Restoration of function after cortical lesion: does it require an internal template?
2014-06-01HolgerSchulze,KonstantinTziridis
Restoration of function after cortical lesion: does it require an internal template?
The brain controls virtually all body functions, both internally and in interaction with the external environment. As the basic body anatomy of all vertebrates has a bilateral symmetry, structures and functions of vertebrate brains are also organized according to this fundamental anatomical principle to meet all sensory, motor, and internal requirements of body control. Consequently, particular parts or functions of the body are controlled by particular brain structures. For mammals whose brains only have a very limited capacity to regenerate (e.g., van den Brand et al., 2012), this organization principle implies that lesions of brain structures often cause dramatic impairments of those functions that have been controlled by the damaged structure (e.g., Witte and Freund, 1999).
On the other hand, many functions are bilaterally organized so the brain may still be able to solve certain tasks after unilateral lesion of a particular brain structure, for example in cases where symmetric sensory systems process redundant information, by using the respective undamaged contralateral structure (Schulze et al., 2014). In addition, undamaged neuronal tissue has a tremendous capacity for neuroplastic reorganization (e.g., van den Brand et al., 2012) so that impaired functions may be restored to some degree by compensatory neuroplasticity within undamaged brain structures. Nevertheless, total loss of functions may occur in cases where complementary structures are lesioned bilaterally (Deutscher et al., 2006) or after unilateral lesion of structures highly specialized for a particular task without a contralateral counterpart, like for example speech centers in human cerebral cortex that usually are localized in the left hemisphere.
From a therapeutic point of view, clinicians are confronted with the challenge to fi nd the best possible rehabilitation strategy for each individual patient with brain damage to ensure maximal improvement of lost function. In this perspective, we argue that every optimal rehabilitation strategy has to take into account the type of functional organization principle (unilateral/bilateral) of the structure underlying the damaged function. In particular, we propose that brain structures that previously have not or only marginally been involved in a particular task or function may still be enabled to solve the task via compensatory neuroplasticity. We think that this process requires an internal “teaching structure”, that is, a brain structure that is still intact and able to solve the task so that the processing capabilities of this teacher may be used as a template by the brain which may then be transferred to a “learning structure”. This idea is based on the following observations:
We have previously demonstrated in our animal model, the Mongolian gerbil (Meriones unguiculatus), that a particular auditory discrimination task, namely the discrimination of fast amplitude modulations (AM), is critically dependent on an intact auditory cortex (AC).
The gerbil AC is a well characterized structure, located bilaterally within the temporal lobes, and consists of at least seven fi elds that may be distinguished based on their spectral frequency representation and temporal response properties (e.g., Ter-Mikaelian et al., 2007). It has been demonstrated to represent complex stimulus features relevant for coding of speech like vowels or temporal envelope information (e.g., Ter-Mikaelian et al., 2013) in normal listening or “cocktail-party”-like situations (Kurt et al., 2008).
In the auditory discrimination task investigated here, the animals had to discriminate AM with periodicities of 160 vs. 320 Hz in a shuttle-box GO-NOGO paradigm. In contrast to slow AM with periodicities of 20 vs. 40 Hz, it was completely impossible for the animals to solve the task after bilateral lesion of AC (Deutscher et al., 2006). This was true for animals that received bilateral AC ablation prior to the fi rst training session and also for those that had learned to solve the task during 15 days of training with single daily training session prior to the bilateral lesion surgery (Figure 1A). As we could demonstrate in a follow-up study, unilateral lesion of the AC did not result in a complete loss of the ability to discriminate fast AM in our animal model (Schul-ze et al., 2014). Obviously an intact AC in one hemisphere was sufficient to solve the task (Figure 1B), although we were able to report subtle differences between left and right AC lesion.
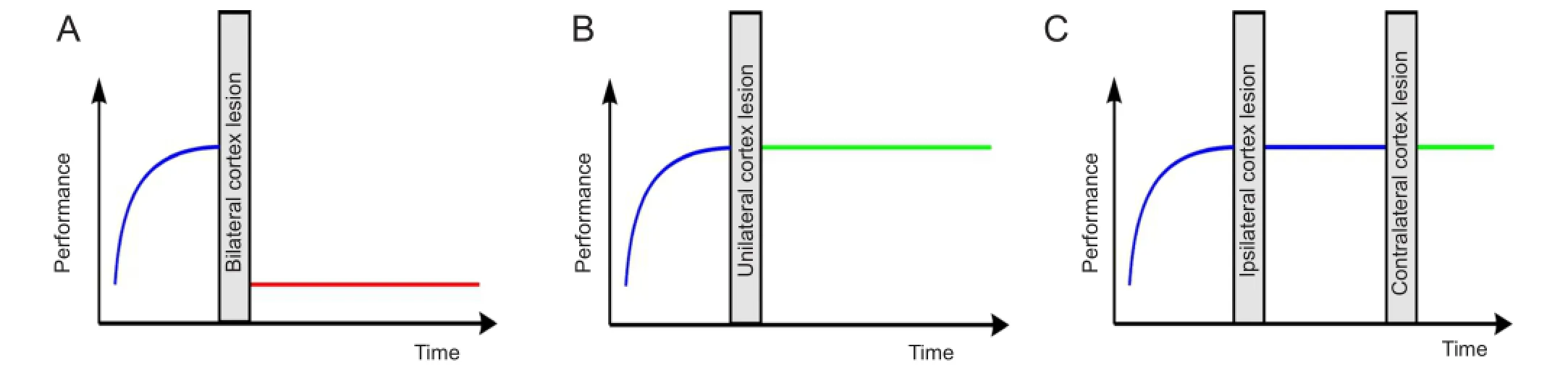
Figure 1 Schematic overview over effects of different avditory cortex (AC) lesion types on discrimination performance of fast amplitude modulations (AM) in Mongolian Gerbil.
Finally, in a third study (Depner et al., 2014), we described the effect of a serial bilateral ablation of AC on the discrimination performance of fast AM in our animal model (Figure 1C). In these experiments, AC was initially ablated unilaterally in pre-trained animals either in the left or the right hemisphere, as it was done in our previous report (Schulze et al., 2014). We then continued to train the animals in the fast AM discrimination task for another 15 training session, followed by a second lesion surgery where the AC contralateral to the side lesioned initially was ablated. Surprisingly we found that these animals were still able to perform the task as well as sham lesioned controls, even after complete lesion of both AC. That is, in contrast to simultaneous bilateral AC lesion that totally extinguished the ability to solve the task in our animals, serial lesion of both AC in two consecutive surgeries with continuing training in between the two unilateral lesions fully conserved the ability to discriminate between the two stimuli (Figure 1C). Similar fi ndings after lesion of other sensory, motor, or prefrontal cortex areas have been referred to as the “serial lesion effect”. It refers to the “ fi nding that serially placed lesions of the brain with some minimum time between lesions result in less severe residual deficits than do simultaneous lesions of equal extent and comparable loci” (Schmidt, 1976). But in contrast to our report the serial lesion effect typically does not lead to fully conserved function of the lesioned loci, but rather enables the system to better recover from brain damage although the function still remains impaired to some extent so that restoration of function is incomplete (e.g., Barbas and Spear, 1976).
In our study, the fi rst lesion obviously triggers some kind of compensatory plasticity that enables the system to still perform the task with no, not even transient, signi fi cant impairment after the second lesion. As a consequence, a function that in healthy animals critically depends on AC can entirely be accomplished by other cortical or sub-cortical structures, given that the two hemispheres are not ablated simultaneously and an adequate training is applied between the lesions. The same training is ineffective if ACs of both hemispheres are ablated simultaneously (Deutscher et al., 2006).
These results clearly demonstrate that after unilateral lesion of the fi rst AC an intact contralateral AC is needed to successfully enable potential substitute structures to take over the lost function by functionally reorganizing themselves. We here propose that the mechanism that underlies this type of compensatory plasticity requires the intact AC as a template whose internal structure and processing algorithms can then be transferred to a substitute structure. That is, the particular processing algorithms needed to solve a task must still be available somewhere in the brain to allow a mechanism of compensatory plasticity to transfer them to another structure that formerly was not-or to a lesser extend-involved in this type of neuronal processing. This view is different from the idea that the substitute structure solves the task using a different processing strategy compared to the original structure, or that the recovery of function seen after serial lesion is based on some kind of redundant representation of the processing algorithm that was already implemented within the substitute structure before the lesion (Stein et al., 1983). Based on our data we are currently not able to identify the cortical or sub-cortical substitute structure relevant here, but it definitely is not within auditory cortical areas as AC was completely destroyed on both sides in our experiments. Nevertheless, data from the visual cortex seem to point to cortical areas as most probable candidates for the substitute structure (Scheff and Wright, 1977).
We believe that our findings give insight into the nature of a particular type of compensatory neuronal plasticity, namely such where a brain structure is damaged that performs a task which is not exclusively represented in that structure alone. In this special case, the brain seems to be able to transfer the function to a new “backup-structure”which thereby gains the ability to perform the task alone in case further damage to the brain occurs that destroys the former “teacher structure”. This compensatory plasticity seems to be triggered by the fi rst lesion, thereby opening a time window for possible new therapeutic interventions that aim to strengthen the proposed transfer process. If the training that we applied in our animal model in between the two sequential AC lesions (Figure 1C) could be a model for such a therapeutic intervention is currently unclear, as we do not know if the compensatory plasticity we described would not have taken place without that training, although it is likely that training indeed is needed (Scheff and Wright, 1977). We currently undertake a new series of new experiments in our laboratory to answer that speci fi c question. In any case, the question remains if the complete preservation of function in our study as opposed to partial restoration of function as in the classical serial lesion effect depends on the speci fi c task investigated or the training strategy applied between consecutive lesions. If the latter would be the case, new therapeutic strategies may be developed to maximize the bene fi t of the serial lesion effect in sensory or motor modalities where rehabilitation after brain lesion is still unsatisfactory.
This work was funded by grants from the Deutsche Forschungsgemeinschaft (DFG), grants SCHU 1272/2-1,2 and-SCHU 1272/4-1.
Holger Schulze, Konstantin Tziridis
Experimental Otolaryngology, Friedrich-Alexander University Erlangen-Nürnberg, Waldstrasse 1, 91054 Erlangen, Germany
Schulze H, Tziridis K. Restoration of function after cortical lesion: does it require an internal template? Neural Regen Res. 2014;9(23):2029-2031.
References
Barbas H, Spear PD (1976) Effects of serial unilateral and serial bilateral visual cortex lesions on brightness discrimination relearning in rats. J Comp Physiol Psychol 90:279-292.
Depner M, Tziridis K, Hess A, Schulze H (2014) Sensory cortex lesion triggers compensatory neuronal plasticity. BMC Neurosci 15:57.
Deutscher A, Kurt S, Scheich H, Schulze H (2006) Cortical and subcortical sides of auditory rhythms and pitches. Neuroreport 17:853-856.
Kurt S, Deutscher A, Crook JM, Ohl FW, Budinger E, Moeller CK, Scheich H, Schulze H (2008) Auditory cortical contrast enhancing by global winner-take-all inhibitory interactions. PLoS One 3:e1735.
Scheff SW, Wright DC (1977) Behavioral and electrophysiological evidence for cortical reorganization of function in rats with serial lesions of the visual cortex. Physiol Psychol 5:103-107.
Schmidt H, Jr. (1976) A theory of the serial lesion effect. Brain Res Bull 1:261-262.
Schulze H, Deutscher A, Tziridis K, Scheich H (2014) Unilateral auditory cortex lesions impair or improve discrimination learning of 1 amplitude modulated sounds, depending on lesion side. PLoS One 9:e87159.
Stein DG, Finger S, Hart T (1983) Brain damage and recovery: problems and perspectives. Behav Neural Biol 37:185-222.
Ter-Mikaelian M, Sanes DH, Semple MN (2007) Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci 27:6091-6102.
Ter-Mikaelian M, Semple MN, Sanes DH (2013) Effects of spectral and temporal disruption on cortical encoding of gerbil vocalizations. J Neurophysiol 110:1190-1204.
van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G (2012) Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336:1182-1185.
Witte OW, Freund HJ (1999) Neuronal dysfunction, epilepsy, and postlesional brain plasticity. Adv Neurol 81:25-36.
Holger Schulze, Ph.D.
Email: Holger.Schulze@uk-erlangen.de.
10.4103/1673-5374.147921 http://www.nrronline.org/
Accepted: 2014-11-07
杂志排行
中国神经再生研究(英文版)的其它文章
- “Standby” EMT and “immune cell trapping” structure as novel mechanisms for limiting neuronal damage after CNS injury
- Angioplasty and stenting for severe vertebral artery ori fi ce stenosis: effects on cerebellar function remodeling veri fi ed by blood oxygen level-dependent functional magnetic resonance imaging
- Sulindac for stroke treatment: neuroprotective mechanism and therapy
- A novel functional electrical stimulation-control system for restoring motor function of post-stroke hemiplegic patients
- Neurotrophins and their receptors in satellite glial cells following nerve injury
- Dynamic reactive astrocytes after focal ischemia
