Evaluation of hollow fiber T-type zeolite membrane modules for ethanol dehydration☆
2017-05-28XueruiWangJiJiangDezhongLiuYouquanXueChunZhangXuehongGu
Xuerui Wang,Ji Jiang,Dezhong Liu,Youquan Xue,Chun Zhang,Xuehong Gu*
State Key Laboratory of Materials-Oriented Chemical Engineering,College of Chemistry and Chemical Engineering,The Synergetic Innovation Center for Advanced Materials,Nanjing Tech University,5 Xinmofan Road,Nanjing 210009,China
1.Introduction
Zeolite membranes show great potential applications in organic solvent dehydration[1–3],gas permeation[4],membrane reactors[5],seawater desalination[6]and so on.In the past two decades,intensive efforts have been made for the fabrication and application of zeolite membranes.Several commercial companies,such as Mitsui Engineering and Shipbuilding(Japan),Jiangsu Nine Heaven(China)and GFT Membrane Systems GmbH(Germany),have built up more than 200 industrial plants based on LTA zeolite membranes[7–9].However,high capital investment of the separation equipment(5000–10,000 USD per square meter for an assembled module)has limited their application in niche markets and implementation to large-scale industrial plants that use larger membrane area[10–11].The fabrication cost is mainly attributed to the membrane modules,which is composed of zeolite membranes,sealing materials,and stainless steel.However,at least 70%of the membrane cost is dominated by support rather than membrane layer[12–13].Since there are no suitable alternatives to the expensive ceramic supports[13],an attractive strategy for reducing the investment is to increase membrane permeation flux and membrane packing density in the modules(membrane surface area per unit volume).
Recently,increasing attentions have been drawn to hollow fiber(HF)zeolite membranes due to their high permeation flux as well as high packing density[14–18].The membrane packing density could increase from 30 to 250 m2·m−3for tubes up to 1000 m2·m−3for hollow fibers[14,15].Hollow fiber supports could be easily prepared by a combined phase-inversion and sintering method,which produces a thin wall with asymmetric structure[19,20].Commonly,hollow fiber supported zeolite membranes possess high permeability due to the much lower transfer resistance of hollow fiber supports compared with the conventional tubular supports.So far,much work has been done in the preparation of hollow fiber zeolite membranes,such as LTA[14–16],Silicalite-1[17],CHA[21]and T-type zeolite membranes[18].However,there is very few work devoted to the design of practical membrane modules for hollow fiber zeolite membranes,which is essential to the industrial applications.
Recently,we reported the preparation of high-performance HF T-type zeolite membranes[18].A preliminary module was successfully fabricated for organic solvent dehydration.However,the permeation flux of the membrane module dropped by approximately 60%compared with the single hollow fiber T-type zeolite membranes.We speculated that the module con figuration could have an important effect on the separation efficiency.In this work,we investigated hollow fiber zeolite membrane modules with different con figurations for dehydration of ethanol/water mixtures.The influence of operation parameters on separation efficiency was also evaluated in terms of operation temperature,the water content in feed,and feed flow rate.Computational fluid dynamics(CFD)technique was employed to visualize the flow field distribution of the hollow fiber modules with different con figurations.The cross-section layout of the modules was systematically investigated to optimize module design.Furthermore,the operation stability of membrane module for dehydration of ethanol solution was investigated.
2.Experimental
2.1.Membrane preparation
Hollow fiber T-type zeolite membranes were hydrothermally synthesized on yttria-stabilized zirconia(YSZ)hollow fiber supports with an effective length of~200 mm in batch scale.The YSZ supports were home-made by a wet-spinning method with a calcination temperature of 1550°C for 5 h.The supports had outer/inner diameters of~1.8/1.0 mm,average pore size of~0.9μm and porosity of~30%.Prior to hydrothermal synthesis,T-type zeolite particles(ca.1.4 μm in average particle size)were planted onto the outer surface of hollow fiber substrates by the vacuum-coating method.The process was conducted under the condition of 10 kPa in vacuum degree,1.0 wt%in seed concentration and 40 s in coating time,which has been optimized in our previous work[18].After drying at 60°C overnight,the seeded substrates were used for membrane synthesis.The synthesis precursor was prepared by dissolving sodium aluminate,colloidal silica,sodium hydroxide and potassium hydroxide in D.I.water at room temperature.The molar composition of the synthesis precursor was 1 SiO2:0.05 Al2O3:0.26 Na2O:0.09 K2O:17 H2O.All the chemicals were purchased from commercial companies in China.Hydrothermal synthesis was carried out at 100°C for 40 h.The as-synthesized membranes were washed by D.I.water and dried in an oven overnight before the module assembly.
2.2.Membrane module assembly
Membrane modules were assembled by packing the hollow fiber T-type zeolite membranes bundles inside of the stainless steel cylinders.The open end of the membrane was fixed onto a stainless steel plate by silicon rubber,while the other end was free and capped with silicon rubber.Fig.1 shows the photograph of four membrane modules,which were composed of one(Module A),three(Module B)and seven(Module C and Module D)hollow fiber membrane bundles.The open end of membrane module was connected to a vacuum line for pervaporation(PV).Detailed dimensions of the membrane modules were listed in Table 1.A tubular membrane module(Module E)was also assembled for comparison.The tubular T-type zeolite membranes with a length of 40 cm were provided by Jiangsu Nine Heaven High-Tech Co.,Ltd.

Table 1Dimension parameters for the membrane modules
2.3.Evaluation of membrane and membrane module with PV and CFD simulation
Single hollow fiber membranes were evaluated by gas-tightness test.One end of the membrane was sealed by silicon rubber and the other end was connected to a vacuum line with an initial vacuum degree ofP1.After gas permeation through the membrane layer in dry air for60 s,the vacuum degree in the vacuum line decreased to a certain value ofP2.The pressure difference betweenP1andP2was used to evaluate the membrane quality.
PV performance of the membrane module was identified by dehydration of ethanol/water mixture.Fig.2 shows a schematic diagram of the apparatus for measuring PV performance of the membrane modules.The feed solution was continuously pumped into the shell side of the membrane module and the permeate was removed from the lumens of the membranes by a vacuum pump,which maintained a downstream pressure below 200 Pa throughout the operation.The permeated vapor was collected with two liquid nitrogen traps in parallel for sampling without interruption of the operation.Both of the feed and permeate were analyzed by a gas chromatograph(GC,GC-2014A,Shimadzu)equipped with a thermal conductivity detector.The separation factor(α)for componentiover componentjand the water permeation flux(J)are respectively de fined as:

Fig.1.Photographs of hollow fiber T-type zeolite membrane modules.(a)Module A,(b)Module B,(c)Module C,and(d)Module D.

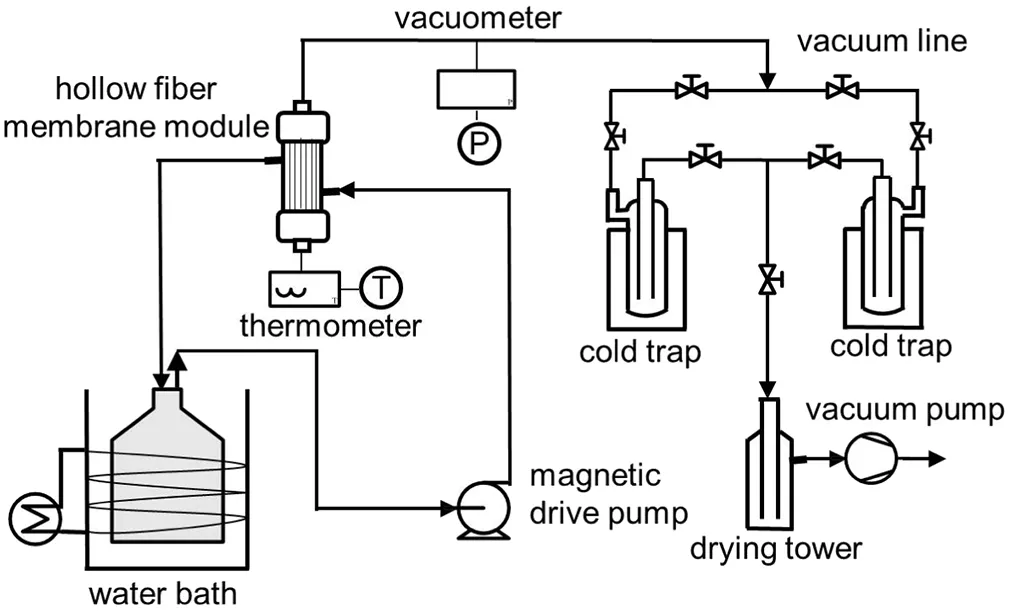
Fig.2.Schematic diagram of the experimental apparatus for evaluating membrane modules.
wherexiandxjare mass fractions of componentiand componentjfor the feed;yiandyjare the corresponding mass fractionsin the permeate;wis the mass of the permeated water,kg;tis the collecting time,h;andAis the active separation area of the membrane,m2.
Computational fluid dynamics(CFD)simulations were performed to visualize the flow rate distribution in the hollow fiber modules with a three-dimensional model.The module geometry was identical to that used for the experiments.The turbulence was modeled by the standardk-εmodel[22]based on the following assumptions:(1)the pure aquatic system was applied;and(2)the mass transport through membranes was ignored.
3.Results and Discussion
3.1.Quick evaluation of membrane quality
SEM images of a hollow fiber T-type zeolite membrane was shown in Fig.3.The T-type zeolite crystals were randomly-oriented and well inter-grown in the membrane layer with a thickness of about 20 μm.Typical PV performances of 5 pieces of the 20 cm hollow fiber T-type zeolite membranes were listed in Table 2.The permeation fluxes were(2.22 ± 0.15)kg·m−2·h−1and the separation factor fluctuated between 351 and 579.The separation performance of T-type zeolite membranes prepared in batch scale is slightly lower than that prepared in laboratory[18].It is important to evaluate the membrane quality by an easy and quick method for the scaled-up production.Thus,we used a gas-tightness experiment to preliminarily evaluate the membrane quality.Generally,high-quality T-type zeolite membranes should have low permeances for nitrogen and oxygen due to molecular sieving effect[23].However,the gas permeances should be high for the membranes with pinholes or defects,which could be judged by a gas-tightness experiment in dry air.To con firm the reliability,over 60 pieces of hollow fiber membranes were subjected to gas-tightness tests and evaluated by PV dehydration of 90 wt%ethanol/water mixture at 70°C.Fig.4 shows the relationship between pressure difference and PV performance of single hollow fiber T-type zeolite membranes.For the membranes with a pressure drop of lower than 50 Pa,the water concentration in permeate was always higher than 90 wt%.The separation performance rapidly decreased when the pressure drop increased from 50 Pa to 100 Pa,indicating the existence of more defects even pinholes in those membrane layers.The results suggested that it is feasible to quickly screen hollow fiber T-type zeolite membranes by the gas-tightness test.In the following experiment,the membranes with a pressure drop of lower than 50 Pa were used for module assembly.

Table 2Typical PV performance of the 20 cm T-type zeolite membranes for 90 wt%ethanol/water mixture at 70°C
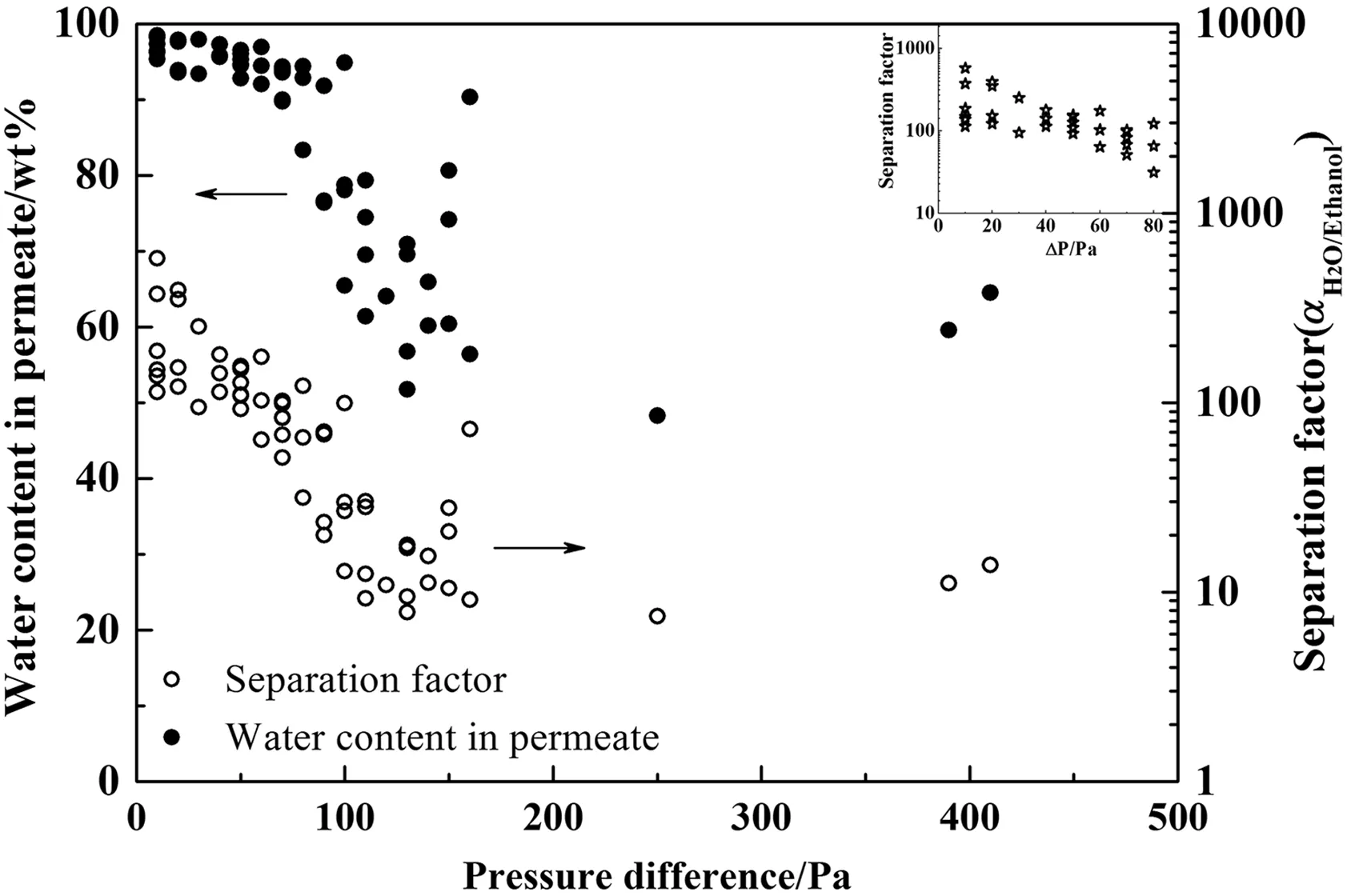
Fig.4.Correlation between gas-tightness and PV performance of single hollow fiber T-type zeolite membranes.

Fig.3.SEM surface(a)and cross-sectional(b)images of hollow fiber T-type zeolite membranes.
3.2.Membrane modules for ethanol dehydration
3.2.1.Effect of temperature
Fig.5 shows PV performance of hollow fiber membrane modules(Module A,B and C)as a function of operation temperature.The feed is 90 wt%ethanol/water mixtures with a constant feed flow of 2 L·min−1.It was observed that the water permeation fluxes increased with operation temperature for all the three membrane modules.Similar phenomenon was also reported for tubular NaA zeolite membrane modules[24].However,the water permeation fluxes for Module B and Module C was enhanced by 20%and 25%compared with Module A at 70°C.The difference in permeation flux should be contributed by the different geometric con figurations.The optimized module geometry could suppress undesirable concentration polarizations caused by uneven flow in shell side and consequently improve the permeation flux for pervaporation[25].It was noted that the achieved separation factors had a consequence of Module A<Module B<Module C.As reported by Bakeretal.[26],concentration polarization could largely reduce the separation factor for pervaporation.As shown in Fig.5,the difference in both permeation flux and separation factor was enlarged at a higher temperature for all the three modules,which should be attributed to the concentration polarizations caused by the enhanced permeation flux.

Fig.5.PV performance of the membrane modules with different con figurations.
3.2.2.Effect of feedflow
In order to con firm concentration polarization,we further evaluated module performance under different feed flows.Fig.6 shows the water permeation fluxes as a function of feed flow over the three modules for dehydration of 90 wt%ethanol/water mixtures at 70°C.Module A exhibited relatively stable water permeation flux when varying feed flow from 0.5 to 2 L·min−1.However,obvious enhancement in water permeation flux was observed for both Module B and Module C.The results indicated that the membrane bundle size within the module had a significant effect on the separation performance of membrane module.For module A,100 pieces of hollow fiber membranes were packed in one bundle,which could be difficult for the feed stream to flow through at such a feed condition.As a result,the module exhibited low mass transfer efficiency.Recently,Liuetal.[27]investigated the flow distribution in hollow fiber module by computational fluid dynamics(CFD)technique.A nonuniform flow distribution was demonstrated inside the bundle of hollow fiber ceramic-PDMS composite membranes.Higher separation efficiency for Module B and Module C was achieved because of the smaller hollow fiber bundles(35 and 15 pieces of membrane in one membrane bundle).As shown in Fig.6,only a slight increase in water permeation flux(<4%)was achieved for Module C at a flow rate of 2 L·min−1compared with Module B,which was due to the minor effect of bundle size on concentration polarization at a smaller size.Although the uniform distribution of HF T-type zeolite membranes might minimize the effect of concentration polarization and lead to high efficiency,it is more difficult to fabricate such a membrane module with high packing density.Furthermore,due to the brittleness and slenderness of HF T-type zeolite membrane,the adhesion of membrane to silicon rubber might be weakened by feed flow during long PV test.In order to con firm the speculation,we fabricated a module equipped with separated 7 pieces of HF T-type zeolite membranes.After several hours of PV dehydration,the separation factor decreased to less than 50,indicating the poor reliability for practical applications.
CFD simulation was further used to analyze the flow distributions in the module with different geometric con figurations.The feed flow was kept at 2 L·min−1.The simulated contours ofxvelocities in the middle(x=0.11 m)of Modules A–C at 70 °C were showed in Fig.7.It was found that the geometric con figuration significantly affected the velocity distributions in the modules.The increased bundles of hollow fiber membranes distinctly improved the bulk flow patterns,which resulted in thinner velocity boundary layer and more homogeneous velocity distributions.Thus,the uniform velocity distributions reduced concentration polarizations and temperature gradient in the modules,which strongly improved their performances.Therefore,higher increase in water permeation flux and separation factor were achieved for Module B and Module C.

Fig.6.PV performances of the membrane modules as a function of feed flow.
3.2.3.Effect of water content
From the perspective of process design,average water permeation flux is an important parameter for estimating required membrane area for specific applications.Fig.8(a)demonstrates the dehydration of 90 wt%ethanol/water mixture(ca.4 kg)at 70°C by three membrane modules under a batch operation mode.Anhydrous ethanol with a water content of approximate 0.1 wt%could be obtained after 67.0 h,40.5 h and 31.5 h for Module A,Module B and Module C,respectively.The average water permeation fluxes during the whole dehydration process were calculated to be 0.07 kg·m−2·h−1,0.12 kg·m−2·h−1,and 0.14 kg·m−2·h−1for Module A,Module B and Module C,respectively.As reported in our previous work,the average water permeation flux of Module B was fairly close to that of the tubular T-type zeolite membrane module(0.11 kg·m−2·h−1)[28].As mentioned above,single hollow fiber zeolite membranes showed much higher water permeation flux than the tubular membranes[18,28].Thus,the results suggested that the separation efficiency was strongly dependent on the module con figuration.The water permeation flux as a function of feed water content was shown in Fig.8(b).The water permeation flux of modules decreased with feed water content,which was in accordance with single hollow fiber T-type zeolite membranes[18].The water permeation fluxes always had a sequence of Module A<Module B<Module C in the range of 0.1 wt%–10 wt%water content.We speculated that it was difficult for the feed to flow through the big membrane bundles.As a result,the water content in the bundles was always lower than the bulk concentration.A schematic diagram of the water molecule distribution is illustrated in Fig.9.
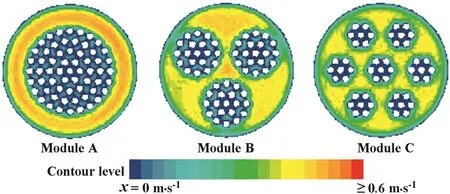
Fig.7.Contours of x velocity(m·s−1)predicted by CFD simulation in Modules A–C.

Fig.8.Dehydration of ethanol solution(4.0 kg)by hollow fiber membrane modules with different geometric con figurations.

Fig.9.Schematic diagram for water molecule distribution in small and big hollow fiber bundles.
3.3.Operation stability
For practical applications,we further assembled a larger membrane module(Module D)to evaluate the reliability during multi-cycle dehydration in batch scale.Each bundle in Module D was composed of 35 pieces of hollow fiber T-type zeolite membranes.The effective membrane area was approximately 0.25 m2.The module was used to dehydrate 3 kg 90 wt%ethanol/water mixtures at 70°C.The feed was pumped to the module at a flow rate of 2 L·min−1and the retentate was recycled to the feed tank.The process was operated continuously until the water content was lower than 0.1 wt%.As can be seen in Fig.10,operation duration for the first cycle was slightly shorter than those in the later cycles.As we know,the molecular size of ethanol(0.43 nm)is very similar to the aperture size of T-type zeolite(0.36 nm×0.51 nm).Therefore,ethanol molecules could continuously enter into zeolitic pores at the initial stage during pervaporation,which would strongly exert on the water molecule diffusion through membrane layer.The operation durations were almost identical in the later three cycles,suggesting good stability of the membrane module for ethanol dehydration.

Fig.10.Dehydration of 90 wt%ethanol solutions with HF zeolite membrane module at 70°C.
3.4.Comparison with tubular membrane module
A tubular membrane module with a membrane area of 0.1 m2(Module E)was also fabricated,which was comprised of 7 tubular T-type zeolite membranes.No turbulence promoters were used in Module E.The module had a water permeation flux of 0.72 kg·m−2·h−1for PV dehydration of 90 wt%ethanol/water mixtures at 70°C,which was similar to the single tubular membrane(0.81 kg·m−2·h−1).It might be due to the large space between the tubes as hypothesized in Fig.9.A comparison between Module E and the hollow fiber membrane modules was shown in Fig.11.Except for Module A,PV performances of hollow fiber membrane modules were comparable to that of the tubular membrane module.However,the packing density of hollow fiber membrane module was 10 times higher than that of tubular membrane module(Table 1).The packing density could be as high as 600 m2·m−3for Module D.Therefore,it is very promising to reduce the facility size and capital investment for PV plants by using hollow fiber zeolite membranes.

Fig.11.Comparison between hollow fiber membrane modules and tubular membrane module.
4.Conclusions
The membrane quality could be quickly evaluated by gas-tightness tests.Hollow fiber T-type zeolite membrane modules were successfully fabricated for dehydration of ethanol solutions.The permeation fluxes of the modules were dependent on the operation temperature,feed flow and feed water content.High permeation flux could be achieved at high feed flow,operation temperature and feed water content.The separation efficiency was strongly dependenton the geometric con figurations of the membrane modules.CFD simulation revealed that strong concentration polarization occurred in the case of big membrane bundle.The membrane module with seven hollow fiber membrane bundles exhibited optimized separation efficiency.The hollow fiber T-type zeolite membrane module had an average water permeation lf ux as high as 0.78 kg·m−2·h−1for 90 wt%ethanol/water mixture at 70°C.Moreover,the packing density could reach as high as 600 m2·m−3.Therefore,hollow fiber membrane modules could reduce the fabrication cost of separation equipment,which has promising applications in organic dehydration.
[1]T.C.Bowen,R.D.Noble,J.L.Falconer,Fundamentals and applications of pervaporation through zeolite membranes,J.Membr.Sci.245(1–2)(2004)1–33.
[2]P.D.Chapman,T.Oliveira,A.G.Livingston,K.Li,Membranes for the dehydration of solvents by pervaporation,J.Membr.Sci.318(1–2)(2008)5–37.
[3]S.L.Wee,C.T.Tye,S.Bhatia,Membrane separation process-pervaporation through zeolite membrane,Sep.Purif.Technol.63(3)(2008)500–516.
[4]N.W.Ockwig,T.M.Nenoff,Membranes for hydrogen separation,Chem.Rev.107(2007)4078–4110.
[5]E.E.McLeary,J.C.Jansen,F.Kapteijn,Zeolite based films,membranes and membrane reactors:Progress and prospects,Microporous Mesoporous Mater.90(1–3)(2006)198–220.
[6]J.Dong,Z.Xu,S.Yang,S.Murad,K.R.Hinkle,Zeolite membranes for ion separations from aqueous solutions,Curr.Opin.Chem.Eng.8(2015)15–20.
[7]Y.Morigami,M.Kondo,J.Abe,H.Kita,K.Okamoto,The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane,Sep.Purif.Technol.25(1–3)(2001)251–260.
[8]J.Gascon,F.Kapteijn,B.Zornoza,V.Sebastián,C.Casado,J.Coronas,Practical approach to zeolitic membranes and coatings:State of the art,opportunities,barriers,and future perspectives,Chem.Mater.24(15)(2012)2829–2844.
[9]Y.S.Lin,M.C.Duke,Recent progress in polycrystalline zeolite membrane research,Curr.Opin.Chem.Eng.2(2013)209–216.
[10]M.Tsapatsis,Toward high-throughput zeolite membranes,Science334(2011)767–768.
[11]J.Caro,Are MOF membranes better in gas separation than those made of zeolites?Curr.Opin.Chem.Eng.1(2011)77–83.
[12]J.Caro,M.Noack,Zeolite membranes-status and prospective,Adv.Nano.Mater.1(2010)1–96.
[13]M.P.Pina,R.Mallada,M.Arruebo,M.Urbiztondo,N.Navascués,O.de la Iglesia,J.Santamaria,Zeolite films and membranes.Emerging applications,Microporous Mesoporous Mater.144(1–3)(2011)19–27.
[14]X.Xu,W.Yang,J.Liu,L.Lin,N.Stroh,H.Brunner,Synthesis of NaA zeolite membrane on a ceramic hollow fiber,J.Membr.Sci.229(1–2)(2004)81–85.
[15]Z.Wang,Q.Ge,J.Shao,Y.Yan,High performance zeolite LTA pervaporation membranes on ceramic hollow fibers by dipcoating-wiping seed deposition,J.Am.Chem.Soc.131(2009)6910–6911.
[16]L.Lai,J.Shao,Q.Ge,Z.Wang,Y.Yan,The preparation of zeolite NaA membranes on the inner surface of hollow fiber supports,J.Membr.Sci.409-410(2012)318–328.
[17]X.Shu,X.Wang,Q.Kong,X.Gu,N.Xu,High- flux Mfizeolite membrane supported on YSZ hollow fiber for separation of ethanol/water,Ind.Eng.Chem.Res.51(37)(2012)12073–12080.
[18]X.Wang,Y.Chen,C.Zhang,X.Gu,N.Xu,Preparation and characterization of highflux T-type zeolite membranes supported on YSZ hollow fibers,J.Membr.Sci.455(2014)294–304.
[19]X.Tan,S.Liu,K.Li,Preparation and characterization of inorganic hollow fiber membranes,J.Membr.Sci.188(2001)87–95.
[20]C.C.Wei,K.Li,Yttria-stabilized zirconia(YSZ)-based hollow fiber solid oxide fuel cells,Ind.Eng.Chem.Res.47(2008)1506–1512.
[21]Y.Hasegawa,C.Abe,M.Nishioka,K.Sato,T.Nagase,T.Hanaoka,Formation of high flux CHA-type zeolite membranes and their application to the dehydration of alcohol solutions,J.Membr.Sci.364(1–2)(2010)318–324.
[22]F.Zhang,W.Jing,W.Xing,Modeling of cross- flow filtration processes in an airlift ceramic membrane reactor,Ind.Eng.Chem.Res.48(2009)10637–10642.
[23]Y.Cui,H.Kita,K.Okamoto,Preparation and gas separation performance of zeolite T membrane,J.Mater.Chem.14(2004)924–932.
[24]M.Kondo,M.Komori,H.Kita,K.Okamoto,Tubular-type pervaporation module with zeolite NaA membrane,J.Membr.Sci.133(1)(1997)133–141.
[25]M.M.Teoh,S.Bonyadi,T.S.Chung,Investigation of different hollow fiber module designs for flux enhancement in the membrane distillation process,J.Membr.Sci.311(1–2)(2008)371–379.
[26]R.W.Baker,J.G.Wijmans,A.L.Athayde,R.Daniels,J.H.Ly,M.Le,The effect of concentration polarization on the separation of volatile organic compounds from water by pervaporation,J.Membr.Sci.137(1–2)(1997)159–172.
[27]D.Liu,G.Liu,L.Meng,Z.Dong,K.Huang,W.Jin,Hollow fiber modules with ceramicsupported PDMS composite membranes for pervaporation recovery of bio-butanol,Sep.Purif.Technol.146(2015)24–32.
[28]X.Wang,Z.Yang,C.Yu,L.Yin,C.Zhang,X.Gu,Preparation of T-type zeolite membranes using a dip-coating seeding suspension containing colloidal SiO2,Microporous Mesoporous Mater.197(2014)17–25.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Influence of Na+,K+,Mg2+,Ca2+,and Fe3+on filterability and settleability of drilling sludge☆
- An optimal filter based MPC for systems with arbitrary disturbances☆
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
