水体中双氯芬酸的分布与生态效应研究
2015-08-22张正华陆光华丁剑楠
张正华,陆光华,丁剑楠
(浅水湖泊综合治理与资源开发教育部重点实验室,河海大学环境学院,南京 210098)
· 综述 ·
水体中双氯芬酸的分布与生态效应研究
张正华,陆光华,丁剑楠
(浅水湖泊综合治理与资源开发教育部重点实验室,河海大学环境学院,南京210098)
双氯芬酸作为消炎类药物,在国内外被广泛使用。作为新兴污染物,双氯芬酸在水体环境中经常被检出,对生态安全及人类健康存在潜在威胁。本文总结了双氯芬酸的水体分布、在水生生物体内的富集效应及代谢,并阐述了双氯芬酸的生物毒理效应。在综述双氯芬酸现有研究成果基础上,提出了双氯芬酸的主要研究方向。
双氯芬酸;生物富集;代谢;生态毒理效应
由于药物在环境中不断被检出,已成为一类主要的新兴污染物。大量文献报道记载了药物活性化合物在水环境中的发生情况[1~4],并且其在自然水体中检出率很高。双氯芬酸的通过抑制环氧化酶活性,从而阻断花生四烯酸转化前列腺素,抑制前列腺素的合成,前列腺素不仅是疼痛感应介质也可以抑制血液凝固、调节血管通透性与血管扩张[5, 6],因此抑制前列腺素合成可以起到消炎止痛作用。
双氯芬酸经生物体吸收、代谢后排出进入环境中。研究表明大部分双氯芬酸以原型或以代谢产物的形式排放到环境中,双氯芬酸代谢产物易在污水处理厂中裂解回原形排放到环境水体中[7]。很多水生生物体内存在与人体中相似的药物靶分子[8],因此双氯芬酸暴露下会对生物产生毒性。虽然双氯芬酸在水环境中浓度不高,一般为ng/L水平,但是因其亲脂性,在水环境中易于生物富集,因此,水环境中较低浓度的双氯芬酸也可能对水生生物产生毒性作用[9, 10]。
1 双氯芬酸在水环境中的分布
双氯芬酸在污水处理厂中的去除效率在0%~80%之间,大部分集中在21%~40%之间[4]。由于在污水处理厂中双氯芬酸的降解并不完全,许多国家在污水、地表水甚至地下水中都发现了双氯芬酸的存在。
1.1在污水厂尾水中的分布
在市政污水厂尾水中,双氯芬酸是最常检测到的药物之一,已有文献报道了双氯芬酸在市政污水处理厂尾水的浓度水平, 范围涵盖了欧洲、北美、非洲和亚洲部分国家。Letzel等[11]在德国美茵河9个污水处理厂尾水采集到的60个样品中都检测出双氯芬酸,检测的最低浓度为120 ng/L,最高浓度达2200 ng/L。在英国,Roberts等[12]对泰恩河污水处理厂尾水中的双氯芬酸进行了分析研究,结果发现双氯芬酸残留浓度范围为36~300 ng/L。Sim等[13]通过对韩国的12个污水处理厂中的药物进行检测,在市政污水处理厂、家畜污水处理厂、医院污水处理厂以及药物制造污水处理厂的尾水中分别检测到双氯芬酸的残留,检出频率最高的是市政污水处理厂,但药物制造污水处理厂尾水中检测浓度最高,达到19.2 μg/L,市政污水处理厂中检出频率高与家庭中消炎止痛药物的大量使用有关。在国内,Sui等[14]检测了北京两个污水处理厂尾水中双氯芬酸的残留情况,最高浓度为0.46 μg/L,最低为0.035 μg/L。
1.2在地表水中的分布
与污水厂尾水中双氯芬酸检测到的浓度相比,地表水中双氯芬酸浓度相对较低,但作为受纳水体,双氯芬酸广泛存在于地表水中。Togola等[15]采集法国塞纳河口不同月份、不同地点的水样,检测到双氯芬酸的的最高浓度为172.5 ng/L,最低浓度为7.1 ng/L,检测浓度随地点以及河流水量的不同而变化。Matamoros等[16]调查了丹麦河流以及恢复湿地中的17种新型污染物,其中双氯芬酸的浓度最高,在布拉布兰湖出口浓度达到156 ng/L,双氯芬酸的浓度因丰水期河水流量大而得到稀释,浓度下降,这一变化规律与Togola等[15]的研究结果一致。
国内对药物的研究起步较晚,Wang等[17]在调查黄河、海河及辽河中的酸性药物残留时发现双氯芬酸有较高的检出率,最高浓度达到717 ng/L。Zhao等[18]在珠江流溪河、石井河以及珠江河段的地表水中检测出双氯芬酸,最高浓度为150 ng/L。关于双氯芬酸在我国各大流域残留水平的报道不多,但资料显示中国存在大量的风湿性关节炎和骨关节炎的患者,对消炎止痛药物的使用量较大,因此,需要加强这方面的分析检测[19]。
1.3在地下水及饮用水中的分布
由于土壤层的净化作用以及饮用水净水厂的进一步处理,饮用水以及地下水中双氯芬酸浓度很低或者低于检测限,但在一些双氯芬酸使用量大的国家,仍能检测到双氯芬酸的存在。Rabiet等[20]在地中海饮用水源中检测到双氯芬酸的浓度为2 ng/L。Heberer等[21]在希腊与柏林调查不同水体中药物的残留时,在饮用水中检测到了双氯芬酸的存在。表1列举了双氯芬酸在部分水体环境中的残留情况。

表1 水体环境中双氯芬酸的残留水平
2 双氯芬酸的富集与代谢
2.1双氯芬酸的生物富集
虽然双氯芬酸在水体中残留的浓度不高,但其亲脂性较强,容易被水生生物吸收并富集,长期暴露使双氯芬酸在水生生物体内的含量升高。
目前对双氯芬酸的富集研究比较少,主要集中在鱼类富集研究。Schwaiger等[6]在实验条件下得到虹鳟鱼 (Oncorhynchusmykiss) 在不同浓度的双氯芬酸暴露28 d的生物富集因子,结果显示双氯芬酸在鱼肝脏中最易富集,生物富集因子范围为12~2732。Mehinto等[9]研究了虹鳟鱼对双氯芬酸的生物富集,0.5 μg /L、5 μg /L和25 μg /L的双氯芬酸暴露21 d在胆汁中的生物富集因子分别为657、 534和509,上述研究结果表明了双氯芬酸在不同鱼组织中产生生物富集效应。而Memmert[29]通过双氯芬酸不同浓度的暴露实验得到虹鳟鱼的稳态、动态以及脂质规范化等3种生物富集因子,范围在2~9之间,生物富集效应不明显。来自不同实验室的生物富集数据存在较大差异,需要进一步开展影响因素分析。
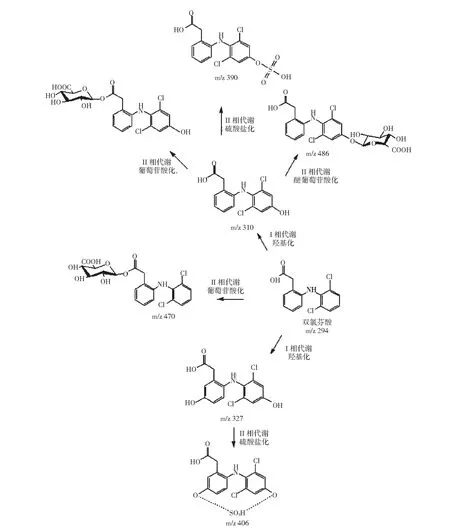
图 双氯芬酸主要代谢途径及代谢产物[35~37]Fig. The major metabolic pathways and metabolites of diclofenac
Ericson等[30]测定双氯芬酸对海洋底栖生物蓝贻贝 (Bluemussels) 的生态效应,不同浓度暴露时,双氯芬酸均在蓝贝体内较高浓度残留,生物富集因子在10到180之间,表明双氯芬酸在底栖生物体内也存在生物富集现象。
2.2双氯芬酸的代谢转化
双氯芬酸在生物体内经代谢酶的作用发生代谢反应,通过对人体及生物的研究,双氯芬酸主要的代谢产物是羟基化、硫酸盐化以及葡萄苷酸化的双氯芬酸。研究表明,哺乳动物、植物以及微生物双氯芬酸的Ⅰ相代谢产物是4’-羟基双氯芬酸和5-羟基双氯芬酸,主要的Ⅱ相代谢产物是酰基葡萄苷酸化的产物[31,32]。
双氯芬酸的代谢产物主要通过排尿排出生物体,且经常在污水处理厂以及自然水体被检测到[33]。Mueller等[34]通过体外实验测定得到双氯芬酸在人体肝脏中的8种代谢产物,羟基化双氯芬酸是主要的Ⅰ相代谢产物,酰基葡萄苷酸化与葡萄苷酸化双氯芬酸是主要的Ⅱ相代谢产物。Kosjek等[35]对活性污泥生物反应器中双氯芬酸的代谢产物进行了分析,测出多种双氯芬酸代谢产物,并测出双氯芬酸酰胺化的Ⅱ相产物。Lahti等[36]在鱼胆汁中检测到9种双氯芬酸代谢产物,包括两种Ⅰ相代谢产物与7种Ⅱ相代谢产物。其中,Ⅰ相代谢产物羟基双氯芬酸的酰基葡萄苷酸化产物是双氯芬酸主要的Ⅱ相代谢产物,这与Mueller等[34]报道的结果一致。上图显示了双氯芬酸的主要代谢途径及代谢产物,m/z为质核比。
3 双氯芬酸对水生生物的毒性
双氯芬酸的毒性影响最早引起关注是由于巴基斯坦秃鹰数量的急剧下降,研究结果表明,秃鹰吃了喂食了双氯芬酸的牲畜尸体,因而引起肾脏疾病导致秃鹰死亡[38]。双氯芬酸对生物体的毒性取决于受试生物、暴露时间以及响应终端。环境中双氯芬酸浓度很低,不易引起急性毒性,但由于长期存在于环境中,它对非靶生物可能存在慢性毒性。研究表明,长期暴露于双氯芬酸会造成生物的组织病理学毒性、细胞毒性以及基因毒性等[10, 39~41]。
表2总结了双氯芬酸暴露对不同生物标志物的毒性影响。Ferrari等测定了几种非甾类药物对月牙藻 (Pseudokirchneriellasubcapitata) 的慢性毒性,得到双氯芬酸对月牙藻生长率的最低可见效应浓度 (Lowest observed effect concentration) 为20 mg/L,并计算得出了预测无效应浓度为116 μg/L,比检测到的环境中双氯芬酸浓度高1000倍[42]。Cleuvers等[43]研究抗生素对藻类的毒性时发现随着双氯芬酸浓度的增加,海藻的生长率递减,半数效应浓度EC50(median effective concentration)为71.9 mg/L,表现为对海藻具有轻微毒性。

表2 双氯芬酸对不同生物的毒性影响
注:LOEC(Lowest observed effect concentration)—最低效应浓度。
Lee等[44]测定双氯芬酸对两种水蚤的毒性实验时发现,双氯芬酸对大型蚤(Daphniamagna) 以及刺裸腹蚤 (Moinamacrocopa) 的首次繁殖时间、子代个数以及死亡率均有明显抑制作用,并表现出浓度差异,两种水蚤的子代个数都呈现随浓度增加而减少的趋势。Ferrari等[42]研究双氯芬酸对浮游动物的急性毒性时发现双氯芬酸对大型蚤和网纹水蚤 (Ceriodaphniadubia) 的活动能力也有一定影响。
除了对浮游生物的毒性研究,Schmidt等[45]将海洋贻贝 (Mytilusspp.) 暴露于1 μg/L及1000 μg/L双氯芬酸中,96 h后谷胱甘肽-S转移酶活性明显增加,脂质过氧化水平作用表达显著上升,表明贻贝出现组织损伤以及氧化应激反应,暴露于1000 μg/L的双氯芬酸96 h后出现DNA损伤。
关于双氯芬酸对鱼类的毒性研究较多,主要为对低浓度暴露下的分子、细胞及组织水平的生物标志物研究。Schwaiger等[6]评价了双氯芬酸引起的虹鳟鱼的组织病理学病变,结果显示暴露浓度为1 μg/L时鱼的肝细胞与肾脏细胞受到损伤,同时鱼鳃中出现柱状细胞坏死现象。Feito等[46]测定了双氯芬酸对斑马鱼胚胎的氧化性损伤,结果表明当双氯芬酸浓度为0.03 μg/L时,脂质过氧化作用明显下降,表明低剂量的双氯芬酸对水生生物产生了氧化损伤。
Islas-Flores等[47]研究发现双氯芬酸会引起鲤鱼 (Cyprinuscarpio) 的肝、鳃、血液等产生氧化应激反应,暴露后的肝与鳃中的脂质过氧化酶的活性与对照组相比明显上升,血液中以及组织中的脂质过氧化酶和超氧化物歧化酶活性均产生变化,文献表明生物体内酶活性变化与其对外源性物质的代谢、毒物的降解及抗氧化作用有关。
Saravanan等[48]将印度大鲤鱼 (Cirrhinusmrigala) 暴露于1、10及100 μg/L的双氯芬酸96 h,发现血浆葡萄糖与血钠离子的水平明显升高,而血浆蛋白与血氯离子的水平下降;在35 d长期暴露时,血钠与血氯离子的水平在所有暴露浓度下均明显上升,而血糖与血蛋白随暴露浓度的不同呈现升高或降低的两向趋势。血浆中离子浓度变化可能由于药物作用导致细胞膜受损因而使血浆离子浓度变化,或是药物毒性引起渗透调节的不平衡,产生代偿效应;而血糖含量变化则是由于双氯芬酸毒性引起能量的需求变化导致[49]。双氯芬酸对鱼类肝、肾的毒性以及由此引起的蛋白质含量变化则与组织损伤及解毒机制有关,肝脏作为外源性污染物质作用的靶器官,容易导致病理改变,使得肝脏损伤,影响蛋白质的合成[50, 51]。
4 结 论
4.1在外源性有机污染物质的生物富集研究中,来自不同实验室的生物富集因子数据差异较大,实验条件如水温、pH值、水体流速等参数可能是污染物富集水平差异产生的原因,因此,对双氯芬酸生物富集的影响因素研究有待加强。
4.2目前针对双氯芬酸的研究主要在实验室开展,而真实水体环境复杂多变,环境中双氯芬酸的迁移转化过程和机制是未来研究的主要方向。
4.3双氯芬酸在食物链及食物网中的累积放大效应还未开展,因此,以后也需要重点研究其在食物链和食物网中的累积放大效应,并对其潜在的生态风险和人类健康风险进行评估。
[1]Boyd G R, et al.Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada[J]. Science of The Total Environment, 2003,311(1-3):135-149.
[2]Gracia-Lor E, et al.Multi-class determination of personal care products and pharmaceuticals in environmental and wastewater samples by ultra-high performance liquid-chromatography-tandem mass spectrometry[J]. Talanta, 2012,99:1011-1023.
[3]Weigel S, Kuhlmann J, and Hühnerfuss H.Drugs and personal care products as ubiquitous pollutants: occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea[J]. Science of The Total Environment, 2002,295(1-3):131-141.
[4]Zhang Y, S U Geiβen, and Gal C.Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies[J]. Chemosphere, 2008,73(8):1151-1161.
[5]Cuklev F, et al.Diclofenac in fish: blood plasma levels similar to human therapeutic levels affect global hepatic gene expression[J]. Environ Toxicol Chem, 2011,30(9):2126-2134.
[6]Schwaiger J, et al.Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: histopathological alterations and bioaccumulation in rainbow trout[J]. Aquat Toxicol, 2004,68(2):141-150.
[7]Heberer T.Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data[J]. Toxicology Letters, 2002,131(1-2):5-17.
[8]Fick J, et al.Therapeutic Levels of Levonorgestrel Detected in Blood Plasma of Fish: Results from Screening Rainbow Trout Exposed to Treated Sewage Effluents[J]. Environmental Science & Technology, 2010,44(7):2661-2666.
[9]Mehinto A C, Hill E M, and Tyler C R.Uptake and Biological Effects of Environmentally Relevant Concentrations of the Nonsteroidal Anti-inflammatory Pharmaceutical Diclofenac in Rainbow Trout (Oncorhynchusmykiss)[J]. Environmental Science & Technology, 2010,44(6):2176-2182.
[10]Triebskorn R, et al.Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchusmykiss) and common carp (Cyprinuscarpio)[J]. Anal Bioanal Chem, 2007,387(4):1405-1416.
[11]Letzel M, Metzner G, and Letzel T.Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input[J]. Environ Int, 2009,35(2):363-368.
[12]Roberts P H and Thomas K V.The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment[J]. Sci Total Environ, 2006,356(1-3):143-153.
[13]Sim W J, et al.Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures[J]. Chemosphere, 2011,82(2):179-186.
[14]Sui Q, et al.Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes[J]. Environ Sci Technol, 2011,45(8):3341-3348.
[15]Togola A. and H Budzinski.Analytical development for analysis of pharmaceuticals in water samples by SPE and GC-MS[J]. Analytical and Bioanalytical Chemistry, 2007,388(3):627-635.
[16]Matamoros V, et al.Occurrence and behavior of emerging contaminants in surface water and a restored wetland[J]. Chemosphere, 2012,88(9):1083-1089.
[17]Wang L, et al.Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of north China[J]. Sci Total Environ, 2010,408(16):3139-3147.
[18]Zhao J L, et al.Occurrence and a screening-level risk assessment of human pharmaceuticals in the Pearl River system, South China[J]. Environmental Toxicology and Chemistry, 2010,29(6):1377-1384.
[19]刘宇.双氯芬酸烷醇胺盐制备及其经皮吸收贴剂的研究[D].沈阳:沈阳药科大学, 2006.
[20]Rabiet M, et al.Consequences of Treated Water Recycling as Regards Pharmaceuticals and Drugs in Surface and Ground Waters of a Medium-sized Mediterranean Catchment[J]. Environmental Science & Technology, 2006,40(17):5282-5288.
[21]Heberer T, et al.Occurrence of Pharmaceutical Residues in Sewage, River, Ground, and Drinking Water in Greece and Berlin (Germany), in Pharmaceuticals and Care Products in the Environment[J]. American Chemical Society,2001,791:70-83.
[22]Togola A and H Budzinski.Multi-residue analysis of pharmaceutical compounds in aqueous samples[J]. J Chromatogr A, 2008,1177(1):150-158.
[23]Ginebreda A, et al.Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain)[J]. Environ Int, 2010,36(2):153-162.
[24]Brozinski J M, et al.The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant[J]. Environmental Science & Technology, 2013,47(1):342-348.
[25]Tixier C, et al.Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters[J]. Environmental Science & Technology, 2003,37(6):1061-1068.
[26]Heberer T.Tracking persistent pharmaceutical residues from municipal sewage to drinking water[J]. Journal of Hydrology, 2002,266(3-4):175-189.
[27]Migowska N, et al.Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography-mass spectrometry and gas chromatography with electron capture detection[J]. Science of The Total Environment, 2012,441:77-88.
[28]Heberer T, et al.Field Studies on the Fate and Transport of Pharmaceutical Residues in Bank Filtration[J]. Ground Water Monitoring & Remediation, 2004,24(2):70-77.
[29]Memmert U, et al.Diclofenac: New data on chronic toxicity and bioconcentration in fish[J]. Environ Toxicol Chem, 2013,32(2):442-452.
[30]Ericson H, G Thorsen, and L Kumblad.Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels[J]. Aquat Toxicol, 2010,99(2):223-231.
[31]Shen S,et al.Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac[J]. Chemical research in toxicology, 1999, 12(2): 214-222.
[32]Osorio-Lozada A, Surapaneni S, Skiles G L, et al.Biosynthesis of drug metabolites using microbes in hollow fiber cartridge reactors: case study of diclofenac metabolism by Actinoplanes species[J]. Drug Metabolism and Disposition, 2008, 36(2): 234-240.
[33]Stülten D, et al.Occurrence of diclofenac and selected metabolites in sewage effluents[J]. Science of The Total Environment, 2008,405(1-3):310-316.
[34]Mueller D, et al.Biotransformation of diclofenac and effects on the metabolome of primary human hepatocytes upon repeated dose exposure[J]. Eur J Pharm Sci, 2012,45(5):716-724.
[35]Kosjek T, et al.Metabolism studies of diclofenac and clofibric acid in activated sludge bioreactors using liquid chromatography with quadrupole-time-of-flight mass spectrometry[J]. Journal of Hydrology, 2009,372(1-4):109-117.
[36]Lahti M, et al.Uptake from water, biotransformation, and biliary excretion of pharmaceuticals by rainbow trout[J]. Environ Toxicol Chem, 2011,30(6):1403-1411.
[37]Kallio J M, et al.Metabolites of the Aquatic Pollutant Diclofenac in Fish Bile[J]. Environmental Science & Technology, 2010,44(19):7213-7219.
[38]Oaks J L, et al.Diclofenac residues as the cause of vulture population decline in Pakistan[J]. Nature, 2004,427(6975):630-633.
[39]Parolini M, A Binelli, and A Provini.Assessment of the Potential Cyto-Genotoxicity of the Nonsteroidal Anti-Inflammatory Drug (NSAID) Diclofenac on the Zebra Mussel (Dreissenapolymorpha)[J]. Water, Air, & Soil Pollution, 2010,217(1-4):589-601.
[40]Triebskorn R, et al.Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchusmykiss)[J]. Aquat Toxicol, 2004,68(2):151-166.
[41]Liu X, et al.Potentials and mechanisms of genotoxicity of six pharmaceuticals frequently detected in freshwater environment[J]. Toxicology Letters, 2012,211(1):70-76.
[42]Ferrari B T, et al.Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid[J]. and diclofenac. Ecotoxicology and Environmental Safety, 2003,55(3):359-370.
[43]Cleuvers M.Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid[J]. Ecotoxicology and Environmental Safety, 2004,59(3):309-315.
[44]Lee J, et al.Chronic exposure to diclofenac on two freshwater cladocerans and Japanese medaka[J]. Ecotoxicol Environ Saf, 2011,74(5):1216-1225.
[45]Schmidt W, et al.Effects of the pharmaceuticals gemfibrozil and diclofenac on the marine mussel (Mytilusspp.) and their comparison with standardized toxicity tests. Mar Pollut Bull, 2011,62(7):1389-1395.
[46]Feito R, Y Valcarcel, and M Catala.Biomarker assessment of toxicity with miniaturised bioassays: diclofenac as a case study[J]. Ecotoxicology, 2012,21(1):289-296.
[47]Islas-Flores H, et al.Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinuscarpio)[J]. Ecotoxicol Environ Saf, 2013,92:32-38.
[48]Saravanan M, and M Ramesh.Short and long-term effects of clofibric acid and diclofenac on certain biochemical and ionoregulatory responses in an Indian major carp, Cirrhinus mrigala[J]. Chemosphere, 2013,93(2):388-396.
[49]Saravanan M, et al.Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp,Cirrhinusmrigala[J]. Environmental Toxicology and Pharmacology, 2012,34(1):14-22.
[50]Kavitha C, et al.Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla[J]. Food Chem Toxicol, 2010,48(10):2848-2854.
[51]Saravanan M, K Prabhu Kumar, and M Ramesh.Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii:Cypriniformes) during acute and chronic sublethal exposure to lindane[J]. Pesticide Biochemistry and Physiology, 2011,100(3):206-211.
[52]Triebskorn R, et al.Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchusmykiss)[J]. Aquatic Toxicology, 2004,68(2):151-166.
[53]Quinn B, et al.Effects of the pharmaceuticals gemfibrozil and diclofenac on biomarker expression in the zebra mussel (Dreissenapolymorpha) and their comparison with standardised toxicity tests[J]. Chemosphere, 2011,84(5):657-663.
Distribution and Ecological Effects of Diclofenac in Water
ZHANG Zheng-hua, LU Guang-hua, DING Jian-nan
(KeyLaboratoryofIntegratedRegulation&ResourcesDevelopmentofShallowLakesofMinistryofEducation,CollegeofEnvironment,HohaiUniversity,Nanjing210098,China)
Diclofenac (DCF) is a common anti-inflammatory pharmaceutical which is widely used at home and abroad. DCF as an emerging contaminant is often detected in aquatic environments which raise a potential threat to the ecological safety and human health. This paper summarized the distribution of DCF in the water, introduced the bio-accumulation effect and metabolism in aquatic organisms, and elaborated the bio-toxicological effect of DCF. Based on the current research progress, the further research direction on DCF is proposed.
Diclofenac; bio-accumulation; metabolism; bio-toxicological effects
2014-07-16
国家自然科学基金(51279061)。
张正华(1990-),男,江西鹰潭人,河海大学环境科学与工程专业2012级在读硕士研究生,主要从事环境毒理学研究。
X703
A
1001-3644(2015)01-0120-07
