]. Nowadays, wastewater contaminated with ordinary biodegradable organics can be treated successfully through several biological treatment processes[3]or chemical catalysis processes[4-5]. However,NB with its strong electron-withdrawing effect of nitro group can reduce the electron density of benzene and lead to a stable chemical property, making itself difficult to be degraded by a stand-alone chemical method (e. g.the Fenton oxidation, ozonation, etc.) or other biological methods (e. g. activated sludge, aerobic/anaerobic bacteria, etc.)[6-7]. When the nitro group becomes amino group, the benzene ring can be activated to facilitate the chemical and biological degradation process. Therefore,a specific multiple-element method that combines the reduction of NB to aniline (AN) with the mineralization of AN to CO2and H2O is a promising alternative to degrade NB. Many studies have shown that the microelectrolysis of the zero valent iron/granular active carbon(ZVI/GAC) is an efficient and cost-effective method for achieving the reduction process, which can continuously convey electrons from ZVI to the organic molecules through forming a large number of primary batteries atthe microscale between ZVI and GAC[8-9]. Meanwhile,ZVI is readily oxidized to ferrous iron ion, Fe2+, in an aqueous system. Thus, ZVI/GAC micro-electrolysis can effectively transform NB into AN to activate the benzene ring, leading to enhancing the further degradation to CO2and H2O[10]. It is pleasantly surprised that the byproduct Fe2+produced by ZVI/GAC micro-electrolysis is an indispensable catalyst for the Fenton oxidation process.Hydrogen peroxide (H2O2) is decomposed catalytically by Fe2+to O2and H2O rapidly with a remarkable yield of hydroxyl radicals, which can fi nally degrade the organic pollutants in wastewater[11-12]. However, with a longer operating time of the ZVI/GAC micro-electrolysis, the surface of ZVI and GAC tends to be covered by some sediments such as iron oxides (Fe2O3and Fe3O4)[13]. The sediments will hinder the electron transfer process of ZVI/GAC micro-electrolysis, and lead to the passivation and inactivation of ZVI and GAC[14]. Therefore, the loss of reactivity over time due to the corrosion products or other precipitates on the iron surface is a great concern[15].Exploring suitable strategies to regenerate the surface reactive sites in situ and significantly improve the efficiency of micro-electrolysis is urgently needed.
Studies have shown that ultrasound (US) can assist ZVI powder in increasing the degradation efficiency of organic pollutants[16-18], and the mechanism follows several aspects: (1) US can effectively remove the oxide layer on the surface of ZVI powder, and continuously recover the reactive sites on the surface of ZVI[19]; (2) The cavitation effect of US generated in the liquid can cause the liquid turbulence, which enhances the mass transfer and accelerates the reaction rate[20]; (3) A certain amount of ·OH radicals could be generated through the cavitation effect of US, which would enhance the reactivity of ZVI through rapid corrosion of ZVI and continuous activation of passive layers[21-22].
This study demonstrated a method for deep degradation of NB wastewater that combined the US-ZVI/GAC micro-electrolysis with the H2O2involved oxidation. The surface reactive sites of ZVI and GAC were continuously regenerated in situ by US and thereby the removal of NB by the micro-electrolysis was efficiently improved.In addition, H2O2solution was added in the subsequent process which could be catalysed by the byproduct Fe2+ions to further degrade the organic pollutants in the wastewater. The specific objectives were: (i) to assess the potential utility of NB reduction by ZVI/GAC microelectrolysis coupled with US, (ii) to gain an insight into the role that US might play in the degradation process,and (iii) to deepen our understanding of the mechanism for degradation of NB in the aqueous system.
2 Experimental
2.1 Chemicals
NB was purchased from the Tianli Chemical Reagent Co. (Tianjin, China) and dissolved in deionized water to the required concentration of 300 mg/L. ZVI with an average particle size of 2.5—4.0 mm was derived from the machining scrap waste in the Shanxi Province Key Laboratory of Higee-Oriented Chemical Engineering(China). To remove the oil film and oxide layer on the material surface, ZVI was soaked in a 10% NaOH solution for 30 min, and then soaked with 10% H2SO4solution for 30 min and finally washed with deionized water to attain a neutral state and was dried off in a vacuum oven.GAC with an average particle size at 1.25—2.5 mm was purchased from the Beichen Fangzheng Reagent Factory(Tianjin, China). GAC was submerged in the NB solution to absorb NB until a saturated concentration of NB was reached. Then, the GAC particles were added into 300 mg/L of NB solution and treated under US repeatedly,until the NB adsorption on GAC was saturated. All the other chemical reagents employed in this study were at least of analytic reagent grade and used without further purification.
2.2 Experimental procedures
The degradation of NB by US-ZVI/GAC was carried out in an ultrasonic generator (SB-3200D) and the setup is schematically shown in Figure 1. A 250-mL conical fl ask,filled with 100 mL of NB solution with a concentration of 300 mg/L, was fixed in the setup (Figure 1). The pH value of NB solution was adjusted by adding diluted H2SO4or NaOH solution. Then, an appropriate amount of activated carbon and iron was added into the conical fl ask. At the designated time interval, the samples to be analysed were withdrawn off with a syringe. After iron/carbon microelectrolysis reaction, the ZVI and GAC were separated by filtering under vacuum, and then a 30% H2O2solution was added to the filtrate in the subsequent process for mineralization of the organic pollutants. During the experiment, the reactor was immersed in a temperaturecontrolled water bath to maintain the temperature at 20 °C.An agitator in the middle of the reactor could ensure that the solution was homogeneous.
2.3 Analysis
The concentration of unreacted substrate and its various reaction products were measured using a high performance liquid chromatography (Ultimate 3000 HPLC, Thermo Fisher, USA) with a C18reverse phase column (4.6 mm × 250 mm ×5 μm). The programs were carried out with a mobile phase composed of methanol and water at a fixed volume ratio of 70/30,operating at a flow rate of 0.9 mL/min, a column temperature of 20 °C, and an injection volume of 20 μL.The total organic carbon (TOC) was determined on an OI 1030 TOC analyzer using the non-purgeable organic carbon (NPOC) method. An ion chromatograph(ICS-1100, Dionex, USA) was used to analyse the concentration of Fe2+and Fe3+species. The efficiency (η)for removal of NB (or TOC) is defined as:

where C0is the initial NB (or TOC) concentration and Ctis the NB (or TOC) concentration at time t. The surface structure of iron and carbon particles were analysed by scanning electron microscopy-energy dispersive X-rays analysis (SEM-EDS) (JEOL-7500F, JEOL, Japan). The intermediates of mineralization process were analysed by a gas chromatograph coupled with a mass spectrometry detector (GC/MS, 7890B-5977A, Agilent Technologies,USA). The dimension of the capillary column was set as 30 m×0.25 mm×0.25 mm (HP-5MS). The temperature program started at 40 °C (held for 2 min) and increased to 280 °C (held for 2 min) at a temperature increase rate of 5°C/min. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The samples (300 mL) were extracted by dichloromethane (100 mL, chromatographic pure grade, Fisher, U.S.A.) twice, and then the extracted liquid was concentrated under a fl owing nitrogen gas to 1 mL before GC/MS analysis.
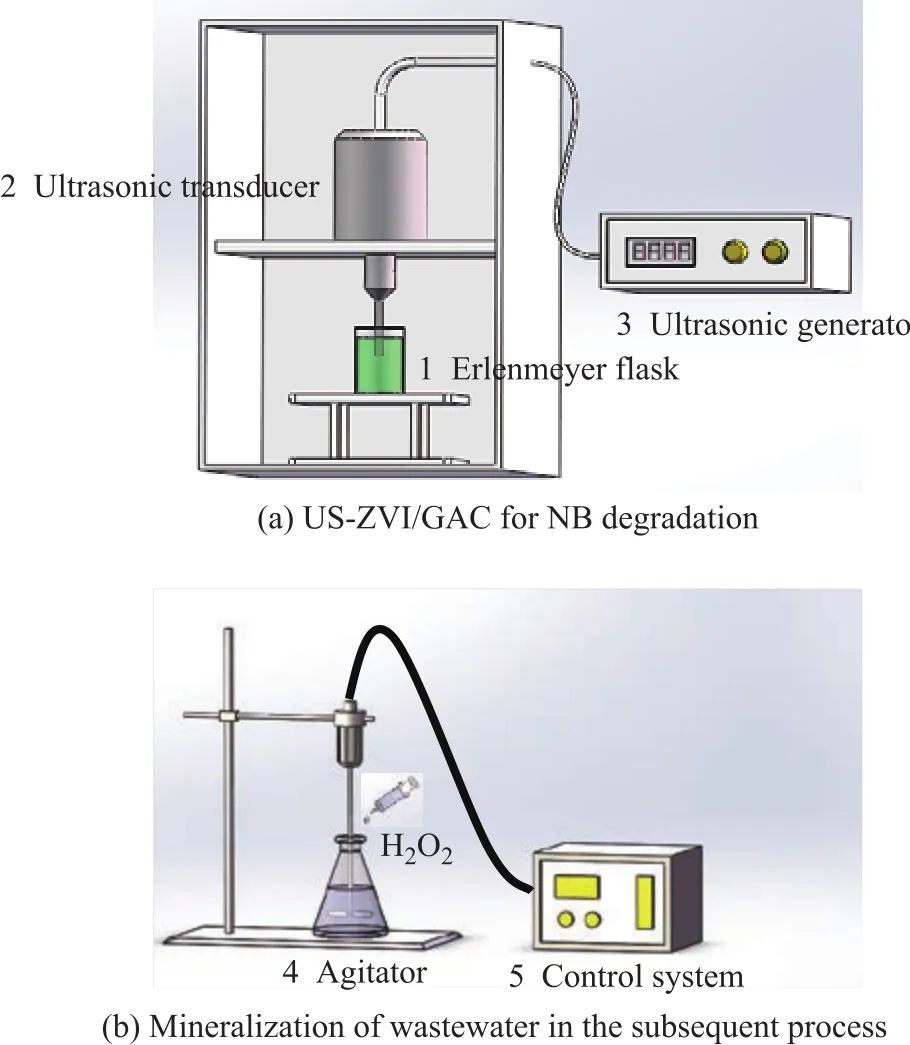
Figure 1 Schematic diagram of the experimental setup
3 Results and Discussion
3.1 Comparison experiments
Batch experiments were conducted to investigate the degradation efficiency of NB by US, ZVI/GAC and a combination of US and ZVI/GAC, respectively. The results showed that lower NB degradation was observed by US or ZVI/GAC alone as compared to the combined US-ZVI/GAC system. As shown in Figure 2, an 11.56%removal of NB was observed in the US system after 80 min of reaction, and the NB removal was realized due to the generated ·OH by virtue of the cavitation effect of US,which could degrade the organic pollutants in wastewater.In the ZVI/GAC degradation experiment, a 45.17% of NB removal was observed, while an 84.5% of NB degradation was achieved in the US-ZVI/GAC system. The results indicated that the NB decomposition efficiency in the USZVI/GAC system was much higher than those in US or ZVI/GAC alone, and thus there might be a synergistic effect between the US and ZVI/GAC micro-electrolysis for the NB degradation.
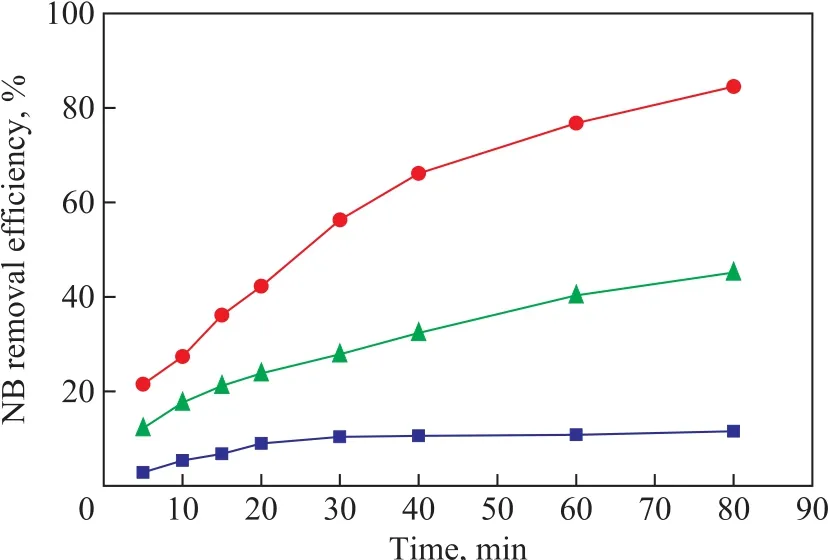
Figure 2 Effect of US, ZVI/GAC and US-ZVI/GAC on thedegradation of NB
The surface morphology and elemental analyses of ZVI and GAC were examined by SEM-EDS before and after the entire reaction process, as shown in Figure 3. The SEM photographs showed that ZVI had cleaner surface after US treatment, and its surface had many cracked layers overlapping together (Figure 3 (b1)). Otherwise,there was a layer of sediments covering the ZVI surface after ZVI/GAC reaction without US treatment (Figure 3(c1)). It is clearly demonstrated from EDS spectra that the iron content of ZVI surface was by about 20% higher in US-ZVI/GAC as compared with ZVI/GAC, while the other elements such as oxygen are lower in US-ZVI/GAC(Figure 3 (a2) - (c2)). The carbon content of GAC was always maintained at a high level during the experiments(Figure 3 (d2) - (f2)). Therefore, US had a prominent competence to clean the surface of ZVI and improve the iron content in the ZVI/GAC to maintain their active sites on the material surface needed in the long-term application.
In addition, it was found that the liquid was black and turbid with many small suspended particles after US-ZVI/GAC treatment. In contrast, the liquid characteristic had slightly changed in the sole ZVI/GAC micro-electrolysis.The characteristic comparison indicated that US could break up the solid particles into the liquid, due to its strong energy to recover more reactive sites on the material surface of ZVI/GAC for NB reduction. Furthermore, the cavitation effect of US could lead to strong micro-jet to burst onto the material surface when cavitation bubbles collapsed, which finally caused violent turbulence of the fluid flow. The turbulent flow could improve the collision probability of ZVI and GAC to accelerate the electron transfer and electrode reaction. Furthermore, on the other hand, the liquid turbulence could strengthen the fluidized state. As a result, the mass transfer of reactants, products or intermediates between the bulk solution and the solid surface could be significantly enhanced in the US system,which could finally accelerate the reaction rate[23].
3.2 Effect of operating conditions
3.2.1 Effect of the ZVI dosage on NB degradation efficiency
The effect of ZVI dosage on NB degradation efficiency of the US-ZVI/GAC system was evaluated by conducting experiments using different ZVI dosages (0—30 g/L).The removal of NB was remarkable as shown in Figure 4. The efficiency for removal of NB increased as the ZVI dosage increased from 5 g/L to 20 g/L, and decreased as the ZVI dosage further increased to an amount exceeding 20 g/L. Meanwhile, the generated AN concentration reached its peak value at a ZVI dosage of 20 g/L.
With the increase of ZVI dosage, the effective amount of the iron-carbon micro-cells also increased, which could finally improve the yield of [H](caused by a “H+” to accept an electron) in the unit time, and accelerate the process of NB reduction. In addition, the NB reduction is limited by the effective surface area of iron and carbon due to its limited surface reaction[24]. The increase of ZVI dosage could increase the effective contact area of iron and carbon, and finally increase the reaction rate.However, when the ZVI dosage is excessive, a serious ZVI accumulation will occur inside the mass contact(between iron and iron, carbon and carbon, or carbon and iron), leading to the reduction of effective active sites on material surface, and finally weakening the reaction rate.Compared with the efficiency for removal of NB with 15 g/L of iron dosage, there was not a significant increase of removal efficiency with 20 g/L of iron dosage. To save the operating cost with less chemical usage to facilitate the system to scale up for future application, an iron dosage of 15 g/L was chosen for further study.

Figure 3 SEM images and EDS spectra of ZVI and GAC
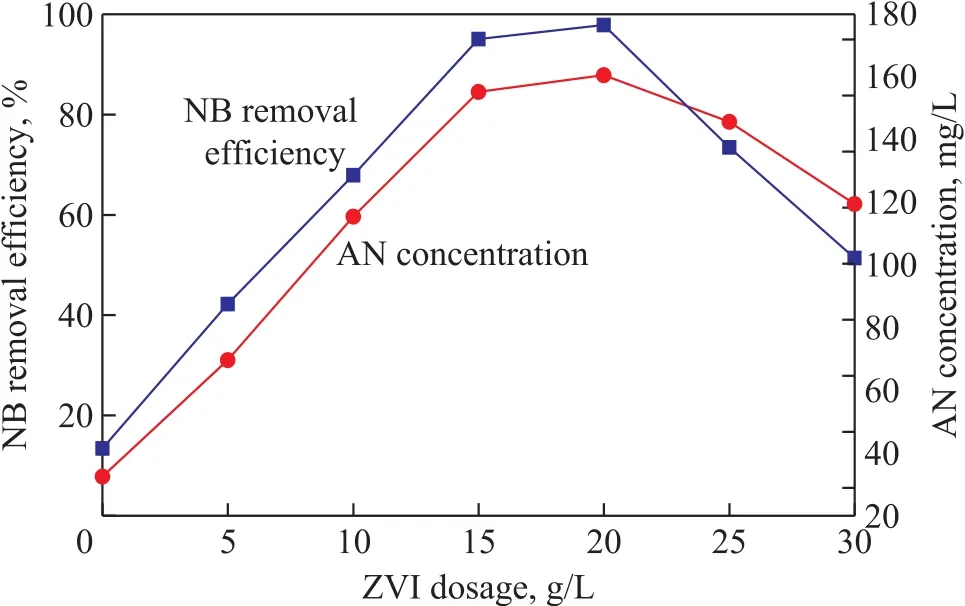
Figure 4 Effect of ZVI/GAC dosage on removal efficiency of NB
3.2.2 ZVI/GAC mass ratio on NB degradation efficiency In order to investigate the effect of ZVI/GAC mass ratio on NB degradation in the US-ZVI/GAC system, five ZVI/GAC mass ratios (viz. 3:1, 2:1, 1:1, 1:2, 1:3, and 1:4)were chosen to test the NB removal efficiency. During all the experiments, the ZVI dosage was fixed at 15 g/L and the GAC mass was adjusted to achieve the designed mass ratio.
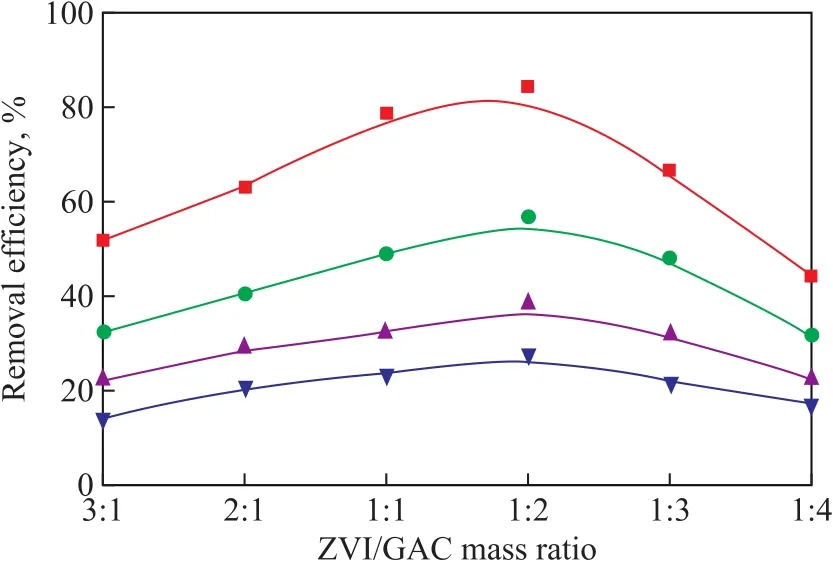
Figure 5 Effect of ZVI/GAC mass ratio on removalefficiency of NB
As shown in Figure 5, the NB removal efficiency increased firstly to a peak value when the ZVI/GAC mass ratio was 1:2, and then decreased with an increasing amount of GAC. Furthermore, the effect of ZVI/GAC mass ratio on NB removal efficiency was greater over the experiment time. At the beginning when the ZVI/GAC mass ratio was more than 1:2, with the increase of GAC,the number of ZVI/GAC micro-cells also increased to enlarge the surface contact area with more active sites to accelerate the reaction rate. However, when the amount of carbon became excessive with ZVI/GAC ratio reducing to less than 1:2, the NB removal efficiency decreased. The ZVI particles would be surrounded by multiple layers of GAC with excessive addition of carbon. As a result, most of [H]generated on the surface of internal carbon would form H2gas by accepting the electrons transferred from GAC before they diffused to the external GAC surface to react with the external NB, and thus their reduction ability was impeded. In other words, the excessive GAC layers could retard the contact between [H]and NB so that the NB removal efficiency would decline. A ZVI/GAC mass ratio of 1:2 was optimal to be chosen for further study.
3.2.3 Effect of initial pH value on NB degradation efficiency
The effect of initial pH value on NB wastewater treatment was also studied in the US-ZVI/GAC system. The experiments were carried out at an initial pH value of 2.0, 3.0, 4.0, 5.0, and 8.0, respectively, which were used to study the effect of initial pH value on the degradation of NB. The results are shown in Figure 6. It can be seen that the NB removal efficiency always decreased at any time with an increasing initial pH value, and the removal efficiency decreased from 91.4% to 46.2% when the initial pH value increased from 2.0 to 8.0 after 80 min of treatment. In addition, it also showed that it tended to be a linear relationship between the NB removal efficiency and the initial pH value when the reaction time increased from 10 min to 80 min, and the linear relationship can be presented by the following equation:

The data ofkand the correlation coefficient (R2) are listed in Figure 6, indicating to a negative relationship between the NB removal efficiency and the initial pH value.
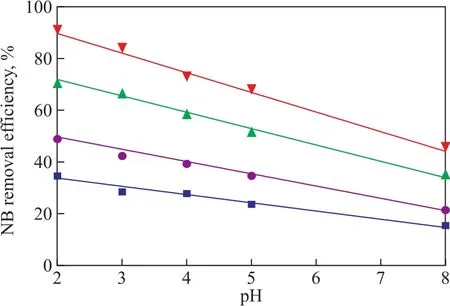
Figure 6 Effect of initial pH value on removal efficiency of NB with different reaction time
The process of NB degradation by US-ZVI/GAC followed a pseudo-first-order kinetics model as shown in Figure 7. It can be seen that the observed reaction rate constant (kobs) increased from 0.00928 to 0.03119 when the initial pH value decreased from 8.0 to 2.0, indicating that H+concentration has a positive effect on ZVI/GAC micro-electrolysis to treat NB. The reason could be explained by the ZVI/GAC micro-electrolysis galvanic cell reaction as shown below[25]:Anode (Fe):

Cathode (C):
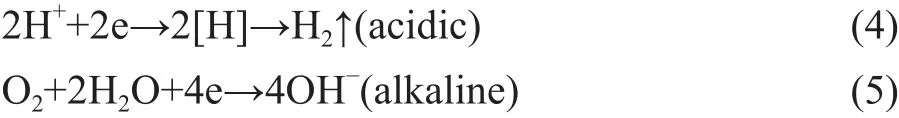
Under acidic condition, it was clear that higher H+concentration could bring forth more production of [H]for reduction of nitro groups. Therefore, a lower pH value is propitious for improving the reduction efficiency of NB. Thus, it was obvious that OH-had no positive influence on the NB removal efficiency because of the lack of the reduction ability. Collectively, the alkaline condition was not favourable for NB removal. However,high equipment requirements were necessary to suit the acidic operating condition, and the low pH value of the solution at the end of experiment was not conducive to the succeeding processes. Thus, a pH value of 3 for the experiment was recommended.
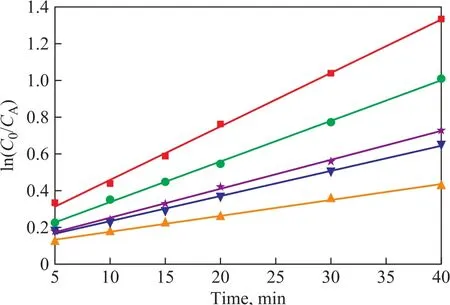
Figure 7 Effect of initial pH on reduction dynamics
3.2.4 Effect of H2O2dosage on mineralization rate
After the US-ZVI/GAC micro-electrolysis reaction,the main substances and water quality after wastewater treatment were measured, with the results given in Table 1.
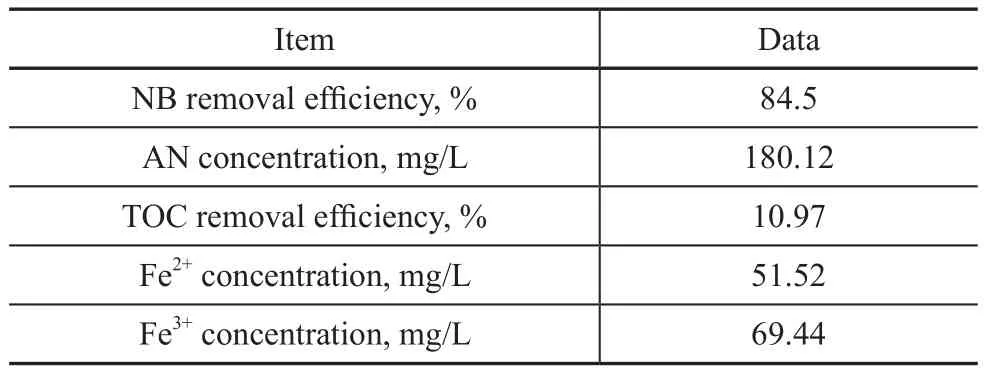
Table 1 Wastewater quality indicators and main substances
It can be seen that ZVI/GAC micro-electrolysis could effectively transform NB into AN, while producing the byproduct Fe2+ions. However, the mineralization rate was very low and the TOC removal efficiency was only 10.97%, which was mainly ascribed to the existence of AN. We expected to increase the mineralization rate of wastewater through adding H2O2that could be catalyzed by Fe2+ions with a large yield of hydroxyl radicals. The produced hydroxyl radicals could finally degrade the remaining organic pollutants in wastewater. In this case,the NB-containing wastewater could be further treated in depth by the utilization of byproduct Fe2+ions and H2O2.In order to optimize the H2O2dosage, different H2O2dosages (1, 2, 3, 4, 5, and 6 mL, resapectively) were chosen to conduct the experiments. As shown in Figure 8, adding H2O2was an effective method of AN mineralization, and the TOC removal efficiency was significantly improved as compared with the case without the addition of H2O2. This could occur because AN with the electron-donating group could activate the benzene ring which would make the benzene ring susceptible to the degradation initiated by the chemical and biological degradation processes[26]. The effect of H2O2dosage on the mineralization rate was investigated to verify that the TOC removal efficiency increased with an increasing H2O2dosage until an optimum ratio was reached.However, further increase of H2O2amount might reduce the AN removal efficiency. An optimum H2O2dosage of 4 mL gave a better TOC removal efficiency (about 73.42%)in this study, while the NB removal efficiency increased from 93.14% to 97.72%. Reasons could be attributed to the ·OH radicals generated by the decomposition of H2O2as shown in Equation (6), which could promote the removal efficiency and improved the effluent quality[27].However, if H2O2dosage was greater than the optimum value, excessive H2O2would react with hydroxyl radicals in Equations (7)—(9), which would contribute to the deterioration of effluent quality[28-29].
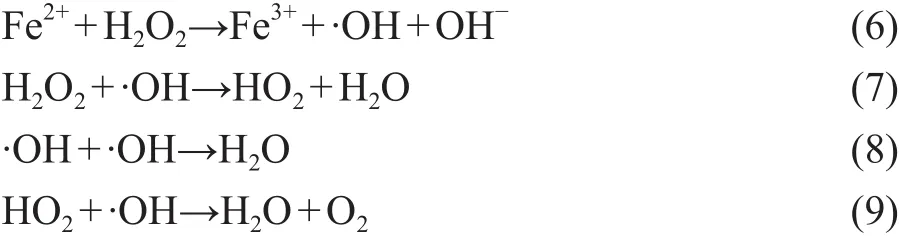
3.3 Analysis of degradation products
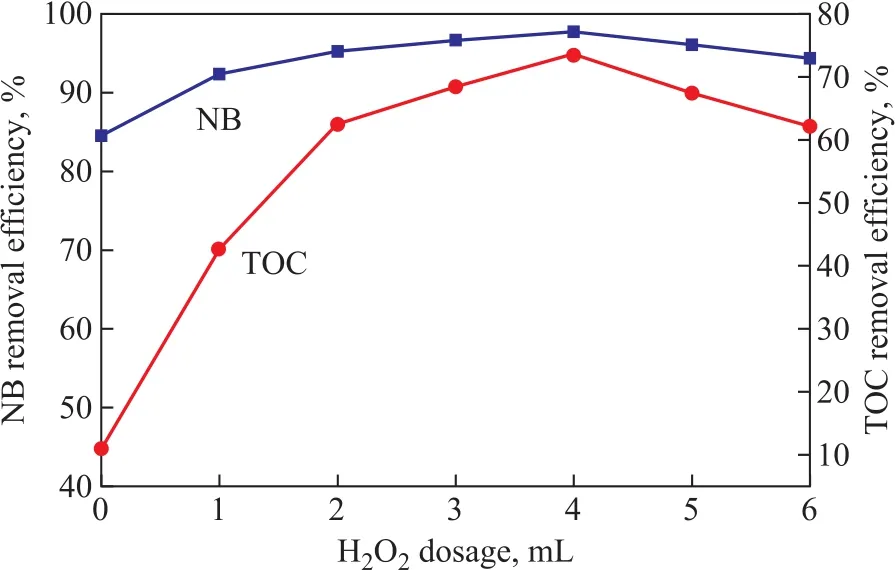
Figure 8 Effects of H2O2 dosages on the NB and TOC removal efficiency
The HPLC pictures of NB solution in the US-ZVI/GAC system at different reaction time are presented in Figure 9. Peaks were identified by comparison with the retention time of standard compounds, such as a retention time of 4.118 min for AN, 6.732 min for NB, and 7.657 min for nitrosobenzene. The phenylhydroxylamine was the most likely intermediate corresponding to an unidentified peak (with a retention time of 3.792 min)[10]. It was clear that the concentration of NB decreased with a prolonged reaction time, while the concentration of AN increased. Table 1 shows that the production of AN was 180.12 mg/L in the US-ZVI/GAC system after 80 min of reaction, denoting that more than 79.4% of NB was transformed into AN. The accumulation of nitrosobenzene was always very small during the experimental period, and similar behaviour was observed in the previous study[30], which might be caused by the faster reaction rate of nitrosobenzene to phenylhydroxylanine in comparison with the reaction rate for transforming phenylhydroxylanine to AN. Based on the HPLC results and the information previously reported on intermediate analyses in the literature, the mechanism of NB degradation in the US- ZVI/GAC system has been proposed and shown in Figure 10[10], which is similar to the reduction process of aromatic nitro-compounds by zero-valent iron metal.
The intermediates of mineralization in the Fenton process with hydroxyl radicals were identified by GC/MS, and the GC/MS total ion chromatograms at different reaction time, with the identified intermediates shown in Figure 11. NB, AN, benzoquinonimine,p-benzoquinone,p-nitrophenol and the small molecular organic acids were identified. Based on the time of production of the wastewater and the sequence of substance disappearance, a possible mechanism of mineralizationprocess has been proposed and shown in Figure 12.The residual NB in the wastewater was converted top-nitrophenol by the attack of ·OH firstly, followed by denitrification top-benzoquinone. Due to the instability ofp-benzoquinone, the small molecular organic acids were generated finally. To go into details, during the AN degradation, the ·OH radicals attacked the amino group first, and then the benzene ring. The ring opening of the aromatic intermediates caused the formation of organic acids[31].
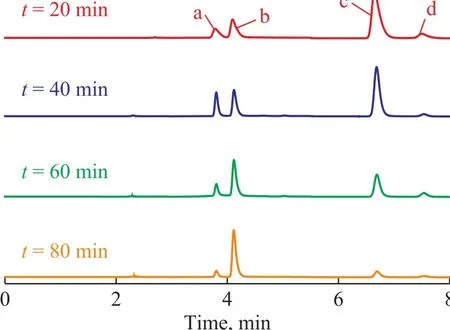
Figure 9 HPLC of NB solution in the US-ZVI/GAC systemat different reaction time
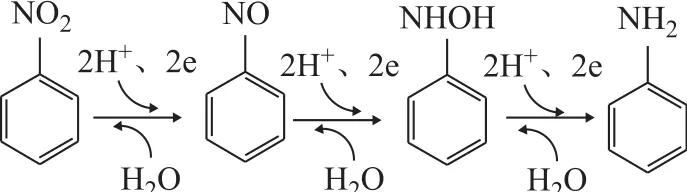
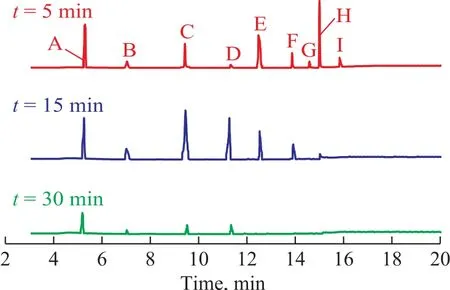
Figure 11 GC/MS total ion chromatograms for mineralization at different reaction time
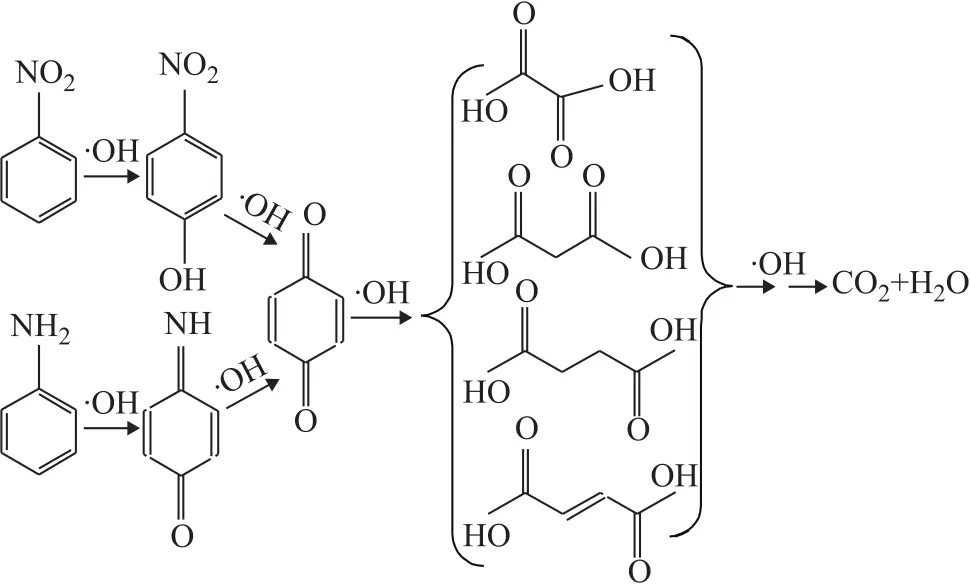
Figure 12 Main reaction pathways for mineralization in the Fenton process
4 Conclusions
The experiments for degradation of the NB-containing wastewater by US-ZVI/GAC system were carried out,and then H2O2was added in the subsequent process which was finally catalysed by byproduct Fe2+ions for further degradation of the remaining organic pollutants in the wastewater. The results of SEM-EDS showed that US not only could regenerate the active sites of the ZVI, but also might improve the reducing capacity of ZVI. Effects of the ZVI dosage, the ZVI/GAC mass ratio, the initial pH value and the H2O2dosage on the NB degradation efficiency were studied in the related experiments. The optimal operating conditions for NB degradation were determined to cover a ZVI dosage of 15 g/L, a ZVI/GAC mass ratio of 1:2; an initial pH value of 3, and a H2O2dosage of 4 mL. In this case, the NB removal efficiency was 97.72% and the TOC removal efficiency reached 73.42% at a NB concentration of 300 mg/L. The reduction of NB by US-ZVI/GAC followed the pseudo-first-order kinetics model, and the pseudo- first-order rate constants were given under different initial pH values. The reaction intermediates AN, benzoquinonimine, p-benzoquinone,p-nitrophenol and organic acids were detected by HPLC and GC-MS, and a working mechanism about how NB can be degraded to final organic acids has been proposed.
Acknowledgements: This work was supported by the Natural Science Foundation of China (U1610106), the Excellent Youth Science and Technology Foundation of Province Shanxi of China(2014021007), the Specialized Research Fund for Sanjin Scholars Pragram of Shanxi Prouince (201707), and the North University of China Fund for Distinguished Young Scholars (201701).
[1]Jiao W Z, Liu Y Z, Shao F, et al. Degradation of wastewater containing nitrobenzene by high gravityultrasonic/ozonation/electrolysis technology[J]. China Petroleum Processing and Petrochemical Technology,2012, 14(3): 96-101
[2]Shen Y Y, Zhao S L, Li Y, et al. A feasible approach to dispose of soil washing wastes: Adsorptive removal of chlorobenzene compounds in aqueous solutions using humic acid modified with monoolein (HA-M)[J]. RSC Advances, 2017, 7(16): 9662-9668
[3]Xue J L, Liu G M, Zhao D F, et al. Inhibition effects of pentachlorophenol (PCP) on anaerobic biological system[J]. Desalination and Water Treatment, 2013,51(28/30): 5892-5897
[4]Zhang W R, Tang A D, Xue J L. Preparation of Cr-MnOx/cordierite and their properties for catalytic oxidation of 1,2-dichlorobenzene[J]. Spectroscopy and Spectral Analysis, 2016, 36(9): 3075-3082
[5]Orge C A, Faria J L, Pereira M F R. Photocatalytic ozonation of aniline with TiO2-carbon composite materials[J]. Journal of Environmental Management, 2017,195: 208-215
[6]Xie G, Zhou L C, Gao W J, et al. Organic additives enhance Fenton treatment of nitrobenzene at near-neutral pH[J]. Environmental Science and Pollution Research,2015, 22(9): 7082-7092
[7]Hu X W, Li A M, Fan J, et al. Biotreatment of p-nitrophenol and nitrobenzene in mixed wastewater through selective bioaugmentation[J]. Bioresource Technology, 2008,99(10): 4529-4533
[8]Lai B, Zhou Y X, Yang P, et al. Degradation of 3,3’-iminobis-propanenitrile in aqueous solution by Fe0/GAC microelectrolysis system[J]. Chemosphere, 2013, 90(4): 1470-1477
[9]Luo J, Song G, Liu J, et al. Mechanism of enhanced nitrate reduction via micro-electrolysis at the powdered zerovalent iron/activated carbon interface[J]. Journal of Colloid and Interface Science, 2014, 435: 21-25
[10]Agrawal A, Tratnyek P G. Reduction of nitro aromatic compounds by zero-valent iron metal[J]. Environmental Science and Technology, 1996, 30: 153-160
[11]Wang L Q, Yang Q, Wang D B, et al. Advanced landfill leachate treatment using iron-carbon microelectrolysis-Fenton process: Process optimization and column experiments[J]. Journal of Hazardous Materials, 2016, 318:460-467
[12]Xu X Y, Cheng Y, Zhang T T, et al. Treatment of pharmaceutical wastewater using interior microelectrolysis/Fenton oxidation-coagulation and biological degradation[J]. Chemosphere, 2016, 152: 23-30
[13]Lai B, Zhou Y, Yang P. Passivation of sponge iron and GAC in Fe0/GAC mixed-potential corrosion reactor[J]. Industrial and Engineering Chemistry Research, 2012, 51(22): 7777-7785
[14]Liu H, Li G, Qu J, et al. Degradation of azo dye Acid Orange 7 in water by Fe0/granular activated carbon system in the presence of ultrasound[J]. Journal of Hazardous Materials, 2007, 144(1-2): 180-186
[15]Yang S, Liang Z, Yu H, et al. Chemical oxygen demand removal efficiency and limited factors study of aminosilicone polymers in a water emulsion by ironcarbon micro-electrolysis[J]. Water Environment Research,2014, 86(2): 156-162
[16]Zhou H, Lv P, Shen Y, et al. Identification of degradation products of ionic liquids in an ultrasound assisted zerovalent iron activated carbon micro-electrolysis system and their degradation mechanism[J]. Water Research, 2013,47(10): 3514-3522
[17]Zhang H, Duan L J, Zhang Y, et al. The use of ultrasound to enhance the decolorization of the CI Acid Orange 7 by zero-valent iron[J]. Dyes and Pigments, 2005(1): 39-43, 65
[18]Hung H M, Ling F H, Hoffmann M R. Kinetics and mechanism of the enhanced reductive degradation of nitrobenzene by elemental iron in the presence of ultrasound[J]. Environmental Science and Technology,2000, 34(9): 1758-1763
[19]Lopes R P, de Urzedo A P F M, Nascentes C C, et al.Degradation of the insecticides thiamethoxam and imidacloprid by zero-valent metals exposed to ultrasonic irradiation in water medium: electrospray ionization mass spectrometry monitoring[J]. Rapid Communications in Mass Spectrom., 2008, 22(22): 3472-3480
[20]Lastre-Acosta A M, Cruz-Gonzalez G, Nuevas-Paz L,et al. Ultrasonic degradation of sulfadiazine in aqueous solutions[J]. Environmental Science and Pollution Research, 2015, 22(2): 918-925
[21]Guo X, Yang Z, Dong H, et al. Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water[J].Water Research, 2016, 88: 671-680
[22]Ji Q Q, Li J, Xiong Z K, et al. Enhanced reactivity of microscale Fe/Cu bimetallic particles (mFe/Cu) with persulfate (PS) for p-nitrophenol (PNP) removal in aqueous solution[J]. Chemosphere, 2017, 172: 10-20
[23]Hung H M, Hoffmann M R. Kinetics and mechanism of the enhanced reductive degradation of CCl4by elemental iron in the presence of ultrasound environment[J].Environmental Science and Technology, 1998, 32(19):3011-3016
[24]Cheng I F, Muftikian R, Fernando Q, et al. Reduction of nitrate to ammonia by zero-valent iron[J]. Chemosphere 1997, 35(11): 2689-2695
[25]Zhang S, Wang D, Zhou L, et al. Intensified internal electrolysis for degradation of methylene blue as model compound induced by a novel hybrid material: Multiwalled carbon nanotubes immobilized on zero-valent iron plates (Fe0-CNTs)[J]. Chemical Engineering Journal, 2013,217: 99-107
[26]Jiao W Z, Qin Y J, Luo S, et al. Continuous preparation of nanoscale zero-valent iron using impinging stream-rotating packed bed reactor and their application in reduction of nitrobenzene[J]. Journal of Nanoparticle Research, 2017,19: 52
[27]Zeng Z Q, Zou H K, Li X, et al. Ozonation of phenol with O3/Fe (II) in acidic environment in a rotating packed bed[J]. Industrial and Engineering Chemistry Research,2012, 51(31): 10509-10516
[28]Ku Y, Huang Y J, Chen H W, et al. Decomposition of acetone by hydrogen peroxide/ozone process in a rotating packed contactor[J]. Water Environment Research, 2011,83(7): 588-93
[29]Li G J, He J J, Wang D D, et al. Optimization and interpretation of O3and O3/H2O2oxidation processes to pretreat hydrocortisone pharmaceutical wastewater[J].Environmental Technology, 2015, 36(5/8): 1026-1034
[30]Feng Z R, Jiao W Z, Liu Y Z, et al. Treatment of nitrobenzene-containing wastewater by iron-carbon microelectrolysis[J]. CIESC Journal, 2014, 66(3): 1150-1155
[31]Huang Y, Su C, Yang Y, et al. Degradation of aniline catalyzed by heterogeneous Fenton-like reaction using iron oxide/SiO2[J]. Environmental Progress and Sustainable Energy, 2013, 32 (2): 187-192
杂志排行
中国炼油与石油化工的其它文章
- Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
- Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance
