Non-target Screening and Quantitation of Organic Chlorides in Oil field Chemicals with Comprehensive Two Dimensional Gas Chromatography Coupled with Time of Flight Mass Spectrometry
2018-01-19ZhengKewenGuoRuiRenGuofaXiaLingyanXiongWeiZhangJinChuXiaodongYuZhiqiang
Zheng Kewen; Guo Rui; Ren Guofa; Xia Lingyan; Xiong Wei; Zhang Jin;Chu Xiaodong; Yu Zhiqiang
(1. Institute of Environmental Pollution and Health, School of Environmental and Chemical Engineering,Shanghai University, Shanghai 200072; 2. SINOPEC Shengli Oil field Branch, Dongying 257000)
1 Introduction
Various kinds of oilfield chemicals, such as heavy oil improver, viscosity reducer, demulsifier, bactericide, etc.,have been used recently in the petroleum extraction and relevant industries of China[1-3]. Among them, a plenty of chemical agents are found to have chlorides, which are easily mixed with crude oil in the process of oil exploration, transportation, and storage. Chlorides are harmful when crude oil is processed. There are two forms of chlorides existing in crude oil, namely the inorganic and organic chlorides. Inorganic chlorides mainly consist of inorganic chloride salts which could be removed by an electric desalting device before processing. However,unlike inorganic chloride salts, organic chlorides cannot be removed by using the electric desalting device[4]. Since organic chlorides in crude oil primarily emerge from the chemical agents[5], it is necessary to establish an effective technology to measure some potential organic chloridescontaining chemical agents in the crude oil.
Nowadays, the organic chloride content in oilfield chemicals is determined by the following equation:organic chloride content = total chlorine content -inorganic chloride content[6], which cannot directly characterize the composition of organic chloride. Up to now, there are only few researches relating to the occurrence of organic chlorides in the crude oil and its distillates[4,7-9]. The occurrence states of organic chlorides in oilfield chemical agents are unknown. At the same time, the limited data are primarily focused on the pretargeted organic chlorides. The main embarrassment is that there are no effective methods for separation of organic chlorides and reliable measurement methods.
GC×GC has been proved to be an extremely powerfultechnique for the analysis of complex samples[10].Theoretically, much higher peak capacities can be obtained from GC×GC in comparison with the conventional GC, leading to the possibility for the separation of target compounds from the matrix. The time of- flight mass spectrometric detection (TOFMS) is capable of presenting full-scan mass spectra with a high scanning rate. Coupling GC×GC with TOFMS opens the possibility for the simultaneous identification and quantitation of nontarget organic chlorides as well as target organic chlorides in all kinds of oilfield chemicals[11]. In this study, we describe an advanced analytical methodology based on GC×GC-TOFMS for investigation of organic chlorides contained in different oil field chemicals.
2 Experimental
2.1 Instruments
The Pegasus 4D comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry(GC×GC-TOFMS, LECO, USA) was used.
2.2 Standards and agents
Target determination was initially carried out for 8 suspected organic chlorides, including chloroform,1,2-dichloroethane, carbon tetrachloride, epichlorohydrin,1,3-dichloropropane (purchased from the Energy Chemical Company, Shanghai, China), 2-chlorotoluene,2-chlorophenol, and 1,2-dichlorobenzene (obtained from the Sinopharm Group Limited Company, Shanghai,China). 22 organic chloride compounds were identified and verified in the real samples by comparison to pure standards, which including 1,2-dichloroethane,3,3-dichloro-1-propene, 1,1-dichloro-propane,1,2-dichloro-propane, epichlorohydrin, (Z)-1,3-dichloro-1-propene, (E)-1,3-dichloro-1-propene, 1-chloro-2-hexene, 3-chloro-1,5-hexadiene, 1,2,2-trichloropropane, 2-bromo-3-chloropropene, (Z)-1,2,3-trichloro-1-propene, (E)-1,2,3-trichloro-1-propene, chlorobenzene,1,3-dichloro-2-propanol, 1,2,3-trichloro-propane,2,3-dichloro-1-propanol, benzyl chloride, bis(1,3-dichloro-2-propyl)ether, bis(2,3-dichloro-1-propyl)ether, 2-chloro-1-chloromethylethoxypropane, and 1-chloroundecane (purchased from the Energy Chemical Company, Shanghai, China). All the standards were obtained as neat compounds with a purity of 99.5%.Hexane, which was an organic HPLC-grade solvent, was purchased from Merck (Darmstadt, Germany).
2.3 Sample collection and treatment
Thirty-nine different brands of oilfield chemicals with a wide range of functions were kindly provided by the Sinopec Shengli Oil field located in east China. All samples were liquid and collected in early August 2014. Based on the solubility of samples in n-hexane, the samples were divided into two categories, namely hydrosoluble samples, such as biocide, acidizing mutual solvent and resin, and liposoluble samples, such as demulsifier,corrosion inhibitor, oil skimmer and cleanup additive.The samples were sealed in brown glass jar at -4 °C until being analyzed. Table 1 lists the detailed information on the samples. For the hydrosoluble samples, a liquid-liquid extraction method was adopted for the enrichment of organic chlorides. Brie fl y, isopyknic hexane and the sample to be tested were first mixed in a Te fl on separating funnel and centrifuged at a rotational rate of 3 600 r/min for 10 min, and then the supernatant was decanted into another centrifuge tube. The residual substance was extracted again following the same procedure and combined with the previous extract. For the liposoluble samples, no additional pretreatment was required.
2.4 Optimized instrument parameters
The standards and samples were analyzed using the comprehensive two-dimensional gas chromatographytime of flight mass spectrometer (GC×GC-TOFMS).The GC column set used thereby was made of the combination of a DB5-MS (15 m in length, 250 μm in internal diameter, and 0.25 μm in film thickness, made by Agilent) as the 1D column and a high temperature HT-8 (1.2 m in length, 250 μm in internal diameter,and 0.10 μm in film thickness, made by SGE) as the 2D column. Ultra-pure helium was used as the carrier gas under the constant flow mode at a flow rate of 1.2 mL/min. Manual injections (1 μL) were made in the pulse splitless mode, with the injection temperature set at 250 °C and the transfer line temperature set at 280 °C.During chromatographic separation, the primary GC ovenwas programmed as follows: it was at first preheated to 50 °C (4-min hold), and was increased to 210 °C(1-min hold) at a rate of 12 °C/min, then to 250 °C at a rate of 5 °C/min (5-min hold), then to 275 °C at a rate of 0.5 °C/min (5-min hold), and finally to 300 °C at a rate of 15 °C/min (5-min hold). The 2D column was coiled into the secondary oven that was by 20 °C higher than the primary oven and was operated in the iso-ramping mode. The temperature of the modulator had an offset of 35 °C as compared with the temperature of the primary GC oven. Modulation was carried out on the very beginning of the 2D column. The modulation period was 3 sec with a hot-pulse duration set at 900 ms and a cooling time between stages of 600 ms. The mass spectrometer was operated in the electron ionization (EI+)mode. The mass range of 50 Da–500 Da was acquired at a data acquisition rate of 50 Hz. The obtained contour plot and surface plot of full scan chromatogram of the mixture of 8 selected organic chlorides is shown in Figure 1.

Table 1 The detailed information and amount of organic chlorides in different oil fi eld chemicals
2.5 Non-target screening
Non-target screening of potential organic chlorides was based on the mixed sample, which was prepared by isopyknic mixing the samples (for the liposoluble samples)or sample extracts (of the hydrosoluble samples). Full-scan screening of the mixed samples in search of ‘unknown’followed by identification of all chemicals was done using the commercially available NIST Mass Spectral Libraries, which led to a large list of compounds to be identified. Because the goal of this work was to investigate non-target organic chlorides, special care was taken with the identification of these compounds based on the following mentioned criteria. First, it was necessary to sort the list by name and delete the chlorine-free compounds.Second, it was necessary to sort the list by similarity and delete the compounds with a library match similarity of<750. Finally, the identified compound was manually verified by comparison to pure standards in each instance.Table 2 lists a summary of compounds identified using this strategy for studying the mixed samples.
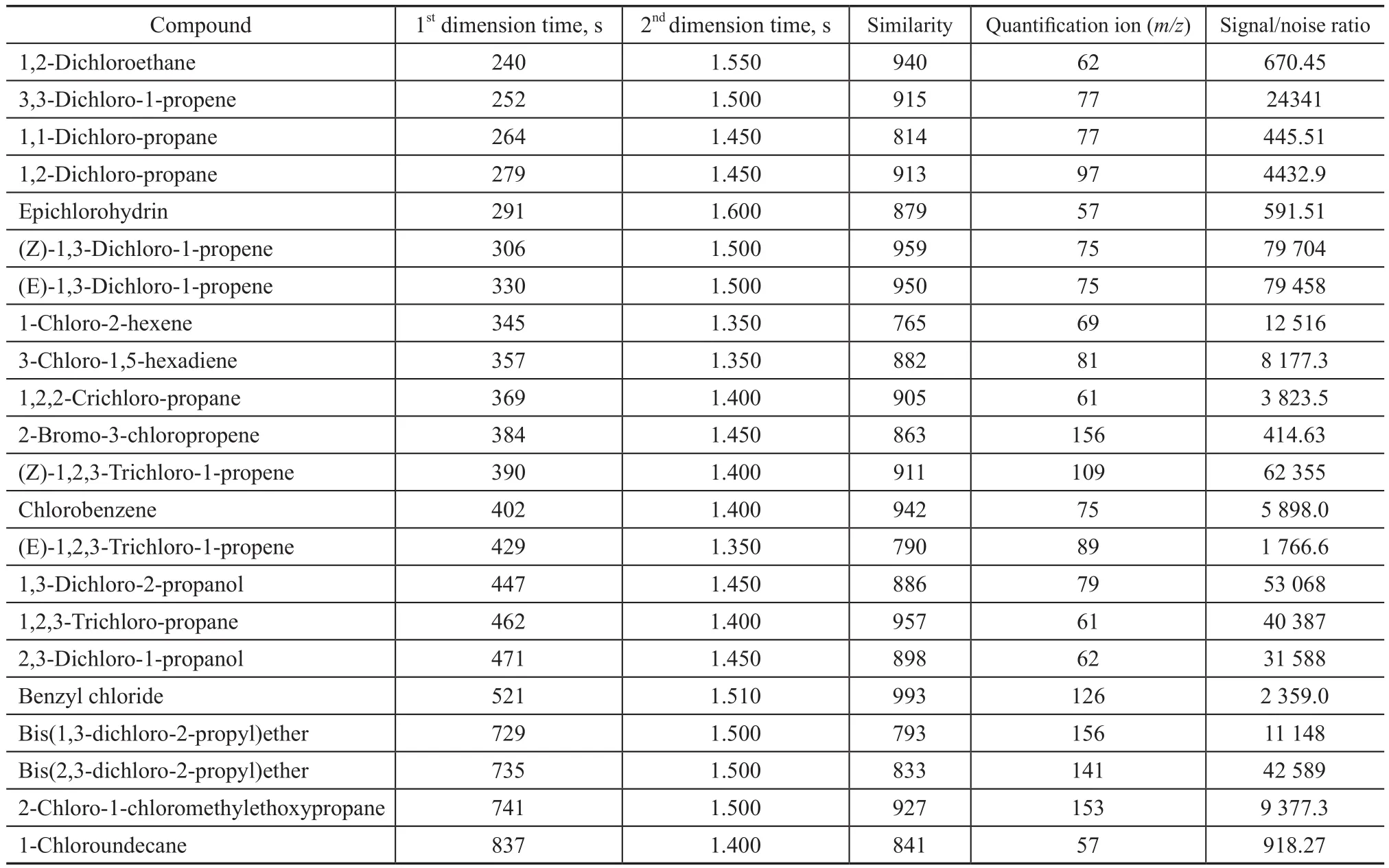
Table 2 The major organic chlorides identified by GC×GC-TOFMS
2.6 Quantitative analysis
Individual solutions of the 22 organic chlorides were mixed and were dissolved inn-hexane to serve as the stock solution (at a concentration of 500 mg/L). The stock solution was then diluted to a series of standard solutions at levels of 0.5, 1, 2,10, 25, 50, and 100 mg/L. Because of the wide range of organic chlorides concentrations in some oilfield chemicals, the samples were diluted to the required concentrations withn-hexane. The peaks were identified and quantified only if the GC×GC retention times matched those of the standard compounds within 10 s and 0.1 s required by the 1D and 2D retention time, respectively, and the signal-to-noise ratio should be >30. A seven-point calibration curve was established for individual compound and the correlation coefficient (R2) should be above 0.99 for all the analytes. A typical regression curve for (E)-1,3-dichloro-1-propene was illustrated in Figure 1.
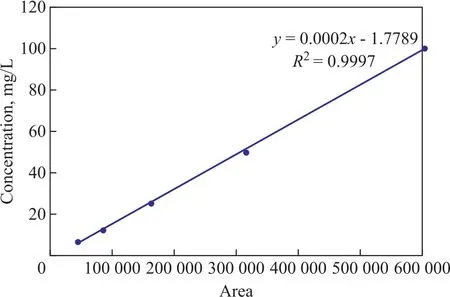
Figure 1 A typical regression curve for (E)-1,3-dichloro-1-propene
3 Results and Discussion
3.1 Optimized instrument parameters
Under the optimized conditions, the peak width in the first dimension was approximately 10—15 s. The peak width in the second dimension was approximately 100 ms. The chosen modulation time of 3 s generally resulted in three to four slices across each 1D peak. The obtained contour and surface plot of selected organic chlorides mix, recorded in the total ion current (TIC) mode, are listed in Figure 2. Target organic chlorides were observed to be distributed on the 2D separation map (between 1.0 and 1.5 seconds in the second dimension),while the column bleed was seen below 1.0 seconds in the second dimension. Data processing was conducted by automatic peak findings using MS deconvolution and spectral searching against the NIST spectral libraries,the detected amounts of the target compounds were low,with their concentrations equating to about 200 μg/L.It was considered to be sufficient in non-target screening of “unknown” of rganic chlorides in the complex matrix.
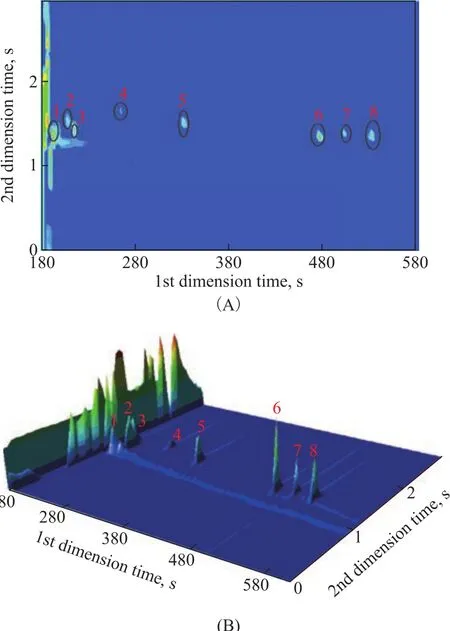
Figure 2 GC × GC–TOFMS contour plot (A), and three dimensional plot (B) of a standard solution containing 8 suspected organic chlorides at a concentration of 1 mg/L
3.2 Non-target screening
Under the same operating conditions, put 1 μL of the mixed sample into the gas chromatograph. Figure 3 displays the GC×GC pattern of the mixed samples, which shows that the matrix compounds are sometimes by several orders of magnitude higher than the target analytes. According to the data processing criterion, 22 organic chlorides, including 1,2-dichloroethane, 3,3-dichloro-1-propene, 1,1-dichloropropane, 1,2-dichloro-propane, epichlorohydrin, (Z)-1,3-dichloro-1-propene, (E)-1,3-dichloro-1-propene, 1-chloro-2-hexene, 3-chloro-1,5-hexadiene, 1,2,2-trichloro-propane,2-bromo-3-chloropropene, (Z)-1,2,3-trichloro-1-propene,(E)-1,2,3-trichloro-1-propene, chlorobenzene, 1,3-dichloro-2-propanol, 1,2,3-trichloro-propane, 2,3-dichloro-1-propanol, benzyl chloride, bis(1,3-dichloro-2-propyl)ether, bis(2,3-dichloro-1-propyl)ether, 2-chloro-1-chloromethylethoxypropane, and 1-chloroundecane, can be identified in the mixed samples (as shown in Table 2). To our knowledge, some of the organic chlorides identified in our study, such as bis(1,3-dichloro-2-propyl)ether, bis(2,3-dichloro-1-propyl)ether, 2-chloro-1-chloromethylethoxypropane, and 1-chloroundecane, have not been identified in the crude oil or in the oil field agents by other researchers. For example, bis(2,3-dichloro-1-propyl)ether was a byproduct of epichlorohydrin and can only be reported in the environmental matrix[12]. In this study, bis(2,3-dichloro-1-propyl)ether was identified in some oil field chemicals. As shown in Figure 4, the resulting library match score (similarity: 833) of this compound was above 80%, which was finally confirmed by the authentic standard.
Figure 3 GC×GC–TOFMS result for a three-dimensional plot of total ion chromatogram of the isopyknic 39 mixed oil field chemicals
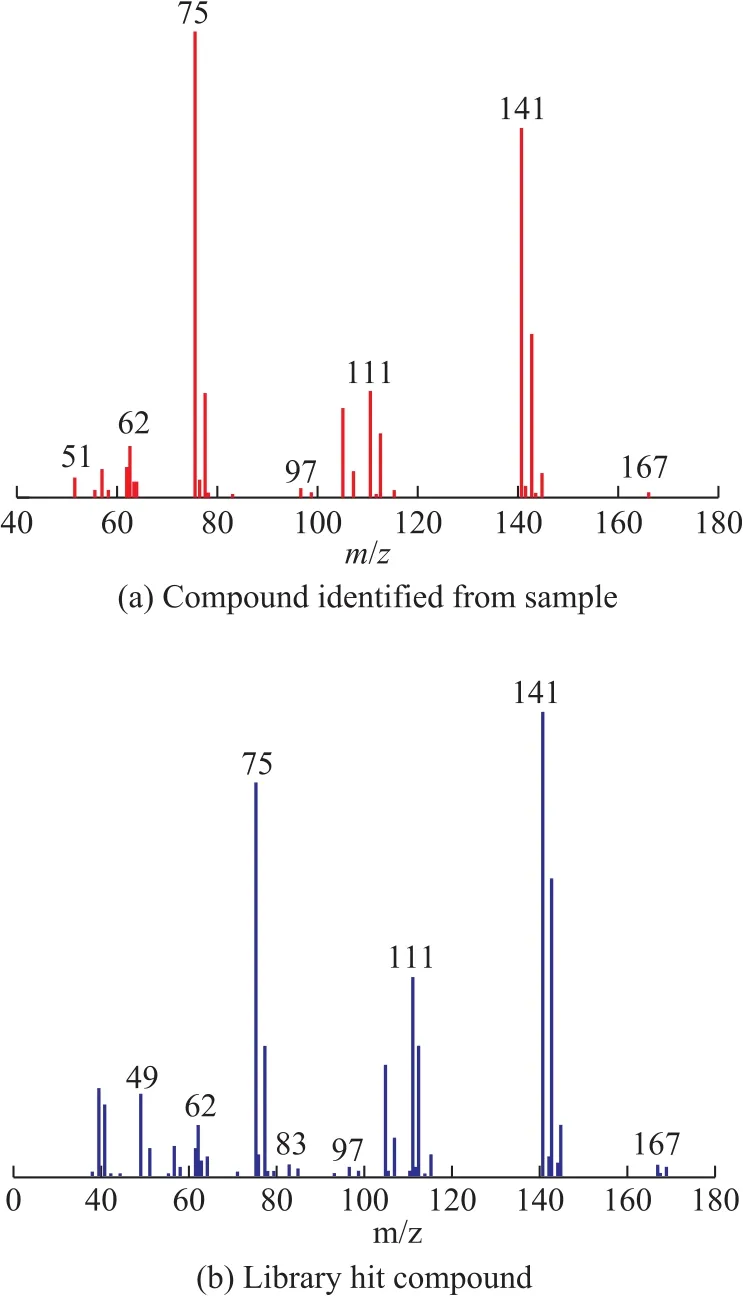
Figure 4 Representative mass spectrum for the identified bis(2,3-dichloro-1-propyl)ether
3.3 Method of evaluation
The efficacy of the proposed methods was tested to determine the precision of the analytical procedure for the organic chlorides in oil field chemicals. We chose the peanut oil as the “blank” sample and 8 suspected organic chlorides as the targeted compounds. The test compounds in the “blank” sample were all below the detection limit.Known amounts of 8 suspected organic chlorides (at a concentration of 50 μg/L in the samples) were added to four sample replicates. These enriched samples were extracted and analyzed with the method described above.The recoveries obtained for the spiked samples were demonstrated in Table 3. Good recovery, ranging from 77.2% to 93.5% with a relative standard deviation (RSD)of less than 7%, had been achieved in the matric spiking experiments.
3.4 Quantitative analysis
The amounts of the organic chlorides were calculated according to the area and amount of the standardsolutions. The results are shown in Table 1. The organic chloride levels in 19 of the 39 tested oilfield chemicals were above the threshold limit of 1 mg/L.
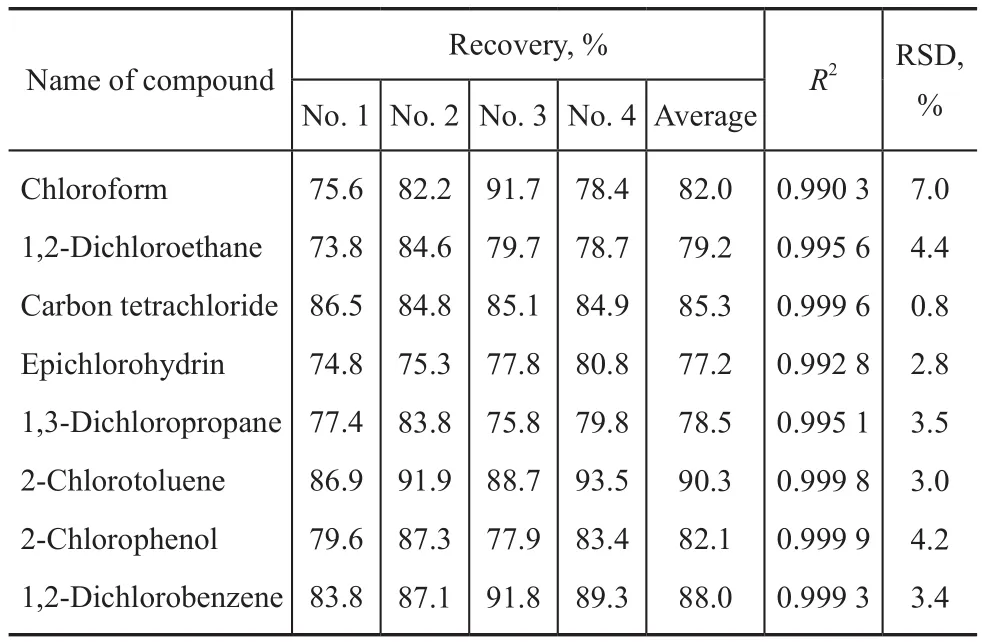
Table 3 Recovery of the selected organic chloride compounds in the matrix sample
4 Conclusions
GC×GC-TOF MS working in full scan mode can provide highly reliable results for non-target screening of organic chlorides that are present in various oil field chemicals.
With the fast growth in demand for all kinds of chemical agents, a growing number of chemical agents would be introduced to the market that might contain organic chlorides, and this technology could be used to discover the origin of the illegal addition of organic chlorides, and guide the implementation of dechlorination technology in the future.
[1]Yang Y Q, Guo J X, Cheng Z F, et al. New composite viscosity reducer with both asphaltene dispersion and emulsifying capability for heavy and ultraheavy crude oils[J]. Energy & Fuels, 2017, 31(2): 1159-1173
[2]Li C Q, Sun P, Shi W G, et al. Synthesis and properties of dendritic long-chain esters as crude oil flow improver additives [J]. China Petroleum Processing & Petrochemical Technology, 2016, 18(1): 83-91
[3]Yu Y, Zhang W, Chen G H, et al. Preparation of petroleumdegrading bacterial agent and its application in remediation of contaminated soil in Shengli Oil Field, China [J].Environmental Science and Pollution Research, 2014,21(13): 7929-7937
[4]Wu B C, Li Y F, Li X H, et al. Distribution and identification of chlorides in distillates from YS crude oil[J]. Energy & Fuels, 2015, 29(3): 1391-1396
[5]Burns M, Carstens D, Ghosh E, et al. Thinking outside the boxcar: Effective and sustainable combined remedies using single application of multifunctional amendments [J].Ground Water Monitoring and Remediation, 2017, 37(1):42-50
[6]Surma-Zadora M, Grochowalski A. Using a membrane technique (SPM) for high fat food sample preparation in the determination of chlorinated persistent organic pollutants by a GC/ECD method [J]. Food Chemistry,2008, 111(1): 230-235
[7]Fan X J, Zhu J H, Song H F, et al. The identification and quantitation of organochlorine in naphtha by gas chromatography with ECD [J]. Petroleum Science and Technology, 2011, 29(8): 867-872
[8]Ma R, Zhu J H, Wu B C, et al. Distribution and qualitative and quantitative analyses of chlorides in distillates of Shengli Crude Oil [J]. Energy & Fuels, 2017, 31(1): 374-378
[9]Concha-Grana E, Fernandez-Martinez G, Fernandez-Villarrenaga V, et al. A study of large-volume oncolumn injection GC-ECD for the ultratrace analysis of organochlorine pesticides in water [J]. Talanta, 2009,78(3): 764-771
[10]Pierce K M, Mohler R E. A review of chemometrics applied to comprehensive two-dimensional separations from 2008-2010 [J]. Separation and Puri fi cation Reviews,2012, 41(2): 143-168
[11]Ren G F, Wang Z, Yu Z Q, et al. Primary investigation on contamination pattern of legacy and emerging halogenated organic pollutions in freshwater fish from Liaohe River,Northeast China [J]. Environmental Pollution, 2013, 172:94-99
[12]Franke S, Meyer C, Specht M, et al. Chloro-bispropyl ethers in the Elbe River - Isomeric distribution and enantioselective degradation [J]. Journal of High Resolution Chromatography, 2015, 21(2): 113-120
杂志排行
中国炼油与石油化工的其它文章
- Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
- Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance
