Neurotrophins and their receptors in satellite glial cells following nerve injury
2014-06-01ChristianBjerggaardVaegter
Neurotrophins and their receptors in satellite glial cells following nerve injury
Peripheral neuropathy is a condition where damage resulting from mechanical or pathological mechanisms is inflicted on nerves within the peripheral nervous system (PNS). Physical injury is the most common cause and may result in nerves being partially or completely severed, crushed, compressed or stretched. Other causes include metabolic or endocrine disorders, with e.g., 50—60% of people with diabetes eventually suffering from various peripheral neuropathies. In contrast to the central nervous system (CNS), the PNS possesses a unique ability to regenerate. However, despite the considerable capacity for regrowth upon injury, PNS regeneration is far from complete and functional recovery rarely returns to pre-injury levels. Furthermore, induced neuropathic pain is often permanent and complex to control, and the common consequence of nerve injury is thus a signi ficant impact on quality of life and daily activities.
Scienti fi c research in the molecular and cellular effects following PNS injury has largely focused on neurons and Schwann cells, whereas considerably less attention has been given to the satellite glial cells (SGCs) — a cell type in intimate contact with the sensory neurons and with a neuron supporting function. Growing evidence over the past decade is, however, establishing SGCs as important components of PNS functionality and in post-injury responses such as chronic pain states. Several SGCs, interconnected by gap junctions, together form a sheath around one neuronal soma (Figure 1), leaving a gap of just 20 nm between the SCGs and the neuron — the width of a synapse. A layer of connective tissue encloses this functional unit of neuron soma and SGCs. The non-neuronal face of the slimly fl attened SGCs has a basement membrane, which together with the endothelial basement membrane constitutes a blood-neuron barrier (Pannese, 2010). Through expression of a number of receptors and channels, SGCs are believed to stabilize the neuronal microenvironment, supporting normal neuron function and providing electrical insulation. This intimate and isolated structural arrangement between SGCs and soma strongly implies that soma-SGC communication is a major determinant of neuronal activity, and that understanding injury-induced changes in this communication is important for understanding the neuronal changes and activity following peripheral nerve injury.
Profound changes can be observed in the soma of dorsal root ganglion (DRG) neurons upon peripheral nerve injury, ranging from altered protein expression, structural changes and even cell death depending on the degree of injury and the neuronal subtype. A series of morphologic changes can be observed as early as a few hours after injury, entailing cell body and nucleolar swelling as well as nuclear eccentricity. The purpose of these changes is to initiate an appropriate response to traumatic stress, switching the neurons from the steady-state production of neurotransmitters and housekeeping proteins to a “regrowth mode”. This involves production of components for cytoskeletal and axonal reconstruction and growth-related proteins including nitric oxide, adenosine triphosphate, neuropeptides, neurotrophic factors and their receptors. Several biological factors influence the communication between peripheral nerves and tissues following injury, including a variety of cytokines and growth factors. Similar conditions can be observed during the development of the nervous system where the PNS strongly depends on trophic stimulation for survival, differentiation, sprouting and maturation (Geuna et al., 2009). As damage to the adult PNS induces cellular mechanisms that control neuronal differentiation as well as growth and connectivity during development, “studies of neurotrophic factors represent one of the most promising areas of research aimed at fi nding new, more effective treatments for peripheral neuropathies” according to National Institute of Neurological Disorders and Stroke.
The family of neurotrophins consists of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4), which can bind to two structurally unrelated receptors: the neurotrophin receptor p75NTRand the tropomyosin receptor kinases (TrkA, -B, and -C). Binding of neurotrophins to the Trk receptors is selective with NGF binding to TrkA, BDNF and NT-4 to TrkB and NT-3 to TrkC. However, many neurons co-express Trk receptors and p75NTR, which together strengthen the affinity and specificity of the neurotrophins for the Trk receptors. The neurotrophins bind to their respective Trk receptors to mediate survival and differentiation but can also provide survival signaling or affect cytoskeletal organization and neurite outgrowth via p75NTR. Yet, p75NTRis better recognized for its ability to induce cell death signaling via binding of various intracellular adaptor proteins (Chao, 2003). Uninjured SGCs have been reported to express neurotrophins and their receptors. Thus, TrkA and p75NTRare found in the SGC cytoplasm and in the SGC-neuron border, and expression of TrkB, more speci fi cally truncated TrkB without the cytosolic kinase domain, has also been described. Furthermore, TrkC mRNA and immunoreactivity in some SGCs surrounding large neurons has also been reported. Finally, NT-3, NGF and BDNF have been observed in SGCs both at mRNA and protein levels.
In general, the SGCs respond rapidly when injury is in fl icted on the neuron, which they encapsulate. Dynamic neuron-SGC communication by P2X receptors, NMDA receptors and glutamate transporters in SGCs increases SGC-SGC communication via gap junctions and initiates a general increase in proliferation and cytokine communication. This SGC response suggests that SGC involvement in post-injury events, such as regeneration and the initiation and/or maintenance of neuropathic pain, is a highly rational assumption. In accordance with this, knockdown of the gap junction protein connexin 43 as well as the use of pharmacological gap junction blockers has been demonstrated to decrease pain behavior in rodents (Hanani, 2005). Several observations also demonstrate that SGCs, but not neurons, increase NGF and NT-3 mRNA following injury. Intriguingly, administration of gap junction inhibitors during injury is found to significantly decrease the number of NGF-positive SGCs. Although the direct effects of SGC-derived NGF on DRG neurons are still to be demonstrated, other observations indicate involvement of SGCs in neurotrophin signaling. Thus, NGF dependent sprouting of sympathetic axons into sensory ganglia following nerve injury is believed to underlie the phenomenon of sympathetically maintained pain, where increased pain is triggered when sympathetic activity is induced.
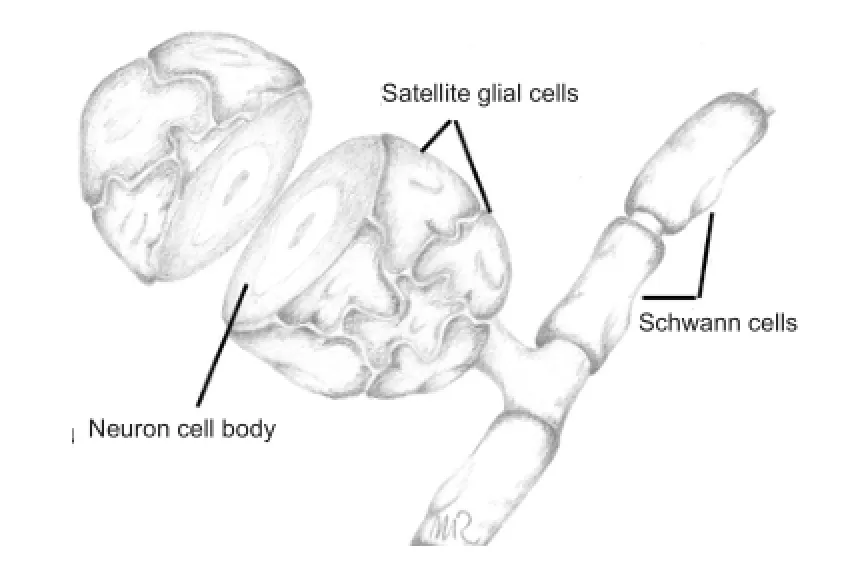
Figure 1 The soma of a dorsal root ganglion sensory neuron closely surrounded by satellite glial cells.
Following nerve injury, sprouts of sympathetic terminals originating from the vasculature form synaptic terminals or “baskets” around selected large DRG sensory neurons. Sympathetic nerves express and respond to neurotrophins, and the observed sympathetic sprouting clearly depends on NGF as antibodies against NGF or NT-3, delivered by an osmotic mini-pump to the DRG via the transected nerve, was found to reduce sprouting signi fi cantly. It has been reported that uninjured and even contralateral DRGs to some extent can be affected by sympathetic sprouting, although this might re fl ect that any surgical procedure per se may initiates local tissue in fl ammation and, thus, induce systemic responsiveness to released NGF. In general, the sympathetic sprouting around the axotomized neurons is associated with p75NTR-positive SGCs. Based on the L5spinal nerve ligation (L5—SNL) model, nerve injury results in reduced p75NTRexpression in injured (L5) neurons, whereas the expression increases in uninjured but affected L4neurons. However, whereas injured neurons decrease p75NTRexpression, the surrounding SGCs experience a dramatic upregulation of p75NTRlevels in response to injury. Increased expression of p75NTRis primarily found in SGCs surrounding large neurons, and co-localization studies further demonstrate that sympathetic sprouting mainly associates with the p75NTR-positive SGCs surrounding these large neurons. Furthermore, other fi ndings show that sympathetic fi bers wrap up around the SGCs rather than around the neurons, and that p75NTRknockout mice display impaired sympathetic sprouting into the DRGs. Thus, increased expression of NGF and p75NTRby SGCs potentially provides an explanation for the abnormal growth of sympathetic fibers in DRGs after peripheral nerve injury. However, whether increased NGF expression by SGCs is restricted to the p75NTR-positive SGCs surrounding large injured neurons and how SGC p75NTRis involved in the supposed SGC-derived NGF attraction of sympathetic sprouts is still to be explained. Only half of the p75NTR-positive neuron-SGC units attract sympathetic sprouts, and p75NTR-positive SGCs surrounding small diameter neurons do not attract sympathetic sprouts, suggesting that sympathetic sprouting mechanisms include, but extend beyond, SGC-related increases in NGF and p75NTRexpression (Richner et al., 2014).
Interestingly, a recent study suggests that the neuronal expression level of p75NTRis critical for SGC responsiveness. Thus, reduction of neuron p75NTRlevels by oligonucleotides to simulate neuronal injury induced a reactive state in the corresponding SGCs, as measured by increased expression of p75NTR, GFAP and connexin 43 (Nadeau et al., 2014). This fi nding clearly suggests that the neurotrophin system is active in the adult organism, and that neurotrophin mediated signaling between neurons and SGC could be involved in injury states.
A major challenge in the exploration of SGC function lies in their intimacy with the sensory neurons, forming a functional unit. Recent experiments have shown that isolation of SGCs in culture results in morphological changes and a loss of selected marker proteins such as glutamine synthetase whereas another used SGC marker, P2X7, is largely unchanged (Belzer et al., 2010). This result indicates the limited use of isolated primary SGC cultures as a meaningful experimental system to explore the expression and regulation of SGC speci fi c proteins. Consequently, in vivo analysis in which the injured neurons trigger SGC activation and the subsequent bidirectional communication is, however challenging it may be, the appropriate system. To progress along this avenue, selective knock-down of signaling molecules or their receptors in either the neurons or the SGCs in the adult mice could be very informative. The development of the tamoxifen-inducible Advillin-Cre mouse allows specific and acute knock-out of proteins of interest in adult DRG sensory neurons. Future development of a corresponding SGC-speci fi c and inducible Cre would, paralleled by the Advillin-Cre model, allow very detailed analysis of the functions of a large variety of proteins in vivo and unravel details on the impact of SGC signals on the neuronal soma and vise versa. Subjecting such mice to various injury and in fl ammatory paradigms of the PNS could be the next path to further study the impact of neurotrophin signaling in the SGC-neuron system on chronic pain states and regenerative issues.
To conclude, neurotrophin signaling is essential for key phases of PNS development, including cellular survival, differentiation and neuronal sprouting. Following injury to peripheral nerves, neurotrophin signaling pathways are reactivated in neurons and SGCs, presumably partaking in the signaling processes facilitating nerve regeneration and functional recovery. Unfortunately, nerve recovery is often far from complete, and unwanted side effects of neurotrophin signaling in e.g., the spinal cord further initiate processes that lead to chronic neuropathic pain symptoms. Thus, there is an immense need to increase our understanding of fundamental biological aspects of neurotrophins and their receptors in neuronal and glial biology as well as their inter-cellular communication. Hopefully, future research will facilitate the development of better treatments to increase functional nerve recovery after injury and to prevent or alleviate neuropathic pain.
Christian Bjerggaard Vaegter
Danish Research Institute of Translational Neuroscience
DANDRITE, Nordic EMBL Partnership, and The Lundbeck
Foundation Research Center MIND, Department of
Biomedicine, Aarhus University, Ole Worms Allé 3, 8000
Aarhus C, Denmark
Belzer V, Shraer N, Hanani M (2010) Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol 6:237-243.
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299-309.
Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M (2009) Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol 87:27-46.
Hanani M (2005) Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev 48:457-476.
Nadeau JR, Wilson-Gerwing TD, Verge VMK (2014) Induction of a reactive state in perineuronal satellite glial cells akin to that produced by nerve injury is linked to the level of p75NTR expression in adult sensory neurons. Glia 62:763-777.
Pannese E (2010) The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol 6:3-10.
Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB (2014) Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol 50:945-970.
Christian Bjerggaard Vaegter, Ph.D.
Email: cv@biomed.au.dk.
10.4103/1673-5374.147924 http://www.nrronline.org/
Accepted: 2014-12-02
Vaegter CB. Neurotrophes and their receptors in satellite glial cells following nerve injury. Neural Regen Res. 2014;9(23):2038-2039.
杂志排行
中国神经再生研究(英文版)的其它文章
- “Standby” EMT and “immune cell trapping” structure as novel mechanisms for limiting neuronal damage after CNS injury
- Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
- Sulindac for stroke treatment: neuroprotective mechanism and therapy
- A novel functional electrical stimulation-control system for restoring motor function of post-stroke hemiplegic patients
- Dynamic reactive astrocytes after focal ischemia
- Restoration of function after cortical lesion: does it require an internal template?
