伴胸腺瘤重症肌无力发病机制研究进展
2017-04-25王中魁黄旭升
王中魁 黄旭升
伴胸腺瘤重症肌无力发病机制研究进展
王中魁 黄旭升
重症肌无力(myasthenia gravis,MG)是以胸腺为靶器官的器官特异性自身免疫性疾病,伴胸腺瘤MG与不伴胸腺瘤MG发病机制不同。近年来发现伴胸腺瘤MG在T细胞数量和功能、自身抗体的种类以及遗传学等方面与不伴胸腺瘤MG存在差异。本文旨在结合文献从胸腺微环境、T细胞发育、自身抗体及遗传学等方面在伴胸腺瘤MG发病机制中作用进行综述。
重症肌无力;胸腺瘤;发病机制
重症肌无力(myasthenia gravis,MG)是T细胞辅助、乙酰胆碱受体抗体(AChR-Ab)介导的神经肌肉接头传递障碍的自身免疫性疾病,也是一种以胸腺为靶器官的自身免疫性疾病[1]。MG多伴有胸腺病变,包括胸腺瘤、胸腺增生和胸腺萎缩[2],其中约15%为胸腺瘤。胸腺瘤是一种来源于胸腺上皮细胞的良性或低度恶性肿瘤,是成年人最常见的前纵隔肿瘤。流行病学结果显示大约20%~25%胸腺瘤伴发MG,大约10%~20% MG患者伴发胸腺瘤[3]。胸腺瘤与MG关系最为密切,也是伴发自身免疫病最多的人类肿瘤[4]。伴胸腺瘤MG与不伴胸腺瘤MG比较,临床特点、治疗反应及预后均不尽相同,提示伴/不伴胸腺瘤MG发病机制不同。本文对胸腺瘤在MG发病中的免疫学机制进行综述。
1 胸腺瘤微环境异常
胸腺是重要的免疫器官,初始T细胞在胸腺内经历阳性选择和阴性选择发育、分化为成熟T细胞[5]。胸腺瘤是来源于胸腺上皮的肿瘤,根据淋巴细胞和上皮细胞的比例,胸腺瘤分为A、AB、B型(包括B1、B2、B3三型)和C型[6]。以髓质成分为主的A型胸腺瘤很少伴发MG[7],B型胸腺瘤髓质区域内缺乏髓质特征,取而代之以混乱的皮质结构,其中B1型为皮质优势型、富含淋巴细胞,胸腺细胞含量高,伴有胸腺髓质分化,部分肿瘤区域内可有正常的Hassall小体结构;B2、B3胸腺瘤中Hassall小体结构极少[6]。B型胸腺瘤最常伴发MG。C型胸腺瘤侵袭性强,恶性程度高,却极少伴发MG。与胸腺增生相比,胸腺瘤缺乏髓质和生发中心,皮髓质区域分界不清[8],皮髓质结构异常引起T细胞分化、发育紊乱和功能障碍,参与MG发病。与不伴MG胸腺瘤相比,伴胸腺瘤MG胸腺瘤组织可产生并向外周血迁移大量成熟CD4+CD45RA+细胞[9-11],而正常AChR抗体反应性的成熟T细胞再循环到胸腺瘤中后可能被激活并大量释放至外周免疫系统发挥致病作用[12]。Belharazem等[13]发现伴胸腺瘤MG患者外周血及胸腺瘤组织中凋亡抑制蛋白——类FLICE抑制蛋白(c-FLIP)表达量显著升高,c-FLIP可促进CD4+T细胞的分化发育,提示伴胸腺瘤MG发病可能与中枢及外周淋巴系统免疫耐受功能障碍有关。
2 胸腺瘤中T细胞异常发育
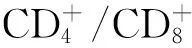
由于胸腺瘤缺乏髓质成分,经历了阳性选择的T细胞不能充分与树突状细胞(DC)和巨噬细胞接触、经历有效的阴性选择。Inoue等[15]的研究表明了胸腺瘤中HLA-DR(MHCⅡ类分子)的重要调控因子二类转化活化因子(class Ⅱ transactivator,CⅡTA)表达降低,而胸腺瘤中CD4+CD8-T细胞亚群中CD3+细胞的比例与HLA-DR和CⅡTA显著相关[18]。由此推测胸腺瘤上皮细胞HLA-DR表达减少引起了胸腺瘤组织中CD4+CD8-单阳性T细胞分化中阴性选择功能障碍。自身免疫调控因子(autoimmune regulator,AIRE)是表达在胸腺髓质上皮细胞上的转录因子,调控胸腺中CHRNA基因位点的AChRα亚单位的表达[19],对于自身反应性T细胞阴性选择和中枢性耐受形成具有重要意义。伴胸腺瘤MG患者胸腺瘤髓质上皮细胞AIRE表达降低,同时胸腺瘤细胞与正常胸腺细胞表达的包括titin抗原表型的多种抗原表型[20]、HLA-Ⅱ类分子[21-23]以及多种AChR亚单位[24-25]均发生改变,这些异常表达的免疫调节因子及抗原表型可能导致针对表达AChRα亚单位受体的T细胞的阳性选择或阴性选择发生偏倚或功能障碍,这些针对AChRα亚单位自身反应性T细胞进入外周免疫系统,攻击神经肌肉接头处AChRα亚单位,引起MG发病[26-27]。
3 伴胸腺瘤MG中自身抗体
除去作用机制研究最清楚的MG自身抗体——AChRAb外,伴胸腺瘤MG患者体内还存在多种其他自身抗体,这些抗体可能也参与了伴胸腺瘤MG的发病。
3.1 抗骨骼肌抗原抗体 连接素(titin)和兰尼碱受体 (ryanodine receptor,RyR)是最主要的自身抗体攻击的靶点。约90%的伴胸腺瘤MG患者Titin抗体或RyR抗体阳性,其中Titin抗体是胸腺瘤的标记性抗体[28-29]。
3.2 抗细胞因子抗体 抗细胞因子抗体是伴胸腺瘤MG常见的血清抗体。体外试验发现抗细胞因子抗体由胸腺瘤细胞产生[30],伴胸腺瘤MG中70%和单纯胸腺瘤患者中30%体内存在针对IFN-α和IFN-ω的中和抗体, 伴胸腺瘤MG中约50%有IL-12的中和抗体[31]。最近一项研究表明伴胸腺瘤MG患者高表达Ⅰ型IFN分子,包括IFN-α2、IFN-α8、IFN-ω和IFN-β[32]。极少部分胸腺瘤患者体内存在针对Th17细胞相关细胞因子的中和抗体,尤其是与控制念珠菌感染相关的IL-17F和IL-22的中和抗体[33]。
4 胸腺瘤的遗传学改变及其与伴胸腺瘤MG发病的相关性
HLA 6p21位点杂合缺失是发生在所有病理类型胸腺瘤的一种遗传学改变,该改变可引起HLAⅡ类分子表达下调[23,34],非HLA基因多态性可以影响CTLA4和蛋白酪氨酸磷酸酶22(PTPN22)[35]等T细胞受体信号通路分子,这些信号分子表达改变可以引起阴性选择功能障碍、自身反应性T细胞的增殖,参与伴胸腺瘤MG发病。Christopoulos等[36]研究发现胸腺瘤患者存在CD3 zeta链(CD247)基因缺陷,胸腺瘤患者γδ和αβT细胞的TCR复合体中CD247数量减少,初始T细胞和低反应性γδT细胞、αβT细胞数量增高,引起T细胞免疫功能缺陷,增加了对于感染的易感性。Yang等[37]的研究发现HLA DQ等位基因中DQA1*0401和DQB1*0604两种基因型在伴胸腺瘤MG中出现频率高,DQA1*0501和DQB1*0301在不伴MG胸腺瘤中出现频率高。该结果提示HLA DQA和DQB位点突变引起的基因多态性与MG发病的易感性有关,但是具体作用机制仍不清楚。此外,Xu等[38]通过分析T细胞免疫球蛋白和黏蛋白结构域-3(Tim-3)574位点多态性,发现伴胸腺瘤MG的GT+TT基因型出现率及T等位基因出现率显著高于不伴MG胸腺瘤。然而该基因多态性导致MG的具体机制仍需进一步研究。
5 辅助性T细胞与伴胸腺瘤MG
近年来辅助性T细胞,包括调节性T细胞(Treg)和Th17辅助细胞(T helper type 17 cells,Th17细胞)在MG及胸腺瘤发病机制中的作用成为研究热点。Treg细胞是一群具有免疫抑制功能的负调控细胞,可与免疫应答中多种细胞如DC、B细胞等相互作用,通过与免疫效应细胞直接接触的方式抑制效应细胞的功能,发挥免疫抑制作用。Masuda等[39]发现MG患者外周血Treg细胞比例减少,且治疗前后Treg细胞比例变化与MG临床评分变化呈正相关。Scarpino等[40]发现正常胸腺及增生胸腺中CD4+CD25+FoxP3+Treg较多,主要集中在Hassall小体周围,Treg细胞数量与是否合并MG无关;胸腺瘤中CD4+CD25+FoxP3+Treg细胞明显减少,在B1型髓质中Treg细胞相对较多。Wang等[41]发现伴胸腺瘤MG的胸腺瘤组织中FoxP3+Treg细胞较正常胸腺显著减少,FoxP3mRNA转录水平下降,其中B1型转录水平最高,B3型最低,提示FoxP3转录和表达可能与胸腺瘤类型和肿瘤上皮细胞功能有关,胸腺瘤FoxP3mRNA转录水平异常可能导致Treg细胞减少,引起自身免疫耐受紊乱,造成MG的发病。
Th17细胞是近年新发现的特征性表达促炎因子IL-17的功能性CD4+T辅助细胞亚群[42]。Wang等[43]发现伴胸腺瘤MG患者外周血的IL-17、IL-1β、IL-23等促炎细胞因子表达量增加,伴胸腺瘤MG患者外周血Th17细胞的比例与血清AChRAb浓度正相关,且伴胸腺瘤MG组QMG评分与Th17细胞比例呈正相关。 胸腺间质淋巴细胞生成素(TSLP)是一种IL-7样细胞因子,由胸腺髓质Hassall小体上皮细胞分泌,是淋巴细胞发育分化的重要调节因子。Watanabe等[44]发现TSLP可以大量活化cDC,cDC诱导CD4+CD8-CD25-细胞增殖后,约50%的增殖细胞分化为CD4+CD8-CD25-FoxP3+Treg细胞。Salomon等[45]发现CD28是Treg细胞发育必不可少的共刺激信号分子。TSLP是目前所知的惟一可以活化DC表达CD28配体-CD80/CD86的生理信号[46]。Wang等[47]发现miR-19b-5p通过转录后调控机制降低TSLP的表达,证实了miR-19b-5p在胸腺瘤中的异常上调可能是TSLP减少的关键机制,证实miR-19b-5p/TSLP调控通路参与Treg细胞生成减少、Th17细胞增多过程的调控。
滤泡辅助性T细胞(T follicular helper cells,TFH)表型是CD4+CXCR5+,是CD4+T辅助细胞的一个亚群。TFH可以迁移到生发中心内,为B细胞提供刺激信号,促进B细胞分化发育成长期存活的浆细胞和记忆B细胞。TFH细胞引导效应B细胞和记忆B细胞产生自身抗体进而在MG发病中发挥重要作用。TFH高表达可诱导共刺激分子(ICOS),程序性死亡1(PD-1)及其配体,转录因子B细胞淋巴瘤6(Bcl-6)和调控因子等分子。Song等[48]的研究亦发现伴胸腺瘤MG患者胸腺瘤组织中TFH细胞比例升高,而且TFH相关分子ICOS,PD-1及其配体,Bcl-6表达量显著升高。
综上,近年来的研究表明胸腺自身免疫耐受机制的破坏可能是胸腺瘤发生以及包括MG在内的自身免疫性疾病发生的重要原因。阐明这些可能的机制,有助于提高对于MG等自身免疫性疾病的认识,促进此类疾病预防和治疗水平的提高。
[1]Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity[J]. Lancet Neurol,2009,8 (5): 475-490.
[2]Ströbel P, Chuang WY, Marx A. Thymoma-associated paraneoplastic myasthenia gravis. In: Kaminski HJ. Myasthenia gravis and related disorders [M]. Second edition. New York: Humana Press, 2009.105-117.
[3]Lucchi M, Ricciard R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment[J]. Eur J Cardiothorac Surg,2009,35(5):812-816.
[4]Müller-Hermelink HK, Marx A. Thymoma[J]. Curr Opin Oncol,2000,12(5): 426-433.
[5]Kurd N, Robey EA. T-cell selection in the thymus: a spatial and temporal perspective[J]. Immunol Rev,2016,271(1):114-126.
[6]Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 Classification[J]. J Thorac Oncol,2015,10(9):1243-1260.
[7]Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis[J]. J Clin Oncol, 2004,22(8):1501-1509.
[8]Willcox N, Leite MI, Kadota Y, et al. Autoimmunizing mechanisms in thymoma and thymus[J]. Ann NY Acad Sci,2008,1132:163-173.
[9]Strobel P, Helmreich M, Meniouda kis G, et al. Paraneoplastic myasthenia gravis correlates with generation of matur e naïve CD4(+) T cells in thymomas[J]. Blood,2002,100(1):159 -166.
[10]Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease[J]. Blood,2000,96(12): 3872-3879.
[11]Buckley C, Douek D, Newsom-Davis J, et al. Mature, long-lived CD4+and CD8+T cells are generated by the thymoma in myasthenia gravis[J]. Ann Neurol,2001,50(1):64-72.
[12]Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis[J]. Autoimmunity, 2010,43(5-6):413-427.
[13]Belharazem D, Schalke B, Gold R, et al. cFLIP overexpression in T cells in thymoma-associated myasthenia gravis[J]. Ann Clin Transl Neurol,2015, 2(9): 894-905.
[14]Weksler B, Lu B. Alterations of the immune system in thymic malignancies[J]. J Thorac Oncol,2014,9(9Suppl 2): S137-S142.
[15]Inoue M, Okumura M, Miyoshi S, et al. Impaired expression of MHC class Ⅱ molecules in response to interferon-gamma (IFN-γ) on human thymoma neoplastic epithelial cells[J]. Clin Exp Immunol,1999,117(1):1-7.
[16]Inoue M, Fujii Y, Okumura M, et al. T-cell development in human thymoma[J]. Pathol Res Pract, 1999,195(8):541-547.
[17]Fujii Y, Okumura M, Yamamoto S. Flow cytometric study of lymphocytes associated with thymoma and other thymic tumors[J]. J Surg Res,1999,82(2):312-318.
[18]Kadota Y, Okumura M, Miyoshi S, et al. Altered T cell development in human thymoma is related to impairment of MHC class Ⅱ transactivator expression induced by interferon-gamma (IFN-γ) [J]. Clin Exp Immunol,2000,121(1):59-68.
[19]Giraud M, Taubert R, Vandiedonck C, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus[J]. Nature,2007,448(7156): 934-937.
[20]Dardenne M, Savino W, Bach JF. Thymomatous epithelial cells and skeletal muscle share a common epitope defined by a monoclonal antibody[J]. Am J Pathol,1987,126(1):194-198.
[21]Willcox N, Schluep M, Ritter MA, et al. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? [J]. Am J Pathol,1987,127(3):447-460.
[22]Savino W, Manganella G, Verley JM, et al. Thymoma epithelial cells secrete thymic hormone but do not express class Ⅱ antigens of the major histocompatibility complex[J]. J Clin Invest,1985,76(3):1140-1146.
[23]Strobel P, Chuang WY, Chuvpilo S, et al. Common cellular and diverse genetic basis of thymoma-associated myasthenia gravis: role of MHC class Ⅱ and AIRE genes and genetic polymorphisms[J]. Ann N Y Acad Sci,2008,1132:143-156.
[24]Maclennan CA, Vincent A, Marx A, et al. Preferential expression of AChR epsilon-subunit in thymomas from patients with myasthenia gravis[J]. J Neuroimmunol,2008,201-202:28-32.
[25]Kirchner T, Hoppe F, Muller-Hermelink HK, et al. Acetylcholine receptor epitopes on epithelial cells of thymoma in myastheni a gravis[J]. Lancet,1987,1(8526):218.
[26]Kisand K, Lilic D, Casanova JL, et al. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications[J]. Eur J Immunol,2011,41(6):1517-1527.
[27]Willcox N, Leite MI, Kadota Y, et al. Autoimmunizing mechanisms in thymoma and thymus[J]. Ann N Y Acad Sci,2008,1132:163-173.
[28]Romi F, Skeie GO, Gilhus NE, et al. Striational antibodies in myasthenia gravis: reactivity and possible clinical signi ficance[J]. Arch Neurol,2005,62:442-446.
[29]Romi F. Thymoma in myasthenia gravis: from diagnosis to treatment[J]. Autoimmun Dis,2011, 2011:474512.
[30]Shiono H, Wong YL, Matthews I, et al. Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmunization in the tumor[J]. Int Immunol,2003,15(8): 903-913.
[31]Meager A, Wadhwa M, Dilger P, et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis[J]. Clin Exp Immunol 2003,132(1):128-136.
[32]Cufi P, Soussan P, Truffault F, et al. Thymoma-associated myasthenia gravis: on the search for a pathogen signature[J]. J Autoimmun,2014,52:29-35.
[33]Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines[J]. J Exp Med,2010,207(2):299-308.
[34]Zettl A, Strobel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma[J]. Am J Pathol,2000,157(1):257-266.
[35]Gianchecchi E, Palombi M, Fierabracci A. The putative role of the C1858T polymor-phism of protein tyrosine phosphatase PTPN22 gene in autoimmunity[J].Autoimmun Rev,2013, 12(7):717- 725.
[36]Christopoulos P, Dopfer EP, Malkovsky M, et al. A novel thymoma-associated immunodeficiency with increased naive T cells and reduced CD247 expression[J]. J Immunol,2015,194(7):3045 -3053.
[37]Yang H, Hao J, Peng X, et al. The association of HLA-DQA1*0401 and DQB1*0604 with thymomatous myasthenia gravis in northern Chinese patients[J]. J Neurol Sci,2012,312 (1-2):57-61.
[38]Xu G, Zheng K, Lu X, et al. Association between polymorphisms in the promoter region of T cell immunoglobulin and mucin domain-3 and myasthenia gravis-associated thymoma[J]. Oncol Lett,2015,9(3):1470-1474.
[39]Masuda M, Matsumoto M, Tanaka S, et al. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients[J]. J Neuroimmunol,2010,225 (1-2):123-131.
[40]Scarpino S, Di Napoli A, Stoppacciaro A, et al. Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas[J]. Clin Exp Immunol,2007,149(3):504-512.
[41]Wang Zhongkui, Wang Jinghua, Deng Benqiang, et al. Reduction of natural regulatory T cells in thymomas accompanying myasthenia gravis and its possible association with Foxp3 and thymic stromal lymphopoietin[J]. Journal of Medical Colleges of People's Liberation Army of China,2009,24(1):50-55.
[42]Spolski R, Leonard WJ. Cytokine mediators of Th17 function[J]. Eur J Immunol,2009,39(3): 658-661.
[43]Wang Z, Wang W, Chen Y, et al. T helper type 17 cells expand in patients with myasthenia-associated thymoma[J]. Scand J Immunol,2012,76(1):54-61.
[44]Watanabe N, Wang YH, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+regulatory T cells in human thymus[J]. Nature,2005, 436:1181-1185.
[45]Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+immunoregulatory T cells that control autoimmune diabetes[J]. Immunity, 2000,12:431-440.
[46]Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis[J]. Autoimmunity, 2010,43(5-6):413-427.
[47]Wang Z, Chen Y, Xu S, et al. Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas[J]. J Neuroimmunol, 2015,288 (15):34-39.
[48]Song Y, Zhou L, Miao F, et al. Increased frequency of thymic T follicular helper cells in myasthenia gravis patients with thymoma[J]. J Thorac Dis,2016,8(3):314-322.
(本文编辑:邹晨双)
10.3969/j.issn.1006-2963.2017.02.015
100853 中国人民解放军总医院神经科
黄旭升,Email:lewish301@sina.com
R746.1
A
1006-2963(2017)02-0139-05
2016-08-03)
