Drag-reduction behavior of an unusual nonionic surfactant in a circular pipe turbulent flow*
2014-06-01CAIShupeng蔡书鹏
CAI Shu-peng (蔡书鹏)
Institute of High Pressure Water Jet, Hunan University of Technology, Zhuzhou 412007, China Graduate School of Science and Technology, Kobe University, Kobe 657-8501, Japan,
E-mail:windowscsp@sina.com
HIGUCHI Yuta
Graduate School of Science and Technology, Kobe University, Kobe 657-8501, Japan
Drag-reduction behavior of an unusual nonionic surfactant in a circular pipe turbulent flow*
CAI Shu-peng (蔡书鹏)
Institute of High Pressure Water Jet, Hunan University of Technology, Zhuzhou 412007, China Graduate School of Science and Technology, Kobe University, Kobe 657-8501, Japan,
E-mail:windowscsp@sina.com
HIGUCHI Yuta
Graduate School of Science and Technology, Kobe University, Kobe 657-8501, Japan
(Received March 11, 2013, Revised September 15, 2013)
The Alkyl Polyglucoside (APG) is a nonionic surfactant with no toxicity and with high biodegradability, its drag-reduction behavior in a circular pipe flow is measured, and the rheological characteristics are investigated with a rheometer with a cone-plate flow cell. From the measured results, the APG is shown to have a high drag-reduction capacity, whose shear viscosity is shear-ratedependent at high concentrations, while its solution at concentrations with drag-reduction effects is non-viscoelastic as verified by zero relaxation time in the relaxation process of the shear stress, which contradicts the general viewpoint that there is a correlation between the viscoselastic characteristics and the turbulent drag reduction for the drag-reduction surfactant. However, the APG solution is birefringent as observed through a birefringent test, which indicates that there are rod-shaped micelles in the solution under the shearing flow. The higher extensional viscosity inferred from the extensional phenomenon observed in the measurements of the shear viscosity could be responsible for the drag reduction property of this nonionic surfactant.
drag reduction, shear stress relaxation, relaxation time, viscoelasticity
Introduction
In a real fluid-transporting engineering system, the flow pattern is almost turbulent, so to reduce the turbulent frictional drag is important for reducing the energy consumption and, thus, for keeping the nature environment from the air pollution. Certain surfactants or polymer additives into a water flow can reduce the frictional drag by up to 80%, which was found firstly by Toms half a century ago, and called the Toms effect. This promising technology has recently been successfully used to reduce the pumping power of district heating-cooling systems in the western countries and Japan, which attach importance to energysaving[1-4].
The experimental and theoretical studies of the mechanism of surfactant drag reduction focus on therelations between the drag reduction and the alterations in the rheological properties or in the turbulence structures. A typical hypothesis is that the viscoelasticity under a shear flow in a surfactant drag-reducing solution is responsible for the surfactant’s drag-reduction property. By measuring the rheological characteristics of a cationic surfactant drag-reduction solution, Hoffman et al.[5-11], proposed that the shear-induced structure (ISI) formed under sufficiently high shear rates makes it viscoelastic, which interacts with the turbulence, suppressing the small scale turbulent eddies, thus reducing the turbulent frictional drag.
However, it should be noted that so far most surfactant drag reducers used for studies and practical engineering applications are cationic surfactants, with a strong toxicity and a very poor biodegradability so that the wasted solutions lost their drag-reducing ability after a long-term practical drag-reducing operation, and the leakage of them need to be specially addressed. Furthermore, since the head groups of cationic surfactants carry positive charges, the addition of negatively charged counter-ions with hydrophobicity, whichcan penetrate the organic core of the micelle, and whose negative charges can screen the positive charges of cationic head groups, can stabilize the micellar structure. Therefore, a proper organic counterion such as sodium salicylate should be added to the solvent in order to realize the turbulent frictional drag reduction, even if it is not desired for lowering the economic cost in a practical drag-reduction application. Since the hydrophilic head of a nonionic surfactant is electrically neutral, one might expect that it can lower the frictional drag independently without counter-ions. Then, many attempts were made to develop the drag-reduction technology by using nonionic surfactants with no toxicity and with high biodegradability[12–16].
In our previous studies, it was found[12]that with using a nonionic surfactant, Oleyldimethylamineoxide (ODMAO), a relatively weak viscoelasticity is obtained sufficient to realize the turbulent drag reduction. Hu and Matthys[13]studied the rheological properties of a nonionic surfactant (SPE95285) solution at a concentration of 3 000 ppm, and found that its shear viscosity is shear-history-dependent, and its solution is flow birefringent and displays the SIS behavior. But the rheological property at low concentrations used for the other drag-reducing studies was not mentioned in their studies. Cho et al.[14]confirmed the dependence of the drag reduction ability on both temperatures and concentrations, using a nonionic surfactant (Stearyl amine oxide + Betaine) solution. However, the studies carried out so far are not enough for a clear understanding of the mechanism of the drag reduction by nonionic surfactants. For a nonionic surfactant, the relationships between the drag reduction and the rheological properties, in particular, between the viscoelasticity and the drag reduction were not adequately studied.
In the present study, the surfactant drag-reducer is a nonionic surfactant APG (Alkyl Polyglucoside, Glucopon 600UP), denoted by the Japan Cognis Chemicals. It is based on plant-derived chemicals (carbohydrate molecular structure) and is readily biodegradable and nontoxic, and, thus, does not add to the Earth’s CO2burden. The drag-reduction measurements are made in a circular pipe of 0.005 m in diameter at mass concentrations of 3 000 ppm and
6 000 ppm, and at temperatures of 20oC, 30oC and 40oC by an air-driven fluid resistance test device. The shear-rate-dependent and temperature-dependent characteristics of the shear viscosity and the shear stress relaxation characteristics are determined to see whether the solution is visvoelastic or not by a rheometer and the drag-reduction mechanism is explored for APG solutions. Also, the flow birefringence is measured under a constant shearing force to see whether the shape of micellar structures in the solution is rod-shaped or not. From these experimental results, the relation between the drag-reduction and the viscoelasticity is revealed. All experiments are performed in the laboratory of Japan Kobe University. much slower than that in a Newtonian fluid[5-7]. Therefore, in order to obtain a fully developed turbulent flow in the test section where the pressure drop is measured, the length from the inlet of the pipe to the front end of the test section is taken as 360 times of the pipe’s inner diameter, much longer than that required for a Newtonian fluid. The apparatus with a temperature-controlled machine is temperature-adjusted to allow the experiment to be performed at different temperatures. To ensure a constant temperature during the experiment, a proper heat-insulating material is wrapped around the reservoir tank and on the surface of the flow passage.
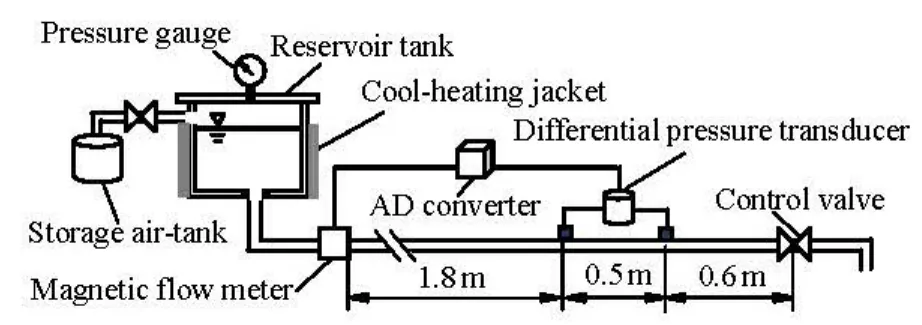
Fig.1 Schematic diagram of drag reduction testing apparatus
The measurements of the shear viscosity and the first normal stress difference are carried out with a MR301 stress-controlled rheometer by Germany Anton Paar, of a cone-plate flow geometry with a cone angle of 0.0395 radians, cone diameter of 0.05 m and a gap of 9.9×10-5m.
The relaxation characteristics of the shear stress are measured with an ARES-LS G2 strain-controlled rheometer of a cone-plate of 0.04 radians, 0.05 m in diameter and 3.98×10-5m in gap.
The flow birefringence measurements are performed with a birefringence test device with a Couette flow geometry cell, which has a stationary inner cylinder of 0.03 m in outside diameter and 0.028 m in height, and an outer cylinder which forms a gap of 0.0015 m with the inner cylinder.
1.2Experimental processes
For measuring the drag reduction effects of the
1. Experimental apparatus and procedure
1.1Experimental apparatus and procedure
The flow resistance test apparatus driven by air is shown in Fig.1. This system consists of a reservoir tank, an electromagnetic flow meter (IMF5800 by Tokyo Instrument), a differential pressure transducer (DP15-38 by Validyne in the U.S.A), a hydraulically smooth and circular stainless pipe of 0.005 m in internal diameter and 1.9 m in length, and other necessary units. Some observations show that the development of the flow in a surfactant drag-reducing solution isAPG solutions, a semi-dilute surfactant solution is firstly prepared by mixing a certain amount of APG with 500 ml of water, heated up to 70oC, diluted to the desired concentration of 3 000 ppm or 6 000 ppm, and stirred for 30 min, then added to the reservoir tank. After the APG solution is adjusted to the experiment temperature and the reservoir tank is pressurized, the control valve is opened to let the APG sample flow through the test pipe. When the sample flows through the test pipe, both the pressure in the storage tank and the flow rate would decrease gradually due to the constant volume of the reservoir tank. The pressure loss in the test section and the flow rate are synchronously measured by the differential pressure transducer and the electromagnetic flow meter, respectively.
For the measurements of the shear viscosity and the first normal stress difference, the APG solution sample is loaded between the upper cone and the lower plate, and is adiabatically kept for 30 min to assure a thermal equilibrium. The sampling frequencies for taking the readings of the shear viscosity and the first normal stress difference are rationally set to make the reading at each shear rate reach an equilibrium value. The shear viscosity and the first normal stress difference are taken as the average reading of clockwise and counter clockwise measurements.
Measurements of the shear stress relaxation are performed with an ARES-LS G2 strain-controlled rheometer with a cone-plate. A constant shear rate is instantaneously applied to the APG solution loaded between the upper cone and the lower plate until the shear stress reaches an equilibrium value. It is then removed instantaneously and the transient time variation of the shear stress is recorded.
Measurements of the flow birefringence are conducted with a birefringence test device with a Couette flow geometry cell. The laser light is applied along the vorticity axis. A constant shear rate is imposed to the APG test sample by the rotation of the outer cylinder of the Couette flow cell.

Fig.2 Friction factor versus Reynolds number for the 3 000 ppm APG solution
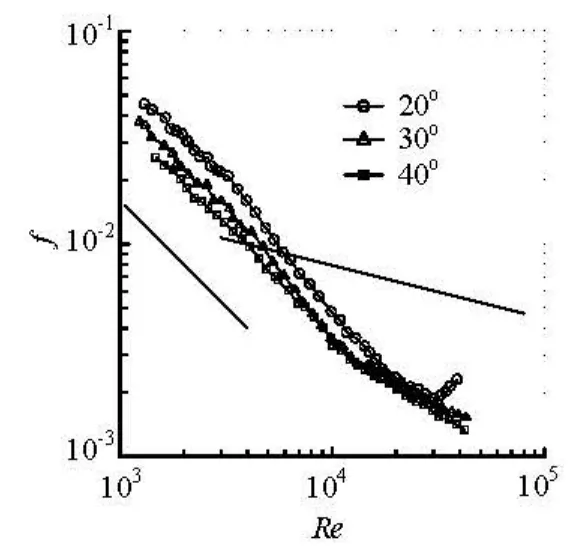
Fig.3 Friction factor versus Reynolds number for the 6 000 ppm APG solution
2. Experimental results and analyses
2.1Drag-reduction characteristics
Since the flow in the test tube is non-steady, a pseudo-steady state assumption is used in the calculation of the frictional drag factor from the recorded pressure drops and the flow rates. The Reynolds number (Re), is defined based on the bulk velocity, the water viscosity and the pipe diameter. The drag reduction behaviors of the APG solutions of concentrations of 3 000 ppm and 6 000 ppm, and at temperatures of 20oC, 30oC and 40oC are compared in Figs.2 and 3, in which the Blasius empirical formula and the laminar theoretical curve for the friction factors in a Newtonian fluid circular pipe flow are also plotted for comparison.
It can be seen from the measured data that for the APG drag-reducing solution, the dependence of the drag reduction effectiveness on the concentration is similar to that for cationic surfactants whose drag-reduction effectiveness increases with the increase of the concentration. For the APG 6 000 ppm solution at 30oand 40o, the critical Reynolds number corresponding to the maximum drag reduction rate could not be obtained due to the limited reservoir tank volume in our experimental apparatus, but evidently, it would exceed 50 000 and the maximum drag reduction rate would be over 70%. Also, as shown in Figs.2 and 3, the drag reduction rate of the APG solutions increases with the increase of temperature, due to the fact that as the temperature rises, the micellar structures essential for a surfactant to have the drag reduction property are stabilized so that their ability against the shear is enhanced.
2.2Shear viscosity and first normal stress difference
It is usually known that if the viscosity of a fluid varies with the shear rate, its molecules are assembledto form large scale aggregated structures in the shear flow.
The shear viscosity of the APG solutions with drag reduction effects is shown in Figs.4 and 5. As presented in Fig.4, for the APG solution of 3 000 ppm concentration with a lower drag reduction effect, in the range of measured shear rates, the shear viscosity does not vary with the shear rate, similar to Newtonian fluids.
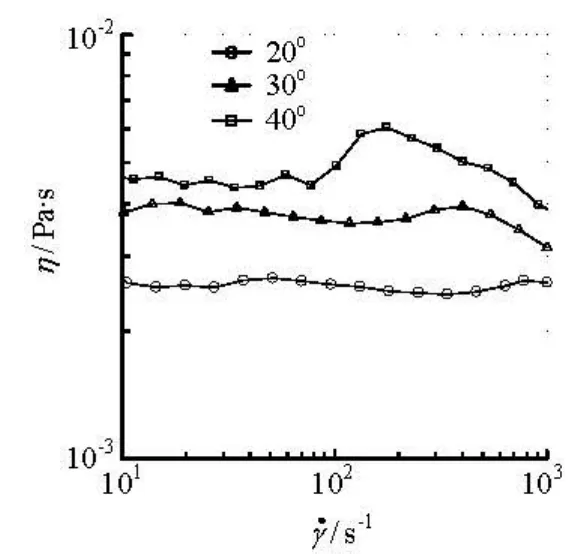
Fig.5 Shear viscosity versus shear rate for the 6 000 ppm APG solution
However, in Fig.5, for the APG solution of 6 000 ppm with a higher drag reduction effect, its shear viscosity at low shear rates is identical to water, while at a high shear rate above the critical shear rate which is affected by the temperature, it increases with the increase of the shear rate, then follows a shear-thinning with a further increase in the shear rate. Such a shear-rate-dependent behavior in the shear viscosity is similar to some cationic surfactant drag reducers. The aggregated structures with the rheological property that its shear viscosity suddenly increases at a critical shear rate are called the shear-induced structure (SIS). It is generally believed that under the SIS state, a surfactant drag-reduction solution exhibits viscoelasticity which is responsible for the occurrence of the drag reduction. This is the vicoelasticity-induced drag reduction hypothesis suggested by many investigators[13-15]. In addition, from the measured results, the first normal stress differences is almost zero. It is generally known that a fluid with non-zero first normal stress difference is viscoelastic, while a viscoelastic fluid may have no first normal stress difference. Then, it is still an unsolved question whether the APG solution is viscoelastic or not.
2.3Viscoelasticity and shear stress relaxation
By observing the development of the shear stress after instantaneously applying a constant shear rate to a surfactant solution, the transient time evolution of the shear stress after instantaneously removing the constant shear rate can be used to predict whether the solution is viscoelastic or not, and whether the micellar structure in the solution can be changed or not. The relaxation time of the shear stress is an important time scale parameter for a viscoelastic fluid. It is generally believed that a long shear stress relaxation time is associated with a strong viscoelastic property. The shear stress relaxation data for the APG solution with a concentration of 6 000 ppm is shown in Fig.6, in which those for a cationic surfactant drag-reducer solution, Ethoquoad O/12, with a strong viscoelasticity is also plotted for comparison. It can easily be noticed that the shear stress relaxation of the APG solution is identical to that for water without viscoelasticity, which shows that the APG solution is non-viscoelastic because no shear strain elastic energy is accumulated while being sheared, contradicting the general belief that there exists a good correlation between the viscoelasticity and the drag reduction.
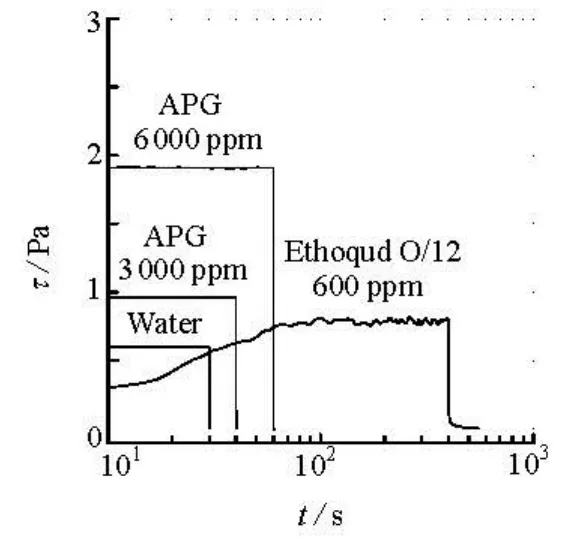
Fig.6 Development and relaxation of shear stress
2.4Flow birefringence measurements
For a surfactant solution with a spherical micelle, the birefringence would not be observed due to the isotropic nature of the solution, while for a surfactant solution with a rod-like micelle under a shear, the flow birefringence would be observed. Hence, the flow birefringence can make sure whether the micelle shape is rod-like or not. The flow birefringence measurement for the APG solution at the concentration of 6 000 ppm is shown in Fig.7. It is clearly seen that it is indeed flow birefringent, which indicates that its mi-celle shape in the solution is rod-shaped.

Fig.7 Flow birefringence measurements for APG solution of 6 000 ppm concentration
2.5Drag-reducing mechanism of the APG solution
From the measured results, the APG solution with concentrations of 3 000 ppm or higher has dragreduction property, and its micellar shape is rod-like, but, it is not viscoelastic, and, thus, not the same as cationic surfactant drag-reducers. Therefore, by using the viscoelasticity-induced drag redution hypothesis, its drag reduction mechanism can not be explained. Then, some other rheological properties responsible for drag reduction might exist.
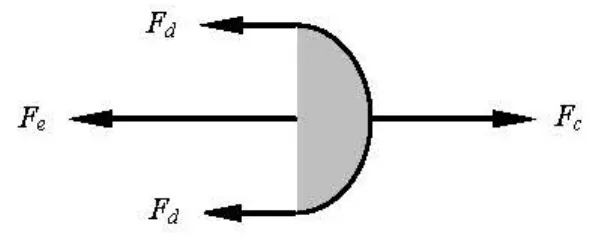
Fig.8 Force equilibrium of tested fluid at the edge while the solution is separated out from the cone-plate
While measuring the shear viscosity of the APG solutions, it is found that a much higher shear rate than that for water is needed to make the solution loaded between the upper cone and the lower plate separated out from the cone-plate. An analysis of force equilibrium for this phenomenon is shown in Fig.8, whereFeis the extensional force,Fcis the centrifugal force, andFdis the frictional force on the cone and plate surfaces. For a test fluid (water or APG solution), when the sum of the extensional force due to the extensional deformation, the frictional drag on the cone and plate surfaces, and the surface tension is less than the centrifugal force for the fluid being kept between the upper and lower plates, the fluid would be separated out from the cone-plate. Since the shear rate for making the APG solution separated out from the con-plate is much greater than that for making water separated out, the centrifugal force for the APG solution is much greater than that for water. Furthermore, since both the frictional drag and the surface tension for the APG solution are smaller than those for water because the APG solution has a high drag-reduction capability and also has the ability to reduce the surface tension, the extensional force for the APG solution with the drag-reduction ability is much greater than that for water, and the extensional viscosity is thus much higher that for water. However, the methods for accurately measuring the extensional viscosity of dilute fluids with low shear viscosity such as the APG drag-reducer solution have not been found so far.
Surfactant drag reduction could be attributed to the suppression of the small scale eddies in a turbulent flow. For a wall-induced turbulent flow, in the processes of burst characterized by the ejecting of low speed fluids and sweeping of high speed fluids, the extensional motions dominate[11]. By adding surfactant additives into water, the extensional viscosity is greatly increased, and, thus, the resistance in the eddy against the extensional flow is increased so that the formation and the growth of small scale eddies are suppressed. It is widely known that in a turbulent flow, the energy loss is mainly caused by viscous dissipations of small scale eddies formed in the processes of fluid bursting. Therefore, the correlation between the surfactant drag reduction and the high extensional viscosity is probable.
3. Conclusions
In this paper, the drag reduction and the rheological characteristics of an environmentally friendly nonionic surfactant APG solution are studied experimentally. The following conclusions are drawn.
(1) The APG solution without counter-ion has a high drag-reduction capability at concentrations of 3 000 ppm or higher and at temperatures of 20oor higher. Its viscosity at concentrations above 3 000 ppm is shear-rate-dependent in the measured shear rare range, which implies that the micelle structure in the solution would be changed in a shear flow.
(2) The relaxation of the APG solution with dragreduction effects is identical with that for water, which indicates that the APG solution is non-viscoelastic. This observation contradicts the usual belief that there exists a relation between the surfactant drag reduction and its viscoelasticity.
(3) The rod-like micelle structures detected through flow birefringence measurements in a shear flow, and the high extensional viscosity inferred by the extensional phenomenon seen in the process of measuring the shear viscosity might be responsible for this nonionic surfactant’s drag reduction property.
Acknowledgement
The authors acknowledge the support provided by Professor Suzuki Hiroshi in Japan Kobe University.
References
[1] INDARTONO Y. S., USUI H. and SUZUKI H. Hydrodynamics and heat transfer characteristics of drag-reducing trimethylolethane solution and suspentions by cationic surfactant[J].Journal of Chemical Engineering of Japan,2006, 39(6): 623-632.
[2] SHAO X., LIN J. and WU T. et al. Experimental research on drag reduction by polymer additives in a turbulent pipe flow[J].Canadian Journal of Chemical Engineering,2002, 80(2): 293-298.
[3] CAI Shu-peng. Drag reduction of a cationic surfactant solution in a circle pipe turbulent flow and its shear stress relaxation[J].Journal of Hydrodynamics,2012, 24(2): 657-661.
[4] USUI H., KAMADA T. and SUZUKI H. Surfactant drag reduction caused by a cationic surfactant with excess addition of counter-ions[J].Journal of Chemical Engineering of Japan,2004, 37(10): 1232-1237.
[5] SUZUKI H., NGUYEN H. P. and NAKAYAMA T. et al. Development characteristics of fluctuating velocity field of drag-reducing surfactant solution flow in a duct[J].Rheologica Acta,2005, 44(4): 457-464.
[6] ROZANSKI J. Flow of drag-reducing surfactant solutions in rough pipes[J].Jouranl ofNon-Newtonian Fluid Mechanics,2011, 166(2): 279-288.
[7] SUZUKI H., FULLER G. G. and NAKAYAMA T. et al. Development characteristics of drag-reducing surfactant solution flow in a duct[J].Rheological Acta,2004, 43(2): 232-239.
[8] MIZUMURA H., KOBAYASHI T. and TOMINAGA S. Drag reduction and heat transfer in surfactant solutions with excess counterion[J].Journal ofNon-Newtonian Fluid Mechanics,2010, 165(2): 292-298.
[9] OHSHIMA T., OHTA T. and KAJISHIAMA T. Influence of surfactant on the elementary motion in turbulent flow[J].JSME International Journal Series B,2007, 73(732): 1647-1654.
[10] ARAGA K., AZUMA T. Effect of surfactant Additives on the transtion in pipe flow[J].JSME International Journal Series B,2005, 72(717): 1137-1145.
[11] LIN Z., ZHENG Y. and DAVIS H. T. et al. Unusual effects of counter-ion to surfactant concentration ratio on viscoelasticity of a cationic surfactant drag reducer[J].Journal ofNon-Newtonian Fluid Mechanics,2000, 9(2): 363-373.
[12] CAI Shu-peng, SUZUKI H. Drag-reduction of a nonionic surfactant aqueous solution and its rheological characteristics[J].Science China Technology Sciences,2012, 55(3): 772-778.
[13] HU Y., MATTHYS F. Rheological and rheo-optical characterizationof shear-induced structure formation in a nonionic drag-reducing surfactant solution[J].Journal of Rheology1997, 41(1): 151-166.
[14] CHO Sung-Hwan, TAE Choon-Seob and ZAHEERUDDIN M. Effect of fluid velocity, temperature, and concentration of non-ionic surfactants on drag reduction[J].Energy Conversion and Management,2007, 48(3): 913-918.
[15] TAMANO S., ITOH M. and YOKOTA K. Drag-reducing effect of nonionic surfactant solutions[J].JSME International Journal Series B,2009, 75(756): 1598-1607.
[16] GURKA R., LIBERZON A. and HETSRONI G. Characterization of turbulent flow in a flume with surfactant[J].Journal of Fluid Engineering,2004, 126(6): 1054-1057.
10.1016/S1001-6058(14)60045-7
* Project supported by the Natural Science Foundation of Hunan Province and Zhuzhou City (Grant No. 13JJ9032).
Biography: CAI Shu-peng (1963-), Male, Ph. D., Professor
DOI: 10.1016/S1001-6058(14)60046-9
杂志排行
水动力学研究与进展 B辑的其它文章
- Numerical simulation of landslide-generated impulse wave*
- A random walk simulation of scalar mixing in flows through submerged vegetations*
- Scaling of maximum probability density function of velocity increments in turbulent Rayleigh-Bénard convection*
- Dispersion in oscillatory electro-osmotic flow through a parallel-plate channel with kinetic sorptive exchange at walls*
- Evaluation of the use of surrogateLaminaria digitatain eco-hydraulic laboratory experiments*
- A numerical study on dispersion of particles from the surface of a circular cylinder placed in a gas flow using discrete vortex method*
