Photosynthetic response dynamics in the invasive species Tithonia diversifolia and two co-occurring native shrub species under fluctuating light conditions
2024-04-12JuLiShuBinZhngYngPingLi
Ju Li ,Shu-Bin Zhng ,Yng-Ping Li ,*
a CAS Key Laboratory of Tropical Forest Ecology,Xishuangbanna Tropical Botanical Garden,Chinese Academy of Sciences,Menglun,Mengla 666303,Yunnan,China
b University of Chinese Academy of Sciences,Beijing 100049,China
Keywords: Invasive plant Photosynthetic induction Photosynthetic relaxation Carbon gain Stomatal traits Tithonia diversifolia
ABSTRACT To determine the invasiveness of invasive plants,many studies have compared photosynthetic traits or strategies between invasive and native species.However,few studies have compared the photosynthetic dynamics between invasive and native species during light fluctuations.We compared photosynthetic induction,relaxation dynamics and leaf traits between the invasive species,Tithonia diversifolia and two native species, Clerodendrum bungei and Blumea balsamifera,in full-sun and shady habitats.The photosynthetic dynamics and leaf traits differed among species.T.diversifolia showed a slower induction speed and stomatal opening response but had higher average intrinsic water-use efficiency than the two native species in full-sun habitats.Thus,the slow induction response may be attributed to the longer stomatal length in T.diversifolia.Habitat had a significant effect on photosynthetic dynamics in T.diversifolia and B.balsamifera but not in C.bungei.In shady habitat, T.diversifolia had a faster photosynthetic induction response than in full-sun habitat,leading to a higher average stomatal conductance during photosynthetic induction in T.diversifolia than in the two native species.In contrast,B.balsamifera had a larger stomatal length and slower photosynthetic induction and relaxation response in shady habitat than in full-sun habitat,resulting in higher carbon gain during photosynthetic relaxation.Nevertheless,in both habitats, T.diversifolia had an overall higher carbon gain during light fluctuations than the two native species.Our results indicated that T.diversifolia can adopt more effective response strategies under fluctuating light environments to maximize carbon gain,which may contribute to its successful invasion.
1.Introduction
Biological invasion is a key threat to global biodiversity and ecosystem function(Diagne et al.,2021).Invasive plants may have different functional traits or strategies that allow them to succeed in the introduced ranges.Among these,photosynthesis is generally regarded as an important process for supporting plant growth and received more attention.Over the last decade,many studies have compared steady-state photosynthetic processes between invasive and native species.However,the light intensity in the field frequently changes from shade to sun and sun to shade in seconds(Pearcy,1994;Zhu et al.,2004;Slattery et al.,2018).Fluctuating light may lead to dynamic photosynthesis and influence total carbon gain in the field (Pearcy,1990).Modulating photosynthesis under fluctuating light conditions may more accurately reflect the photosynthetic process in the field.However,few studies have focused on comparing photosynthetic dynamics between invasive and native species under fluctuating light conditions.
The process by which leaves begin to increase the assimilation of CO2as light transitions from low(shade)to high(sun)is defined as photosynthetic induction,which features a lag due to time required for the regeneration of ribulose 1,5-bisphosphate (RuBP),the synthesis of carbon metabolite intermediates,activation of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco),and stomatal opening when photosynthesis shifts to a steady state(Pearcy,1994;Mott and Woodrow,2000).Because of the delay effect,the leaf photosynthetic rate (A) is lower throughout induction than at steady state,which results in a considerable loss of daily carbon gain (Taylor and Long,2017;Tanaka et al.,2019).Electron transport and activation have been shown to limit CO2assimilation (A) during short flecks (Soleh et al.,2017;Taylor and Long,2017) because of the rapid activation of RuBP regeneration and Rubisco (Mott and Woodrow,2000;Kaiser et al.,2016;Deans et al.,2019).In addition,diffusion triggered by stomatal conductance (gs) and mesophyll conductance (gm) mainly constrainsAduring longer light periods(Pearcy,1990).Bothgsandgminfluence dynamic photosynthesis under light fluctuation and,in turn,affect CO2provision to the RuBP carboxylation site (Sakoda et al.,2022).
Leaf stomata predominantly control CO2uptake for photosynthesis and transpiration and further determine plant productivity and water-use efficiency.The balance between photosynthesis and transpiration relies on internal signals and environmental sensitivity of stomata,and the synchrony of stomatal movement relative to the CO2requirement of the mesophyll (Lawson and Blatt,2014).The response ofgsto light fluctuation is slower commonly than the biochemical process,which is a significant limitation ofAduring the induction phase(Kaiser et al.,2017;Sakoda et al.,2022).The response ofgsis primarily explained by the stomatal movement via the regulation of signal transduction and metabolic processes(Lawson and Blatt,2014).gsis also affected by the morphological features of the stoma,such as the size and shape (dumbbell and elliptical shaped)of a single stoma,stomatal density,and stomatal distribution(Sakoda et al.,2022).Several studies have shown that smaller stomata have faster response rates than larger ones (Drake et al.,2013;Lawson and Blatt,2014).However,it is remains controversial.Other studies have not observed a relationship between stomatal behavior and morphology (Elliott-Kingston et al.,2016).Zhang et al.(2019) found that smaller stomata have a slower initial induction speed and lower stomatal conductance during the initial phase of light induction relative to larger stomata.
Tithonia diversifoliaA.Gray,a perennial invasive shrub,is native to Central America (Laduke,1982).It was introduced as an ornamental and green manure plant in many countries,which offered opportunities for its dispersal throughout most of the tropical and subtropical areas worldwide (Morales,2000).This species can reproduce both sexually,with a large production of seeds,and asexually.Once its population is established,it quickly forms dense monospecific stands by inhibiting the germination and growth of neighboring species (Oyerinde et al.,2009;Otusanya and Ilori,2012;Kato-Noguchi,2020),leading to a decrease in biodiversity(Obiakara and Fourcade,2018;Dai et al.,2021).It can invade a variety of habitats.It can grow in open and sunny areas,such as roadsides,wastelands,riverbanks and disturbed sites,and in shady areas,including forest edges and disturbed secondary forests.This suggests that they are likely to respond rapidly and efficiently to heterogenous environments during physiological processes.However,the photosynthetic strategy ofT.diversifoliain different habitats under natural fluctuating light conditions remains unclear.
Here,we measured gas exchange under fluctuating light conditions inTithonia diversifoliaand two native species,Clerodendrum bungeiandBlumea balsamifera,grown in full-sun and shady sites.We selected these two native plants because of their overlapping habitats.C.bungeiis an accompanying species ofT.diversifolia;B.balsamifera,similar toT.diversifolia,belongs to the Compositae family.We also measured the stomatal traits of all species.We hypothesized that (1)T.diversifoliahas a faster and more efficient response to photosynthetic induction than two native species,(2)habitat type under different light intensities affects photosynthetic dynamics,and (3) changes in photosynthetic induction and relaxation speed are associated with leaf traits.
2.Materials and methods
2.1.Study site and species
This study was conducted at Xishuangbanna Tropical Botanical Garden (XTBG),Chinese Academy of Sciences,Yunnan Province,China.The mean day/night temperature in March at XTBG is 22.8°C/15.2°C,with a day/night relative humidity of 64%/100%(Liu et al.,2004).Our study included an invasive shrub,Tithonia diversifolia,and two native shrubs,Clerodendrum bungeiandBlumea balsamifera.Prior to this study,T.diversifoliaandC.bungeigrew naturally in XTBG,andB.balsamiferawas planted for more than 20 years in XTBG.These three species can grow under full-sun light and shady conditions in the field.We identified six locations with similar soil but different light conditions under which the three species grew (see Table 1).For each individual,similar mature leaves (4th-5th from the shoot tip) in the new shoots of the current year were selected.
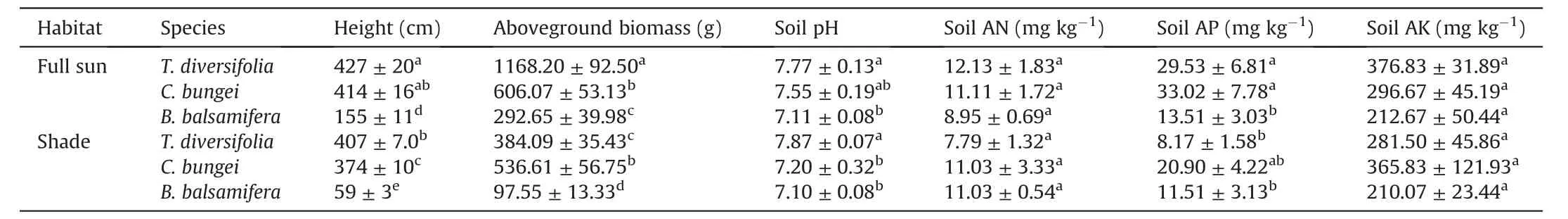
Table 1 Background information of sampling sites.
2.2.Gas exchange measurements
Gas exchange parameters of six individuals per species were measured under full sunlight (≥1200 μmol photons m-2s-1) and shade(≤300 μmol photons m-2s-1)environments using the Li-6800 portable photosynthesis system (LI-COR Inc.,USA) with 27°C air temperature,500 μmol s-1flow rate,1.2-1.8 kPawater vapor pressure deficit(VPD),and 65%relative humidity.The mature fully expanded leaves were selected at random for measuring gas exchange parameters,and the measurement was performed between 10:00-14:00(because of thick fog before 9:30 a.m.) to avoid confounding photosynthesis with any marked circadian effects.The definitions and abbreviations of all the measured traits are specified in Table 2.
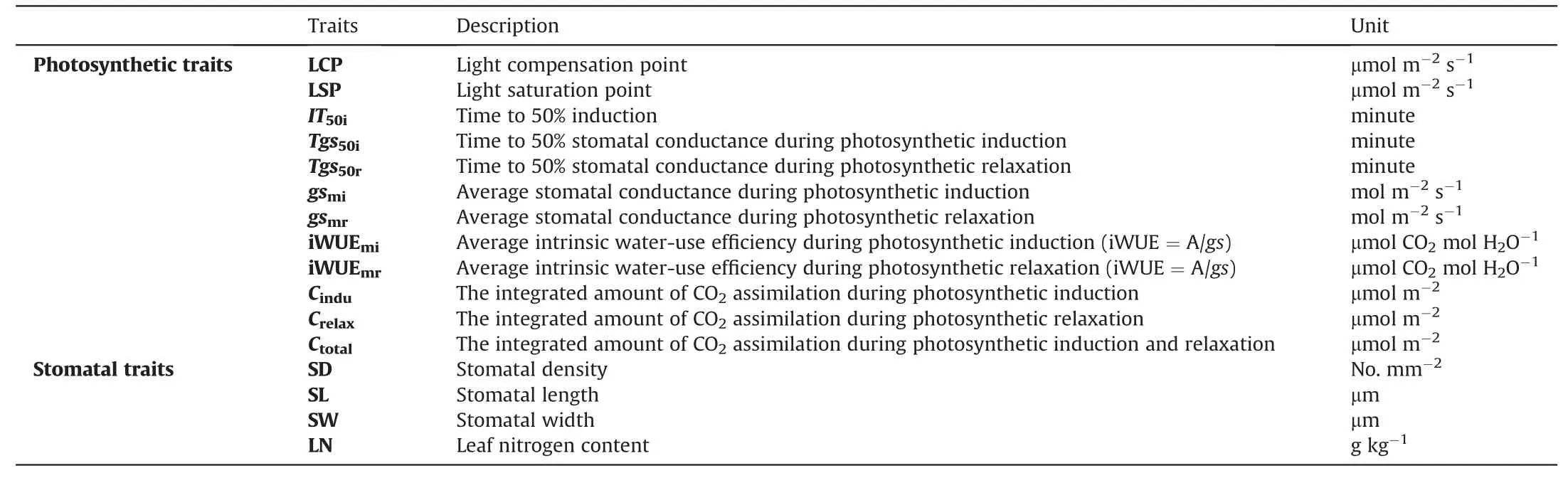
Table 2 A summary of all traits measured and mentioned in the text.Units and light conditions are included.
To determine the range of adaptation to light intensity,we measured the light response curves of the three species.Photosynthetic parameters were measured at saturating CO2concentration(400 μmol mol-1)and light intensities of 0,15,75,150,200,300,500,800,1000,1500,and 2000 μmol m-2s-1.The light saturation point (LSP) and light compensation point (LCP) were fitted using “Photosynthesis” v.1.0.
For induction,leaves were allowed to reach a steady state under low light(50 μmol m-2s-1PPFD)for 5 min,followed by 30 min of high light (1700 μmol m-2s-1PPFD).For relaxation,the light intensity on leaves was decreased back to low light(50 μmol m-2s-1PPFD) for 15 min after 30 min of high light.Gas exchange parameters were logged every minute.
Average stomatal conductance (gsmi) and average intrinsic water-use efficiency (iWUEmi) over 30 min of photosynthetic induction and average stomatal conductance (gsmr) and average intrinsic water-use efficiency (iWUEmr) over 15 min of photosynthetic relaxation were calculated.
The speed of photosynthetic induction was calculated by measuring the time in minutes to 50% relative to steady-state CO2uptake (IT50i).The speed of stomatal opening was assessed as the time to 50% steady-state stomatal conductance during induction(Tgs50i).The initialgsat pre-illumination can constrain the photosynthetic induction response by affecting the activation state of the biochemical response (Kaiser et al.,2016).To eliminate the influence of initial status on induction speed,we regarded the lastAandgsat low light as the initialA0andgs0,and we obtained values of each corresponding ΔAand Δgsby subtracting the initialA0andgs0from the observed value during induction.Finally,we calculated the induction rate ofAand the opening speed of the stoma withseries ΔAand Δgs.The speed of stomatal closure was also evaluated based on the time to 50% of the new steady-state stomatal conductance after returning to low light (Tgs50r).
The integrated amount of CO2uptake (Cindu) during 30 min of light induction,and the integrated amount of CO2uptake (Crelax)during 15 min of light relaxation were calculated using Eq.(1):
whereAtis the transient photosynthetic rate (Xiong et al.,2022).
The integrated amount of CO2uptake (Ctotal) during light induction and relaxation is the sum ofCinduandCrelax.
2.3.Leaf traits measurement
Mature leaves from the same position were sampled.Leaf nitrogen (N,g kg-1) concentration was measured using a C-N elemental analyzer (Vario Max CN,Elementar Analysensysteme GmbH,Hanau,Germany).The leaves (2 × 2 cm) were excised and soaked in a solution (ethanol : acetic acid=1:1,v/v) in a 70°C thermostatic water bath until the leaves were clear,and the abaxial side of the leaf was torn and stained with safranin.Temporary slices for stomatal observations were made.Images were obtained using a light microscope(Leica Microsystems Ltd,Leica DM2500)connected to a digital camera.The stomatal length and width were measured as the major and minor axes of the ellipse,respectively.Stomatal density was defined as the number of stomata per unit of leaf area.
2.4.Statistical analyses
We used a two-way ANOVA to determine the effects of species,habitat type,and their interaction on all traits.Further multiple comparisons among species and habitat types were performed using the new least significant range method (Duncan) (R;“agricolae”).To test pairwise relationships among stomatal traits,induction speed,and carbon gain during photosynthetic induction,we conducted Pearson's correlation analysis for each habitat.
To determine the most important factors affectingCindu,CrelaxandCtotal,we examined a full model consisting of stomatal traits,response speed,averagegs,and average intrinsic water-use efficiency during light induction and relaxation to detect the most important factors.Best regressions were selected using stepwise regression(R;“MASS”).All analyses were performed using R v.4.2.1(R Core Team,2020).
3.Results
3.1.Light response curve
There was a significant difference in the parameters of the light response curve among the three species in the different habitats(Table S1).Tithonia diversifoliahad higher LSP (1682.00 ± 38.83)and LCP(40.67±2.17)than the two native species in sunny habitat(Fig.S1).Habitat also affected the LSP and LCP of the three species(Table S1).In shady habitat,LSP (1276.37 ± 49.24) and LCP(19.33 ± 6.48) ofT.diversifoliaand LSP (1096.01 ± 108.96) ofC.bungeiwere obviously lower relative to the sunny habitats.For LCP (full-sun:26.00 ± 1.16;shady: 25.33 ± 1.69) ofC.bungei,there was no significant difference between the sunny and shady habitats.Therefore,in the shady habitats,T.divesifoliashowed a similar LCP to but higher LSP than the two native species (Fig.S1).
3.2.Gas exchange parameters
The speed of induction differed significantly among the three species(Table S1).In sunny habitats,the speed of induction ofAandgsofT.diversifoliawere slower(higherIT50iandTgs50i)than those inC.bungeiandB.balsamifera(Fig.1).Habitat had a significant effect on induction speed,but this effect differed among species (significant interaction) (Table S1).The time of induction and stomatal opening ofT.diversifoliawas shortened(lowerIT50iandTgs50i),but that ofB.balsamiferawas lengthened (higherIT50iandTgs50i) in shady habitat than in sunny habitat(Fig.1).The trends ingsandAwere tightly coupled,regardless of the species and habitat type during induction (Fig.2).The iWUE first increased and then decreased with increasing light intensity forT.diversifoliain the two habitats and forB.balsamferain shady habitat (Fig.3).InC.bungei,iWUE rapidly increased and was maintained during photosynthetic induction (Fig.3).T.diversifoliawas the topperforming species forgsmiand iWUEmi,and it also achieved the highestCindu,whileB.balsamiferawas the lowest-performing species in all habitats (Table 3).
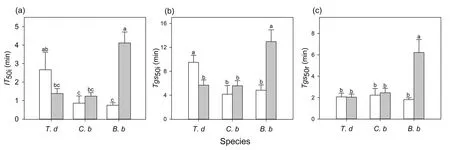
Fig.1.Time to 50% induction of CO2 uptake (IT50i,a),time to 50% induction of stomatal conductance (Tgs50i,b) during photosynthetic induction,and time to 50% relaxation of stomatal conductance(Tgs50r,c).These traits were measured in three species[Tithonia diversifolia(T.d),Clerodendrum bungei(C.b),and Blumea balsamifera(B.b)]in full-sun(white bar) and shady (gray bar) habitats.Each bar is the mean (±SE) of six plants.Different lowercase letters indicate statistically significant differences at P <0.05.
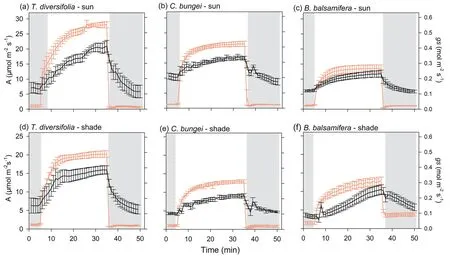
Fig.2.Photosynthetic rate(A)and stomatal conductance(gs)over time showing an increase during photosynthetic induction(transition from low light(50 μmol m-2 s-1)to high light (1700 μmol m-2 s-1) and photosynthetic relaxation (transition from high light to low light).Measurements were taken in three species [Tithonia diversifolia (a,d), Clerodendrum bungei(b,e)and Blumea balsamifera(c,f)]in full-sun and shady habitats.Red line and black line represent A and gs,respectively.Periods of low light are shown by the gray areas in the figure,and the period of highlight is shown in white.
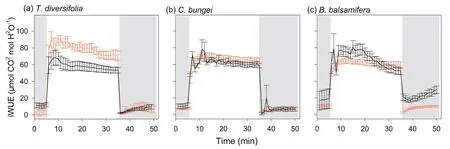
Fig.3.Intrinsic water-use efficiency (iWUE) over time during photosynthetic induction (transition from low light (50 μmol m-2 s-1) to high light (1700 μmol m-2 s-1) and photosynthetic relaxation (transition from high light to low light).Measurements were taken in three species [Tithonia diversifolia (a), Clerodendrum bungei (b),and Blumea balsamifera(c)]in the full-sun(red line)and shady(black line)habitats.Periods of low light are shown by the gray areas in the figure,and the period of high light is shown in white.
During the transition from high to low light,there were no significant differences in the speed of stomatal closing (Tgs50r)among species in full-sun habitat.However,in shady habitat,B.balsamiferashowed a slower speed of stomatal closure (higherTgs50r)relative to that in full-sun habitat,and stomatal closure was slower than that inT.diversifoliaandC.bungeiin shady habitat(Fig.1).Inconsistent with light induction,the dynamics ofAandgswere not coupled,withAdecreasing much faster during light relaxation (Fig.2).Except forB.balsamifera,the iWUE also rapidly decreased during photosynthetic relaxation (Fig.3).ForB.balsamifera,the iWUE showed an increasing trend during photosynthetic relaxation (Fig.3).Thegsmrwas not significantly different among species,but the iWUEmrandCrelaxofB.balsamiferawere higher than those of the other two species during photosynthetic relaxation(Table 3).Even so,T.diversifoliahad the highestCtotalduring the entire light fluctuation period (Table 3).
3.3.Leaf traits
Leaf traits differed among the species and habitats(Table S1).In full-sun habitats,T.diversifoliaandC.bungeihad higher leaf nitrogen thanB.balsamifera.In shady habitats,B.balsamiferahad increased leaf nitrogen relative to that in sunny habitats,but bothC.bungeiandB.balsamiferahad lower leaf nitrogen thanT.diversifolia(Fig.4).Stomatal traits were different among species and habitats(Table S1).Regardless of the habitat type,T.diversifoliahad the lowest stomatal density (Fig.4).Compared to the sunny habitats,the stomatal density ofB.balsamiferawas significantly lower in shady habitats (Fig.4).In full-sun habitats,T.diversifoliahad higher stomatal length values than the two native species,and a higher stomatal width thanC.bungei(Fig.4).B.balsamiferadisplayed longer stomatal length in shady habitat than in full-sun habitat.In shady habitat,the stomatal size ofB.balsamiferawas higher than that ofC.bungei,but similar to that ofT.diversifolia(Fig.4).There was also a significant negative relationship between stomatal length and density in full-sun habitats (Fig.5).
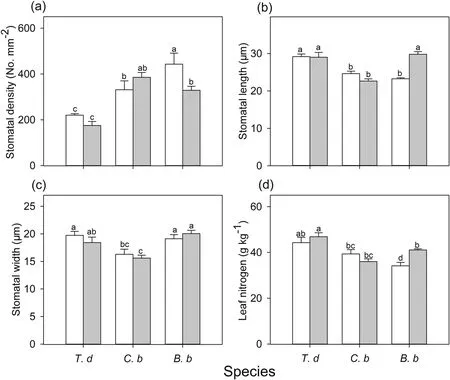
Fig.4.Stomatal density (a),stomatal length (b) and stomatal width (c),and leaf nitrogen (d) of three species [Tithonia diversifolia (T.d), Clerodendrum bungei (C.b) and Blumea balsamifera (B.b)] in full-sun (white bar) and shady (gray bar) habitats.Each bar is the mean (±SE) of six plants.Different lowercase letters indicate statistically significant differences at P <0.05.
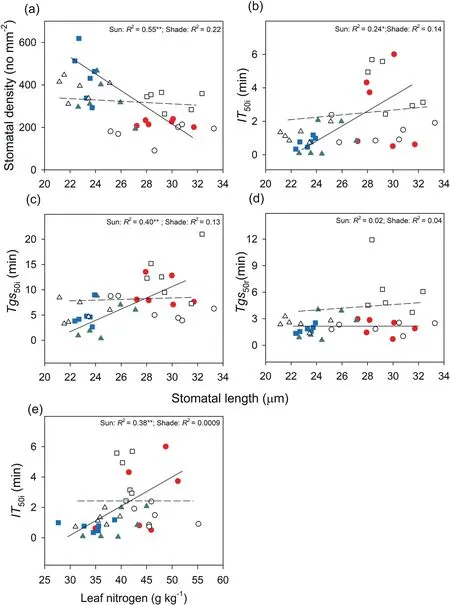
Fig.5.Correlations between stomatal length and stomatal density(a),time to 50%induction of CO2 uptake(IT50i,b),time to 50%induction of stomatal conductance(Tgs50i,c)during photosynthetic induction,and time to 50%relaxation of stomatal conductance(Tgs50r,d)and correlation between leaf nitrogen and time to 50%induction of CO2 uptake(e)in three species[Tithonia diversifolia(full-sun:red solid circle;shade:empty circle),Clerodendrum bungei(full-sun:green solid triangle;shade:empty triangle),and Blumea balsamifera(fullsun: blue solid square;shade: empty square)] in the full-sun (full line) and shady (dotted line) habitats.Each point represents one replication.
3.4.Correlations between gas change parameters and leaf traits
Induction speed(IT50iincreased)decreased with increasing leaf nitrogen,and these relationships were observed in sunny habitats.With increasing stomatal length,the induction speed (IT50iandTgs50iincreased) decreased in full-sun habitats;however,no such relationships were found in shady habitats(Fig.5).Stomatal length had no obvious relationship withTgs50rin either habitat(Fig.5).No significant correlations were found between stomatal density and induction speed (in full-sun habitats,IT50i:R2=0.09,P=0.220;Tgs50i:R2=0.11,P=0.181;Tgs50r:R2=0.008,P=0.720;in shady habitats:IT50i:R2=0.08,P=0.244;Tgs50i:R2=0.07,P=0.318;Tgs50r:R2=0.13,P=0.183),although there was a negative relationship between stomatal length and density.The induction time(IT50iandTgs50i) showed significantly negative relationships withgsmiin shady habitat but not in full-sun habitat (Fig.6).However,induction time (IT50iandTgs50i) showed significant positive relationships with iWUEmiin both habitats (Fig.6).The relaxation time(Tgs50r)showed a significantly positive relationship withgsmrand iWUEmrin shady habitat but not in full-sun habitat (Fig.6).
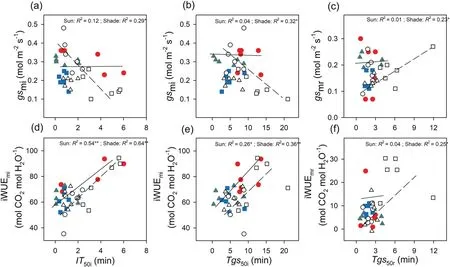
Fig.6.Correlations between average stomatal conductance and time to 50% induction of CO2 uptake (IT50i,a),time to 50% induction of stomatal conductance (Tgs50i,b) during induction,and time to 50%relaxation of stomatal conductance (Tgs50r,c)during relaxation.Correlations between average intrinsic water-use efficiency and IT50i(d),Tgs50i(e),and Tgs50r(f)in three species[Tithonia diversifolia(full-sun:red solid circle;shade:empty circle),Clerodendrum bungei(full-sun:green solid triangle;shade:empty triangle)and Blumea balsamifera (full-sun: blue solid square;shade: empty square)] in full-sun (full line) and shady (dotted line) habitats.Each point represents one replication.
3.5.Modelling trait variation
In full-sun habitat,Cinduwas best predicted bygsmiand iWUEmi;Crelaxwas best predicted by leaf traits (leaf nitrogen,stomatal density and length) and iWUEmr;Ctotalwas best predicted bygsmiand iWUEmi(Table 4).In shady habitat,Cinduwas best predicted byIT50i,gsmiand iWUEmi,Crelaxwas best predicted bygsmrand iWUEmr,Ctotalwas best predicted bygsmi,and iWUEmi,followed byIT50iandTg50r(Table 4).
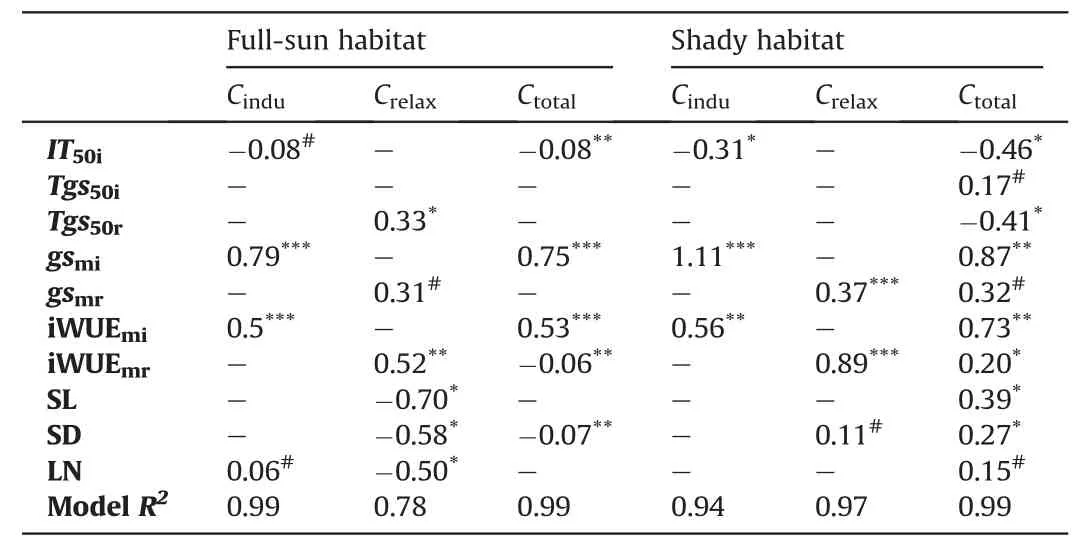
Table 4 Step regression models to predict carbon gain during light fluctuation.
4.Discussion
To understand how the invasive plantTithonia diversifoliaresponds to light fluctuation,we compared the photosynthetic responses to fluctuating light among the invasive plant and two native plants distributed in full-sun and shady environments.We found that the photosynthetic induction dynamics differed among the three species in different habitats.The invasive speciesT.diversifoliashowed different photosynthetic induction in full-sun and shady habitats,whereas the native speciesB.balsamiferadisplayed different photosynthetic relaxations.ForC.bungei,the photosynthetic dynamics were not significantly different between the two habitats.These differences lead to an overall higher carbon gain during fluctuating light for the invasive speciesT.diversifoliain both habitats,which may contribute to its successful invasion.
4.1.Difference in photosynthetic dynamic
Tithonia diversifoliaadopts different strategies to respond to light induction in full-sun and shady habitats to maximize carbon gain during light fluctuation.In full-sun habitat,T.diversifoliashowed slower photosynthetic induction speed ingsandAthan native species (Fig.1).This is attributed to the larger stomata ofT.diversifoliarelative to native species,as suggested by positive relationship between stomatal length and induction speed in full-sun habitat(Fig.5).Stoma controls both CO2uptake and water transpiration(Voelker et al.,2016);when the light intensity increases suddenly,the rapid opening of large stomata may obtain more CO2,but potentially lead to more water loss,leading to a reduction in the photosynthesis rate (Drake et al.,2013).In the field,wilting and drooping leaves have been observed inT.diversifoliaat noon,suggesting that this invasive species may be less resistant to drought than the two native species.Correspondingly,we also observed thatT.diversifoliahad higher iWUEmithan the two native species in fullsun habitat,which contributed to higherCindu(Tables 3 and 4).This is inconsistent with the idea that a slow response to photosynthetic induction may result in a loss of daily carbon gain(Taylor and Long,2017;Tanaka et al.,2019).On the contrary,in shady habitat,T.diversifoliaincreased the induction speed relative to conspecifics in full-sun habitat,and it was faster than that of the native plantB.balsamifera.Thus,faster induction speed plays a key role in maintaining highgsmiin shady habitat (Fig.6) and contributes significantly to carbon gain during photosynthetic induction in Cindu(Table 4).Although the increase in induction speed led to some loss of iWUEmi,this appears to be of less importance in such an environment.The regression analysis results also indicated that iWUEmiis a key factor contributing to carbon gain during light induction in full-sun habitat,whereasgsmiplays an important role in carbon gain during light induction in shady habitat(Table 4).
Light fleck theory predicts that shade-adapted plants should rapidly open stomata in response to light increase to make use of light flecks,while also slowly closing stomata during light decrease to make the most of potential future light flecks(Knapp and Smith,1987).This theory explains the difference in stomata closure speed among the three species in shady habitat.The native speciesB.balsamiferahad lower LSP and LCP values than the other two species,especially in shady habitat (Fig.S1),suggesting that it is more adapted to shady environment than the other two species.Correspondingly,we found that this species showed slower stomata closure (higherTgs50rvalue) from high light to low light(Fig.1).In addition,the slower closing of stomata may maintaingsto some degree and promote photosynthesis.We also observed thatB.balsamiferadisplayed a highergsmrand iWUEmr(Table 3),which contributed to a higherCrelax(Table 4).One possible reason for this is that water is not a limiting factor in shady habitat.Maximum utilization of light energy may be more important in such environments.Nevertheless,the strategy adopted byT.diversifoliain different habitats was more effective for total carbon gain relative to that ofB.balsamifera,because total carbon gain during light fluctuation mainly came from photosynthetic induction rather than photosynthetic relaxation.
4.2.Effect of leaf traits
Stomatal traits are important factors that affect the photosynthetic response dynamics to light fluctuations (Xiong et al.,2022).Our results also indicated relationships between stomatal length and stomatal opening and induction speed;species with large stomata have slower stomatal opening relative to species with small stomata.This is consistent with other studies on many species(Drake et al.,2013;Xiong et al.,2022).However,a relationship between stomatal length and stomatal opening speed was not observed in shady habitat.This could be because the stomatal length ofB.balsamiferawas significantly higher in shady habitat than in full-sun habitat,whereas there was no significant difference in stomatal length between the two habitats for the other two species.Thus,changes in stomatal length may lead to slower stomatal opening and closing speeds inB.balsamiferaduring light induction and relaxation (Figs.1 and 4),suggesting that stomatal morphological traits mediate stomatal behavior species.Drake et al.(2013)also suggested that plants with larger stomata often exhibit a slower response rate to environmental fluctuations (Drake et al.,2013).However,the specific regulatory mechanism for stomatal morphology is still not clear.One possible mechanism is that larger stomata may require more osmotic substances and energy inside the leaves than smaller stomata (Santelia and Lawson,2016).In addition,T.diversifoliashowed faster induction and stomatal opening speed in shady habitat than in full-sun habitat,although no significant differences in stomatal traits were found.This suggests that,in addition to stomatal traits,there may be other factors or mechanisms affecting the induction speed ofT.diversifolia.
Leaf nutrients are essential elements for stromal enzymes and thylakoid proteins (Sudo et al.,2003;Takashima et al.,2004),and can potentially affect photosynthesis.Liu et al.(2021) found that leaf nitrogen content was negatively correlated with the time to 50% and 100% of the maximum photosynthetic rate across eight genotypes ofBrassica napus.Sun et al.(2022) also indicated that tomato seedlings grown under high nitrogen conditions had faster photosynthetic induction speeds.In contrast,in this study,we found a positive relationship between leaf nitrogen content and time to 50% of the maximum photosynthetic rate in full-sun habitats.One possible reason for this is that plants with high leaf nitrogen also have a larger stomatal size (longer stomatal length).Stomatal traits have a greater impact than leaf nitrogen content on photosynthetic induction speed.
Author contributions
J.L.designed and performed the experiments,analyzed the results,and wrote the paper.S.-B.Z.designed and performed the experiments.Y.-P.L.contributed to design,analysis and writing of the paper.
Declaration of competing interest
We have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Data availability statement
The data used in this study will be uploaded to the Science DB database to be shared when the manuscript is accepted.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant number: 32071661).We are grateful to the Institutional Center for Shared Technologies and Facilities of Xishuangbanna Tropical Botanical Garden,(CAS) for measuring the chemicals,and the National Forest Ecosystem Research Station of Xishuangbanna for providing the Li-6800 portable photosynthesis system.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.04.001.
杂志排行
植物多样性的其它文章
- Global patterns and ecological drivers of taxonomic and phylogenetic endemism in angiosperm genera
- Patterns and drivers of plant sexual systems in the dry-hot valley region of southwestern China
- Cryptic divergences and repeated hybridizations within the endangered “living fossil” dove tree (Davidia involucrata) revealed by whole genome resequencing
- Enhanced and asymmetric signatures of hybridization at climatic margins: Evidence from closely related dioecious fig species
- Cryptic diversity and rampant hybridization in annual gentians on the Qinghai-Tibet Plateau revealed by population genomic analysis
- Circumscription of the East Asia clade(Apiaceae subfamily Apioideae)and the taxonomic placements of several problematic genera
