冠状动脉造影及介入术后止血方法对血管并发症的影响
2015-01-19范媛媛郑博王新刚张斌陈明霍勇
范媛媛,郑博,王新刚,张斌,陈明,霍勇
· 论著 ·
冠状动脉造影及介入术后止血方法对血管并发症的影响
范媛媛,郑博,王新刚,张斌,陈明,霍勇
目的评价用于冠状动脉造影或经皮冠状动脉介入术(percutaneous coronary intervention,PCI)的3种血管止血器(angioseal,perclose,桡动脉止血器)及传统人工按压方法的安全性。方法入选2007年1月至2013年1月于北京大学第一临床医院心血管内科心脏导管中心行冠脉造影或冠脉介入术(包括择期及急诊PCI)的患者4717例,男性3018例(64.1%),女性1699例(35.9%),平均年龄(60.6±10.3)岁。根据血管止血方法分为4组,AngioSeal组(1011例),Perclose组(1000例),人工按压组(1677例),桡动脉止血器组(1029例)。比较4组主要及次要血管并发症发生率,分析血管并发症的危险因素。结果研究人群中共出现10例主要血管并发症,均发生于人工按压组。与人工按压组比较,Angioseal组和Perclose组血肿及出血、迷走神经反射及血管痉挛发生率减少,血栓形成及血管闭塞发生率增加,差异有统计学意义(P均<0.05)。与人工按压组比较,Perclose组假性动脉瘤发生率增加,Angioseal组动脉夹层及动静脉瘘发生率下降,差异有统计学意义。以人工按压组为对照,桡动脉止血器(OR=0.35,95%CI:0.25~0.49)和Angioseal(OR=0.26,95%CI:0.25~0.49)发生次要血管并发症的风险明显降低(P均<0.001),Perclose次之(OR=0.71,95%CI:0.54~0.93,P=0.013)。采用桡动脉途径的患者(OR=0.49,95%CI:0.36~0.69)次要血管并发症风险低于经股动脉途径。年龄(OR=1.06,95%CI:1.01~1.04)、女性(OR=2.33,95%CI:1.58~2.64)、高血压(OR=1.81,95%CI:1.23~2.23)、肌酐清除率下降(OR=0.94,95%CI:0.95~0.97)是冠状动脉造影及介入术后次要血管并发症的危险因素。结论血管止血器相对于人工按压显著减少血管并发症。经桡动脉穿刺途径较股动脉途径具有更高的安全性。
血管止血器; 血管并发症;经皮冠状动脉介入术;冠脉造影
随着冠状动脉性心脏病的发病率升高,行冠状动脉(冠脉)造影或经皮冠状动脉介入术(PCI)的人数增多。美国超过75%的冠脉血运重建依赖PCI完成[1]。一项Meta分析(共纳入3662例)结果显示:3.1%~11.4%的行冠脉造影及PCI术的患者出现手术相关的血管并发症[1]。出现手术血管并发症的患者1年死亡率(7.5%)明显高于无血管并发症者(1.1%)[2]。近些年,设计了不同的止血器及穿刺入路途径(经桡动脉、股动脉、肱动脉)以减少血管相关并发症。目前血管止血器如Angioseal,Perclose,Mynx,StarClose,桡动脉止血器等逐渐取代人工按压,但止血器置入或止血失败后仍给予补救性人工按压止血。血管止血器可明显缩短冠脉介入术后患者卧床时间,提高患者舒适度,减少血管并发症。尤其桡动脉止血器因其良好的舒适度、较少血管并发症成为止血的首选。然而,国内外仍有很多研究质疑:血管止血器的安全性是否显著优于人工按压法。本研究利用2007年1月至2013年1月于北京大学第一临床医院心导管中心行冠脉造影或者冠脉介入术(包括择期及急诊PCI)的患者,探究三种常见止血器(AngioSeal,Perclose及桡动脉止血器)和人工按压法发生血管并发症的风险,评价使用血管止血器的安全性。
1 资料与方法
1.1 研究对象和分组选取2007年1月至2013年1月于北京大学第一临床医院心血管内科心脏导管中心行冠脉造影或冠脉介入术(包括择期及急诊PCI)的患者4717例,男性3018例(64.1%),女性1699例(35.9%),平均年龄(60.6±10.3)岁。纳入标准:①诊断为稳定型心绞痛/不稳定型心绞痛/急性ST段抬高型心肌梗死/急性非ST段抬高型心肌梗死;②经股动脉或桡动脉行冠状动脉造影或者冠脉介入术;③病史资料完整。排除标准:①广泛的股动脉钙化及严重斑块形成,无法穿刺;②穿刺部位明显疤痕;③穿刺超过3次;④心源性休克或术中给予主动脉内球囊反搏(IABP)支持;⑤出血倾向:凝血功能异常或严重的血小板减少症;⑥肌酐清除率<30 ml/min;⑦非死亡患者手术当天出院;⑧术中应用比伐卢定抗凝。所有患者分4组,AngioSeal组(1011例),Perclose组(1000例),人工按压组(1677例),桡动脉止血器组(1029例)。急性冠脉综合症(包括不稳定型心绞痛、急性非ST段抬高型心肌梗死、急性ST段抬高型心肌梗死)2593例,行冠脉造影1262例,行PCI术3455例。
1.2 方法
1.2.1 止血方法AngioSeal组使用AngioSeal(1453#,圣犹达)止血器,Perclose组使用Perclose(919#,雅培)止血器,桡动脉止血器组使用(RDP700/800,日本瑞翁)止血器,人工按压组采用传统人工按压方法。
1.2.2 围手术期操作所有患者术前予抗血小板治疗。冠脉造影前及术中普通肝素抗凝。动脉穿刺部位、止血器及动脉鞘管直径,穿刺后抗凝剂的使用(普通肝素、低分子肝素及糖蛋白IIb/IIIa拮抗剂)均由术者决定。是否应用血管缝合器依照患者意愿,并签署知情同意单。Allen试验阳性不能行桡动脉穿刺。冠脉造影、PCI术及止血器置入均由经验丰富的医师,依照规范操作程序完成。人工按压的患者,术后监测凝血时间(PT)到达目标值200~300 s,方拔出股动脉鞘管。拔出鞘管后人工按压30 min。人工按压组需于止血成功后制动24 h,使用Angioseal及Perclose的患者常规制动12 h,桡动脉止血器组穿刺上肢常规制动6 h。PCI术后患者推荐使用阿司匹林100 mg/d和氯吡格雷75 mg/d联合抗血小板治疗至少12月,若无禁忌,阿司匹林需长期服用。对于行PCI术的患者根据冠脉病变情况给予低分子肝素皮下注射1~7 d。住院期间血管止血器的置入及并发症情况由导管室人员及临床医师详细记录。穿刺侧动脉搏动由临床医师严密观察。
1.3 血管并发症的定义主要出血并发症:①致死性的出血事件。②需输注2个或以上的红细胞或者等量的全血。③严重低血压,需要正性肌力药物纠正。④假性动脉瘤、动静脉瘘、腹膜后血肿,局部穿刺部位出血导致严重低血压,或者需要输注至少2单位血红蛋白,且需外科手术干预。⑤引起严重的致残后遗症。⑥颅内或者眼内出血导致严重的视力丧失。⑦血红蛋白下降至少50 g/L。主要出血事件定义参照急性缺血综合症评估策略组织(OASIS)对于冠脉造影或者PCI术后主要出血事件的定义。次要血管并发症:①未达到主要出血事件标准或者需要输注1单位全血,或者需要调整药物方案(比如停用抗血小板或者抗栓治疗)。②局部出血及穿刺部位的血肿形成(股动脉穿刺>5 cm的血肿,桡动脉穿刺>2 cm的血肿),同侧假性动脉瘤,动脉夹层(股动脉、桡动脉或者锁骨下动脉)、动静脉瘘、血栓形成及血管闭塞(股动脉及桡动脉)、腹膜后血肿,无需手术干预。上述并发症需血管超声协助诊断。③局部血管痉挛及血管迷走性反射。
1.4 统计学分析所有的数据由统计学软件SPSS 18进行。计量资料采用均数±标准差(±s)表示,两组间均数的比较采用t检验,计数资料采用例数(构成比)表示,组间比较采用χ2检验。Logistic回归分析确定影响血管并发症的因素。P<0.05为差异有统计学意义。
2 结果
2.1 基线资料比较四组患者性别、血压、合并高血压、冠脉搭桥比例差异无统计学意义(P均>0.05)。与人工按压组比较,Angioseal组、Perclose组、桡动脉止血器组糖尿病、冠脉综合症、PCI比例下降,肌酐清除率上升,差异有统计学意义(P均<0.05)。见表1。
2.2 四组患者血管并发症情况共出现10例主要血管并发症,其中1例腹膜后血肿,出现失血性休克;1例股动静脉瘘和1例股动脉假性动脉瘤,需要手术修补,2例严重股动脉血肿,出现严重低血压。5例患者出现消化道出血,血红蛋白明显下降,经输血支持好转出院。10例均发生于人工按压组。局部血肿和出血是最常见的次要血管并发症,与人工按压组比较,Angioseal组和Perclose组血肿及出血、迷走神经反射及血管痉挛发生率减少,血栓形成及血管闭塞发生率增加,差异有统计学意义(P均<0.05)。与人工按压组比较,Perclose组假性动脉瘤发生率增加,Angioseal组动脉夹层及动静脉瘘发生率下降,差异有统计学意义(P均<0.05)。见表2。
2.3 不同止血方法比较以人工按压组为对照,桡动脉止血器(OR=0.35,95%CI:0.25~0.49)和Angioseal(OR=0.26,95%CI:0.25~0.49)发生次要血管并发症的风险明显降低(P均<0.001),Perclose次之(OR=0.71,95%CI:0.54~0.93,P=0.013)(见表3)。采用桡动脉途径的患者(OR=0.49,95%CI:0.36~0.69)次要血管并发症风险低于经股动脉途径。
2.4 回归分析结果高龄、女性、高血压及糖尿病与次要血管并发症呈显著正相关,正常体质指数及肌酐清除率增加对于减少次要血管并发症有一定保护作用。多因素分析结果显示,年龄(OR=1.06,95%CI:1.01~1.04)、女性(OR=2.33,95%CI:1.58~2.64)、高血压(OR=1.81,95%CI:1.23~2.23)、肌酐清除率下降(OR=0.94,95%CI:0.95~0.97)是冠状动脉造影及介入术后次要血管并发症的危险因素。见表4。
3 讨论
冠脉造影及PCI术后的血管并发症已经成为血管止血器研究的焦点,然而,对于各种血管缝合器及封堵器的安全性评价并不一致。很多研究指出应用于临床的血管止血器的安全性及有效性优于传统的人工按压法[4-6]。Tavris等[7]指出Angioseal和perclose较人工按压明显减少了出血及其他血管并发症的发生率。一项Meta分析发现,一些血管止血器甚至增加血肿及假性动脉瘤的风险[8]。还有一些研究指出,血管止血器增加肢体缺血及腹股沟区感染的风险[9,10]。Nikolsky E等[11]比较了PCI术后血管止血器及人工按压的血管并发症情况,止血器导致血肿发生率增加(9.3% vs. 5.1%,P<0.001),血红蛋白下降增加(5.2% vs. 2.5%,P<0.001)。一项Meta分析指出Angioseal封堵器与人工按压相比,降低血管并发症发生率,而Perclose缝合器增加血肿及出血风险[2,11,12]。
对于股动脉穿刺处的止血,器械止血由于人工按压。但Perclose组的假性动脉瘤发生率高于其他组。Perclose止血器学习曲线长,容易出现不完全的缝合可能是导致动静脉瘘、动脉夹层等的主要原因,尤其在明显动脉钙化的情况下。Angioseal封堵器因血肿、动脉夹层等并发症的发生率低、容易掌握而较广泛应用。使用Angioseal封堵器后穿刺部位局部血栓形成,因此一些病例报道对Angioseal封堵器长期安全性提出质疑[13,14]。

表1 各组患者基线资料比较
因对股动脉穿刺处压迫不当或不充分、穿刺位置判断失误均可继发血管并发症。但桡动脉较股动脉细小浅表,易于定位和按压,无复杂的毗邻结构及重要器官,发生严重血管并发症的几率相对小。然而桡动脉的管径小,在前臂的血管分布及走行往往存在解剖变异(比如存在桡动脉环),穿刺时有发生桡动脉或锁骨下动脉夹层、血管穿孔或痉挛的风险。桡动脉穿刺学习曲线较长,当手术紧急、复杂,多次穿刺失败、桡动脉严重钙化时转为股动脉穿刺途径。
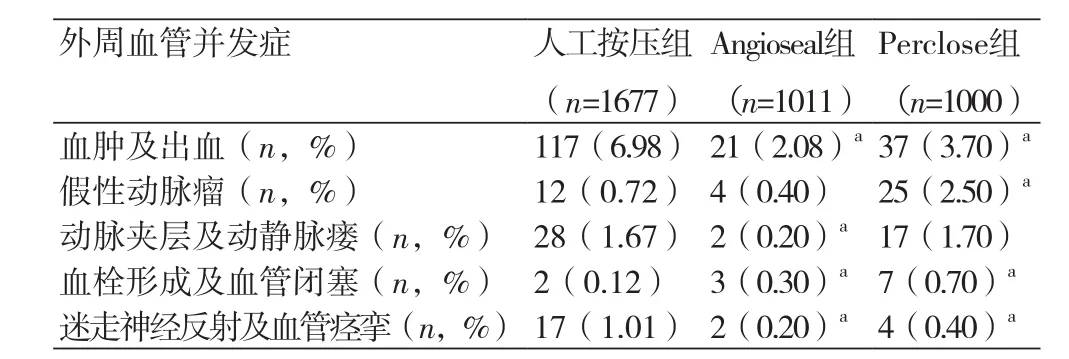
表2 不同止血方法的次要血管并发症

表3 不同止血方法比较

表4 影响次要血管并发症的单因素及多因素的预测因子
本研究中主要血管并发症均发生于经股动脉入路组,次要血管并发症发生率于桡动脉入路组明显降低。多个研究均提示经桡动脉入路在单纯冠脉造影及PCI术中较安全[15-17]。一项Meta分析指出与经股动脉入路相比,经桡动脉入路降低出血风险[18]。同时与经股动脉途径相比,经桡动脉途径显著减少主要出血事件[19,20]、卒中、心肌梗死及死亡[21]的发生率。一项随机平行多中心研究发现:经桡动脉穿刺和股动脉穿刺的血管并发症发生率明显无差异(分别为3.7%和4.0%)[22]。Chodor P等得出类似结论:冠脉介入术后主要心血管不良事件及出血并发症的发生率于桡动脉穿刺途径组分别为2.1%和8.2%,股动脉穿刺途径组分别为1.7%和10.2%[23]。经股动脉穿刺,尤其是人工按压,使患者卧床及制动时间增加,容易出现背痛、尿潴留等不适。经桡动脉途径因其减少疼痛、改善生活质量、缩短住院时间而更易被患者接受。以往医师因经股动脉穿刺可缩短血管开通时间,但随着医师经验的积累和针对异常血管解剖结构的导管及导丝的出现,经桡动脉入路已成为优先选择[24-26]。
本研究发现高龄、女性、高血压及糖尿病病史与次要血管并发症发生率相关。Ayhan等[27]在研究中指出女性及高龄(>75岁)股动脉假性动脉瘤发生率高。Duvernoy等[28]也发现女性术后出现卒中、严重出血事件及死亡的风险增加。与Nipun Arora等[29]的研究结果一致。肾功能不全患者的血管并发症多见于出血。除上述因素外,介入者操作血管止血器的“学习曲线”,可能是影响血管并发症的潜在因素。
其次,根据患者及术者意愿选择止血途径可能会造成偏倚,影响最终研究结果。同时常规超声检查可辅助诊断可能的并发症(动静脉瘘、局部动脉血栓形成等),因此术后未常规行血管超声检查,可能导致无临床症状的血管并发症未被发现。晚期血管并发症如穿刺动脉或深静脉血栓形成,可能在数周或者数月后出现,超出我们的研究时限。综上所述,对于冠脉造影或者PCI术后的患者,器械止血方法(Angioseal和Perclose)较传统人工按压法减少了股动脉穿刺后的血管并发症。而桡动脉穿刺途径较股动脉穿刺途径明显降低血管并发症的风险,值得临床广泛使用。
[1] Epstein AJ,Polsky D,Yang F,et al. Coronary revascularization trends in the United States, 2001-2008[J]. JAMA,2011,305(17):1769-76.
[2] Das R,Ahmed K,Athanasiou T,et al. Arterial closure devices versus manual compression for femoral haemostasis in interventional radiological procedures: a systematic review and meta-analysis[J]. Cardiovascu Intervent Radiol,2011,34(4):723-38.
[3] Omoigui NA,Califf R M,Pieper K,et al. Peripheral vascular complications in the coronary angioplasty versus excisional atherectomy trial (CAVEAT-I)[J]. J Am Coll Cardiol,1995, 26(4):922-30.
[4] Sulzbach-Hoke LM,Ratcliffe SJ,Kimmel SE,et al. Predictors of complications following sheath removal with percutaneous coronary intervention[J]. J Cardiovasc Nurs,2010, 25(3):E1-8.
[5] Applegate RJ,Sacrinty MT,Kutcher MA,et al. Propensity score analysis of vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention 1998-2003[J]. Catheter Cardiovasc Interv,2006,67(4):556-62.
[6] Deuling JH,Vermeulen RP,Anthonio RA,et al. Closure of the femoral artery after cardiac catheterization: a comparison of Angio-Seal, StarClose, and manual compression[J].Catheter Cardiovasc Interv, 2008,71(4):518-23.
[7] Tavris DR,Gallauresi BA,Lin B,et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender[J]. J Invasive Cardiol,2004,16(9):459-63.
[8] Koreny M,Riedmüller E,Nikfardjam M,et al. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization[J]. JAMA,2004,291(3):350-7.
[9] Derham C,Davies JF,Shahbazi R,et al. Iatrogenic limb ischemia caused by angiography closure devices[J]. Vasc Endovascular Surg,2007,40(6):492-4.
[10] Carey D,Martin J AR,Moore CA,et al. Complications of femoral artery closure devices[J]. Catheter Cardiovasc Interv,2001,52(1):3-7.
[11] Nikolsky E,Mehran R,Halkin A,et al. Vascular complicationsassociated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures A meta-analysis[J]. J Am Coll Cardiol,2004,44(6):1200-9.
[12] Vaitkus P T. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention[J]. J Invasive Cardiol,2004, 16(5):243-6.
[13] Kirchhof C,Schickel S,Schmidt-Lucke C,et al. Local vascular complications after use of the hemostatic puncture closure device Angio-Seal TM[J]. Vasa,2002,31(2):101-6.
[14] Dregelid E,Jensen G,Daryapeyma A. Complications associated with the Angio-Seal arterial puncture closing device: intraarterial deployment and occlusion by dissected plaque[J]. J Vasc Surg,2006,44(6):1357-9.
[15] Agostoni P,Biondi-Zoccai G,de Benedictis M,et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials[J]. J Am Coll Cardiol,2004,44(2):349-56.
[16] Jolly S,Amlani S,Hamon M,et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials[J]. Am Heart J,2009,157(1):132-40.
[17] Leonardi R A,Townsend J C,Bonnema D D,et al. Comparison of Percutaneous Coronary Intervention Safety Before and During the Establishment of a Transradial Program at a Teaching Hospital[J]. J Am Cardiol,2012,109(8):1154-9.
[18] Jolly S S,Amlani S,Hamon M,et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials[J]. Am Heart J,2009,157(1):132-40.
[19] Montalescot G,Ongen Z,Guindy R,et al. Predictors of outcome in patients undergoing PCI. Results of the RIVIERA study[J]. Int J Cardiol (Electronic Publication 2007 Dec 3).
[20] Chase AJ,Fretz EB,Warburton WP,et al. The Association Of Arterial Access Site At Angioplasty With Transfusion And Mortality The MORTAL Study: mortality benefit of reduced transfusion after PCI via the arm or leg[J]. Heart,2008,94(3):1019-25.
[21] Vorobcsuk A,Kónyi A,Aradi D,et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction: systematic overview and meta-analysis[J]. Am Heart J,2009,158(5):814-21.
[22] Jolly S S,Yusuf S,Cairns J,et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomized, parallel group, multicenter trial[J]. Lancet,2011,377(9775):1409-20.
[23] Chodor P,Kurek T,Kowalczuk A,et al. Radial vs femoral approach with Star-Close clip placement for primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. RADIAMI II: a prospective, randomized, single center trial[J]. Kardiologia polska,2010,69(8):763-71.
[24] Cantor W, Puley G,Natarajan M,et al. Radial versus femoral access for emergent percutaneous coronary intervention with adjunct glycoprotein IIb/IIIa inhibition in acute myocardial infarction-the RADIAL-AMI pilot randomized trial[J]. Am Heart J,2005,150:543-9.
[25] Louvard Y,Benamer H,Garot P,et al. Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study)[J]. Am J Cardiol,2004,94(9):11 77-80.
[26] Ochiai M,Isshiki T,Toyoizumi H,et al. Efficacy of transradial primary stenting in patients with acute myocardial infarction[J]. Am J Cardiol, 1999,83(6):966-8.
[27] Ayhan E,Isik T,Uyarel H,et al. Femoral pseudoaneurysm in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: incidence, clinical course and risk factors[J]. Int Angiol,2012,31(6):579-85.
[28] Duvernoy CS,Smith DE,Manohar P,et al. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry[J]. Am Heart J, 2010,159(4):677-83.
[29] Arora N,Matheny M E,Sepke C,et al. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices[J]. Am Heart J,2007,153(4):606-11.
Influences of hemostatic methods on vascular complications after coronary angiography or percutaneous coronary intervention
FAN Yuan-yuan*, ZHENG Bo, WANG Xin-gang, ZHANG Bin, CHEN Ming, HUO Yong.*Department of Cardiology, Peking University First Hospital, Beijing 100034, China.
ObjectiveTo review the safety of 3 kinds of vascular hemostats (angioseal, perclose and radial hemostat) and traditional manual compression (MC) applied during coronary angiography (CAG) or percutaneous coronary intervention (PCI).MethodsThe patients undergone CAG or PCI [n=4717, male 3018 (64.1%), female 1699 (35.9%) and average age: 60.6±10.3] were chosen from Jan. 1, 2007 to Jan. 1, 2013, and then divided into 4 groups including angioseal group (n=1011), perclose group (n=1000), MC group (n=1677) and radial hemostat group (n=1029). The incidence rates of major or secondary vascular complications were compared, and risk factors of vascular complications were analyzed.ResultsThere were totally 10 cases with major vascular complications and all of them in MC group. Compared with MC group, the incidence rates of hematoma, bleeding, vagal reflex and vasospasm decreased, and those of thrombosis and vascular occlusion increased in angioseal group and perclose group (all P<0.05). Compared with MC group, the incidence rate of pweudo aneurysm increased in perclose group, and incidence rates of arterial dissection and arterovenous fistula decreased in angioseal group. Taken MC group as control, radial hemostat (OR=0.35,95%CI:0.25~0.49) and angioseal (OR=0.26, 95%CI: 0.25~0.49) had significantly lower risk of secondary vascular complications (all P<0.001), and perclose (OR=0.71, 95%CI: 0.54~0.93, P=0.013) had low risk. The risk of secondary vascular complications was lower in the patients with transradial hemostat (OR=0.49, 95%CI: 0.36~0.69) than that in those with transfemoral hemostat. Age (OR=1.06, 95%CI: 1.01~1.04), female (OR=2.33, 95%CI: 1.58~2.64), hypertension (OR=1.81, 95%CI: 1.23-2.23 and decrease of creatinine clearance rate (OR=0.94, 95%CI: 0.95~0.97) were risk factors of secondary vascular complications after CAG or PCI.ConclusionVascular hemostats, compared with MC, can significantly reduce incidence of vascular complications, and transradial hemostat has higher safety compared with transfemoral hemostat.
Vascular hemostat; Vascular complications; Percutaneous coronary intervention; Coronary angiography
R816.2
A
1674-4055(2015)01-0049-05
2014-06-10)
(责任编辑:姚雪莉)
100034 北京,北京大学第一医院心内科
范媛媛,E-mail:6688qingchuan@sina.com
10.3969/j.1674-4055.2015.01.16
