Sex-specific facilitation and reproduction of the gynodioecious cushion plant Arenaria polytrichoides on the Himalaya-Hengduan mountains,SW China
2024-04-12XufngChenYzhouZhngLishenQinRenyuZhouHngSunJinguoChen
Xufng Chen ,Yzhou Zhng ,Lishen Qin ,Renyu Zhou ,Hng Sun ,** ,Jinguo Chen ,*
a State Key Laboratory of Plant Diversity and Specialty Crops,Kunming Institute of Botany,Chinese Academy of Sciences,Kunming,Yunnan,China
b Yunnan Key Laboratory of Plateau Wetland Conservation,Restoration and Ecological Services,Southwest Forestry University,Kunming,Yunnan,China
Keywords: Facilitation intensity Feedback effect Sex-specific facilitation Nurse plant Population dynamics Reproductive function
ABSTRACT When benefiting other beneficiaries,cushion plants may reciprocally receive feedback effects.The feedback effects on different sex morphs,however,remains unclear.In this study,taking the gynodioecious Arenaria polytrichiodes as a model species,we aimed to assess the sex-specific facilitation intensity of cushion plant by measuring the beneficiary cover ratio,and to assess the potential costs in cushion reproductive functions by measuring the flower and fruit cover ratios.The total beneficiary cover ratio was similar between females and hermaphrodites.Females produced much less flowers but more fruits than hermaphrodites.These results suggested that females and hermaphrodites possess similar facilitation intensity,and female cushion A.polytrichoides may allocate more resources saved from pollen production to seed production,while hermaphrodites possibly allocate more resources to pollen production hence reducing seed production.The surface areas covered by beneficiaries produced less flowers and fruits than areas without beneficiaries.In addition,strong negative correlations between beneficiary cover and flower cover were detected for both females and hermaphrodites,but the correlation strength were similar for these two sex morphs.However,the correlation between beneficiary cover and fruit cover was only significantly negative for females,suggesting that beneficiary plants negatively affect fruit reproduction of females while have neutral effects on hermaphrodites.All the results suggest that to facilitate other beneficiaries can induce reproductive costs on cushion A.polytrichoides,with females possibly suffering greater cost than hermaphrodites.Such differentiation in reproductive costs between sex morphs,in long-term perspective,may imply sex imbalance in population dynamics.
1.Introduction
Severe environmental conditions in alpine ecosystems,such as low temperature and poor soil nutrients,frequently limit the survival and reproduction of many plant species (K¨orner,2003).However,alpine cushion plants can effectively ameliorate the severe micro-environments,including biotic and abiotic,hence act as nurse plants and offer suitable/safe micro-habitats for other less stress-tolerant species,making them assemblage within and/or surrounding cushion canopies (e.g.,Nu~nez et al.,1999;Cavieres et al.,2002;Callaway,2007;Chen et al.,2015b;Molina-Montenegro et al.,2015;Ramirez et al.,2015;Rodriguez-Echeverria et al.,2016;Zhao and An,2021;Liu et al.,2023).As a result,the presence of huge number of cushion plants in the global alpine ecosystems (Aubert et al.,2014) can guarantee the persistence of plant community structures and biodiversity,including plants (e.g.,Badano et al.,2006;Cavieres and Badano,2009;Kikvidze et al.,2015;Chen et al.,2015a,b;Gavini et al.,2020),soil microorganisms (e.g.,Molina-Montenegro et al.,2015;Rodriguez-Echeverria et al.,2016) and arthropods (e.g.,Haddad et al.,2009;Molenda et al.,2012;Rich et al.,2013;Chen et al.,2021).Accordingly,the persistence and/or dynamics of alpine cushion populations may highly affect the dynamics of alpine communities and biodiversity (e.g.,Chen et al.,2023).
Series ecological factors can affect the facilitation functions of cushion plants.In terms of external factors,the stress-gradient hypothesis generally predicts that the importance of facilitation increases with increasing abiotic stress(e.g.,Bertness and Callaway,1994;Callaway et al.,2002;Maestre et al.,2009).While,in terms of internal factors,plant individual size/age distinctly influence the facilitation intensity,with larger/older individuals generally exerting stronger facilitation on associated beneficiaries(e.g.,Bruno and Kennedy,2000;Tewksbury and Lloyd,2001;Yang et al.,2017;Zhao and An,2021).Moreover,some cushion plants are sexual polymorphism and the sex-specific attributes of nurse plants can also influence their facilitating effects (e.g.,Verdu et al.,2004;Montesinos et al.,2007;Cranston et al.,2012;Lortie and Reid,2012).For instance,the gynodioecious cushion plantSilene acaulisis a typical nurse plant and plays important roles in facilitating other beneficiary plants(e.g.,Antonsson et al.,2009;Molenda et al.,2012;Kjaer et al.,2018),and the hermaphroditic individuals of this plant can support greater beneficiary richness and abundance than female individuals (e.g.,Cranston et al.,2012).
When cushion plants facilitate other beneficiaries,they may simultaneously receive feedback effects from beneficiaries (e.g.,Cranston et al.,2012;Sch¨ob et al.,2014a,b,c;Michalet et al.,2016).Generally,such feedback effects are negative.For example,when facilitating other species,cushion plants may suffer reduced physiological status and decreased reproductive outputs,especially when the abundance or cover of beneficiaries become higher(e.g.,Sch¨ob et al.,2014a,b,c;Michalet et al.,2016).In addition,the facilitation intensity could also be sex-specific,few studies,however,have compared the specific feedback effects of beneficiaries between different sex morphs of cushion individuals (but see Cranston et al.,2012).To fill this gap will definitely help for understanding and predicting the population dynamics in a long-term perspective.
Additionally,most cushion plants are sexual reproductive,and the reproductive functions may be sensitive to climate change(e.g.,Alatalo and Little,2014;Cranston et al.,2015).Cushion plants’ reproductive functions might directly determine their future population dynamics.However,previous studies mainly focused on how cushion plants’ compact structures and their long life-spans have enabled them to persist in hostile environments (e.g.,Benedict,1989;Molau,1997;Morris and Doak,1998;Forbis and Doak,2004;Aubert et al.,2014);while,few studies have tried to detect the cushion population dynamics and the underlying driving mechanisms.Chen et al.(2020,2023) suggested that the populations of cushionArenaria polytrichoidesEdgew.(Caryophyllaceae) may experience series dynamics and the process may be affected by ecological constraints,such as temperature,moisture,light,extreme climate events and competition from surrounding species,at different life-history stages.Cranston et al.(2012) predicted that female cushions might be displaced more easily by beneficiaries than hermaphrodites,possibly resulting in lower female frequency in the longterm population dynamics.However,for those plants with sexual polymorphism,females may achieve higher fitness in seed reproduction (including quantity and quality) than hermaphrodites (e.g.,Sakai et al.,1997;Williams et al.,2000;Chen et al.,2016,2017a,b),implying that population recruitment might be highly relied on the reproductive functions of female individuals.Given this is true,the cost in reproductive functions by facilitating other beneficiaries would rise further influences on the long-term dynamics of cushion populations.However,the potential costs of benefiting other species by different sex morphs still remain highly unclear.
Arenaria polytrichiodesis a typical cushion-forming plant.It harbors a gynodioecious sexual system with female and hermaphroditic individuals co-existing in a same population,and the sexual ratio within population differs between populations (Chen et al.,2017a).This species can effectively ameliorate microenvironments hence facilitate both less stress-tolerant beneficiary plants and insects(e.g.,Yang et al.,2010,2017;Chen et al.,2015a,b,2019,2021).In a previous study (Chen et al.,2021),we found that hermaphroditic individuals ofA.polytrichoidescould support higher insect diversity than female individuals and this could be due to that hermaphroditic individuals provide more resources.Chen et al.(2017a) found that the female individuals can achieve fitness mainly through producing seeds,while hermaphroditic individuals achieve fitness mainly through producing pollen.In addition,some populations of this keystone species are experiencing degeneration (Chen et al.,2020,2023).But little is known about how different sex morphs degenerate.To reveal the sexspecific reproductive costs by facilitating other beneficiaries will,therefore,provide important information on understanding the population dynamics of this keystone cushion plant in the alpine ecosystems.In this study,we aimed to detect:i) the difference in reproductive functions between females and hermaphrodites of cushionA.polytrichoides,by determining flower and fruit cover ratios (i.e.,flowering and fruiting areas/total cushion surface area×100%)and the average numbers of flowers and fruits per unit area (cm-2) within cushion canopies;ii) the facilitation intensity between females and hermaphrodites,by determining the beneficiary cover ratios (i.e.,beneficiary covering area/total cushion surface area×100%)on cushion canopies;iii)the costs in reproductive functions between females and hermaphrodites by facilitating other beneficiaries,by correlating the beneficiary cover ratio and the flower/fruit cover ratio,and by comparing the average number of flowers and fruits per unit beneficiary-covered and beneficiaryfree area.
2.Materials and methods
2.1.Target cushion populations
According to the accessibility,we selected eight alpine plant communities dominated by cushionArenaria polytrichoideson the Himalaya-Hengduan Mountains,SW China (Table 1).Four are distributed along an altitudinal gradient in the Pujin pasture on the Baima Snow Mountains.From relatively low to high,we designated them as PJ1,PJ2,PJ3 and PJ4 communities,respectively.Two are situated on another mountain range of the Baima Snow Mountains,ca.7 km from the Pujin pasture.As these two communities are close to an abandoned coal mine,we designated the low-elevation community as CM1 and the high-elevation community as CM2,respectively.Another community,the Puyong community (PY hereafter) is situated on the Puyong pass on the Daxueshan Snow Mountains,ca.57 km from the Baima Snow Mountains.The last community is situated on the first summit of the Yulong Snow Mountains (YL hereafter) near Lijiang city,ca.146 km from the Baima Snow Mountains.See Chen et al.(2023) for further information of those target cushion-dominant communities.
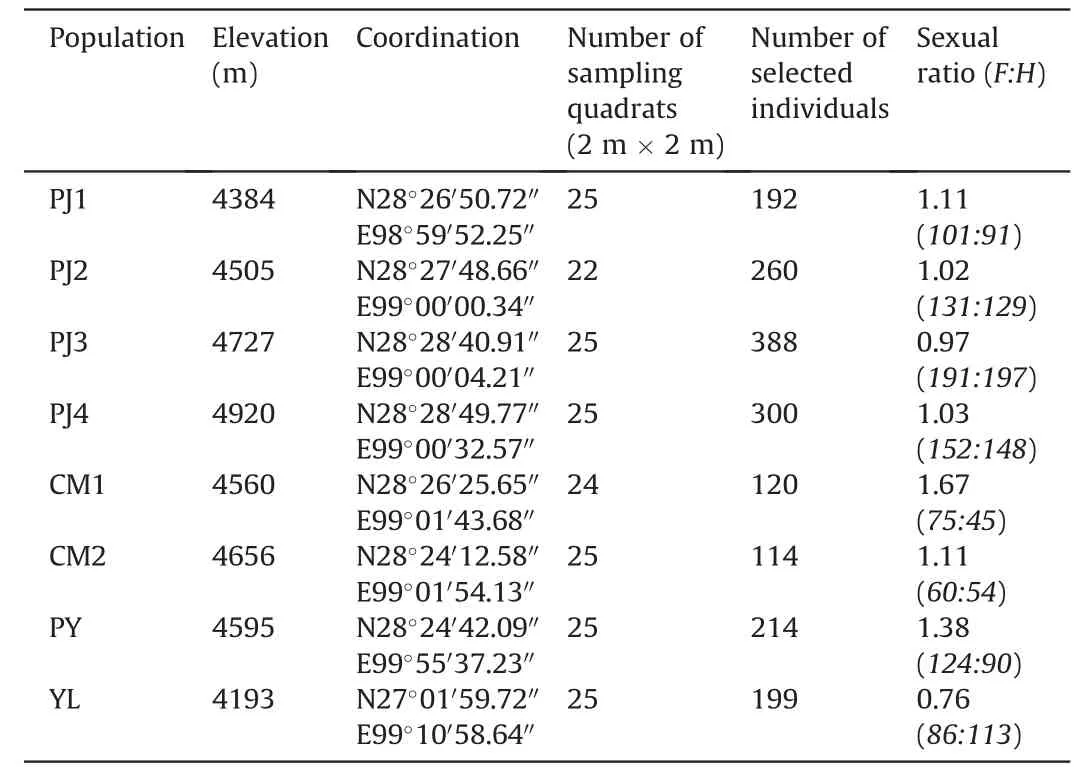
Table 1 Information of study populations of cushion Arenaria polytrichoides.
2.2.Data collection
2.2.1.Flower and fruit reproduction
Field surveys were conducted in 2021 in the sampling quadrats(2 m×2 m)in each community which were previously established(Chen et al.,2023).Specifically,in late June to early July(25th June to 10th July) when the cushionArenaria polytrichoideswas at blooming stage,we checked each flowering cushion individual ineach quadrat,and labeled them as female (F) and hermaphroditic(H),respectively.We ignored those individuals that not flowered,due to the uncertainty of their sex morph.For each labeled individual of each sex morph,we firstly measured its surface length(a)and width (b).Since cushion surface is usually considered as elliptical (e.g.,Cavieres and Badano,2009;Yang et al.,2010;Chen et al.,2015a,b;Pugnaire et al.,2015),these parameters were taken to calculate the cushion individual size(=πab/4 which is the formula for measuring ellipse area) for further analyses.
The cushionArenaria polytrichoides,especially hermaphroditic individual,produces huge number of small flowers within the cushion canopy surface (Fig.1),making it impossible for us to precisely count the total number of flowers of each individual.We thus assessed the flowering ratio (i.e.,flowering area/total individual surface area × 100%) on the surface of each labeled individual and took it as the predictor of the total flower reproduction of a single cushion individual (also see Zhang et al.,2020;Chen et al.,2023).For this,we initially adopted the protocol proposed by Zhang et al.(2020)to assess the flowering ratios for the first 50 randomly chosen individuals in each cushion population,regardless of their sex morphs.Specifically,in each community,we arbitrarily selected the first one individual and did relevant investigation,then by dropping a plastic arrow and selected the nearest one to the arrow vertex as the second individual for investigation.We repeated this process until 50 individuals being investigated.Then,by achieved experience,we only visually assessed the flowering ratios for the remaining individuals due to time constraints.Specifically,we spread a nylon net,which is much more flexible than iron wire net,with 3 m×3 cm mesh as evenly as possible on the surface of each individual(Fig.1).Then,we counted the squares covered the entire surface of the individual and squares covered the flowering areas(Fig.1).We ignored those squares that covered flowering area less than one third of a single square area(9 cm2),because they might enlarge our assessments if taking them as entire flowering squares;otherwise we took them as entire flowering squares,because they might weaken the assessments if being ignored.All opened flowers and unopened flower buds were taken into consideration.Finally,the flowering ratio was calculated as the number of flowering squares to the number of squares covered the total individual cushion surface.We took the same protocol to measure fruit production (i.e.,fruiting ratio) for the same labeled cushion individuals in each population in late growing season in 2021 (20th September to 6th October).

Fig.1.Field protocol for measuring flower/fruit and beneficiary cover ratios on cushion individuals.
2.2.2.Facilitation intensity and reproductive costs
A common approach to assess the facilitation intensity of cushion plants is to compare the composition,abundance and/or richness of associated beneficiaries between within cushion surfaces and in cushion-free areas(e.g.,Cavieres et al.,2002;Cavieres and Badano,2009;Sch¨ob et al.,2013;Chen et al.,2015a,b,2019;Gavini et al.,2020).Such approach could tell us more specific information on how cushion plant facilitate others,such as what and how many species are facilitated.However,considering most previous studies had limited sampling efforts (commonly 30-50 sampling cushion plots and relevant cushion-free plots),such approach could be extremely time-consuming.We had hundreds of selected cushion individuals(plots)in this study(Table 1)which is quite hard to do investigation on species composition,abundance and/or richness for each individual.Considering that vegetation cover could be positively correlated with plant diversity (e.g.,Wiesmair et al.,2017),for the purpose of this study,we took the total beneficiary cover ratio (i.e.,beneficiary covering area/total cushion individual surface area × %) on the cushion surface as the predictor of the facilitation intensity of cushion plants (also see Raath-Krüger et al.,2023).For this,we took the same protocol of assessing flower cover ratio (Fig.1) to assess the total beneficiary cover ratio of each individual.
To assesshow beneficiaryspecies affectthe number of flowers and fruits in a unit cushion surface area,we randomly placed an iron-wire frame (2 cm× 2 cm) on the beneficiary-covered area,and counted and recorded the number of flowers or fruits within the frame.Considering that flower and fruit densities may be varying even at different positions within a single cushion individual surface,we did this for three times at different positions within a single cushion individual.After that,the same iron-wire frame was randomly placed on the beneficiary-free areas for three times,and the number of flowers or fruits were also counted and recorded.Average values of the number of flowers orfruitsper unitsurface areawere achievedfor each cushion individual and used in further analyses.
2.3.Data analysis
We conducted mixed-effect models using the lme()function in the ‘nlme’ package (R Core Team,2022) to test the effects of sex,study site (coupled with elevation) and their interactions on the reproductive functions (flower and fruit reproductions),sampling quadrat ID was coded as a random effect.
We took data from all populations as a synthetical database to test the general differences in cushion’s facilitation intensity and reproductive functions (flowering and fruiting ratios,number of flowers/fruits per unit area)between females and hermaphrodites.Since individual size can influence the facilitation intensity of this cushion plant (e.g.,Yang et al.,2017),we took wilcoxon test to compare whether the individual size between females and hermaphrodites differs.Then,we also took wilcoxon tests to compare the differences in facilitation intensity and reproductive functions between females and hermaphrodites:1)beneficiary cover ratio;2)flower and fruit cover ratios;3) average number of flowers and fruits per unit area (cm-2) in beneficiary-covered areas and beneficiary-free areas.All data were log10transformed to satisfy the assumption of homogeneity of variance.
After checking with Q-Q plotting method,data were generally satisfying Gaussian distribution,we hence conducted Pearson correlations using the rcorr () function in the ‘Hmisc’ package(Harrell,2023) to test the correlations between any two of those involving parameters for females and hermaphrodites,respectively.In addition,we also conducted Principal Component Analysis(PCA)using the pca () function in the ‘FactoMineR’ package (Sebastien et al.,2008) to test how these parameters separate between sex morphs,between study sites and explore the relationships between those involving parameters.Since the PCA results indicated that the first three PCA axes accounted for over 88% of the total variation and the involving parameters could be significantly separated into two groups (study site and sex morphs),namely,parameters about flower reproduction showed high colinearity and parameters about fruit reproduction showed high colinearity.Thus,we extracted the first PCA axis of flower reproduction parameters and fruit reproduction parameters to compared the reproductive functions between females and hermaphrodites for each study population,respectively.
Finally,to compare the strength of correlations between reproductive functions and facilitation intensity between sex morphs and between study populations,we also conducted Pearson correlations for each sex morph in each study population.
All analyses were conducted with R v.4.1.3 (https://www.rproject.org/).The ‘ggplot2’ package (Wickham,2016) was used to plot all reported figures and the layout of figures was designed with Adobe Illustrator 2021.
3.Results
The female frequency varies between populations,with generally decreasing female frequency along with increasing elevation on a same mountain range (PJ populations and CM populations),but it also could be influenced by other undefined factors.For example,the YL population locates at the lowest elevation but shows the lowest female frequency as well (Table 1).
The size of selected cushion individuals does not differ between females and hermaphrodites(wilcoxon test,same for the following,P=0.07;Fig.2a).Also,the beneficiary cover ratio does not differ between females and hermaphrodites (P=0.29;Fig.2b).These results implied that females and hermaphrodites had similar facilitation intensity on other beneficiary plants,and the size effects could be ignored at least in this study.
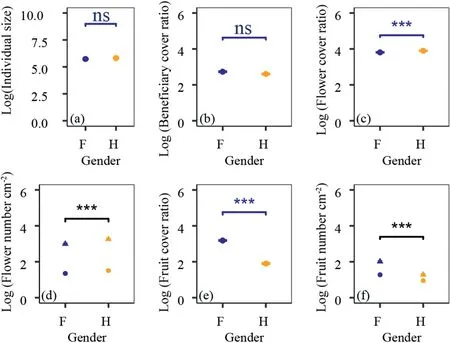
Fig.2.Sex-specific differences in individual size(a),facilitation intensity(b)and reproductive functions(c-f).(a)individual size;(b)facilitation representing by beneficiary cover ratio;(c) flower cover ratio;(d) flower number per unit area (cm-2) in beneficiary-covered surface area (solid circle) and in beneficiary-free surface area (solid triangle);(e) fruit cover ratio;(g)fruit number per unit area(cm-2)in beneficiary-covered surface area(solid circle)and in beneficiary-free surface area(solid triangle).Data were log10 transferred.
Study site,sex and their interactions all had significant effects on the reproductive functions of cushion individuals (Table 2).Generally,hermaphrodites produce much more flowers,either in terms of total flower cover ratio (P<0.001;Fig.2c) or in term of average number of flowers per unit surface area(P<0.001;Fig.2d).However,females produce much more fruits than hermaphrodites(P<0.001;Fig.2e-f),indicating fruit set of hermaphrodites is much lower than that of females.
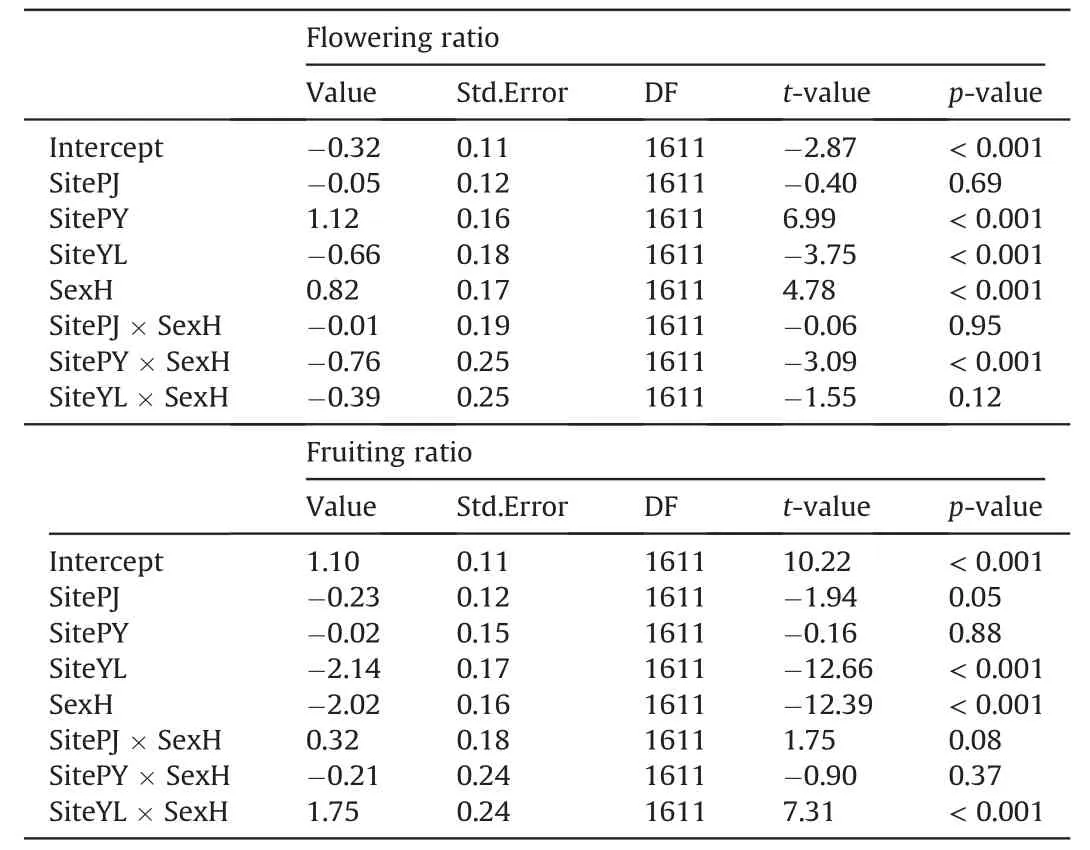
Table 2 Results of the mixed-effects models of the effect of study site,sex and their interactions on the flower and fruit reproductions.
Generally,positive correlations exist between flower and fruit reproductions for both females (r=0.36,P<0.001;Fig.S1a) and hermaphrodites (r=0.10,P<0.001;Fig.S1b),indicating that the initial flower reproduction can directly determine the final fruit reproduction of cushion individuals.The first three PCA axes accounted for more than 88% of the total variation in facilitation and cushion reproductive functions(Fig.3).Specifically,the flower reproduction-related and fruit reproduction-related parameters could be significantly separated into groups between sex morphs(Fig.3a) or between study sites (Fig.3b).Moreover,both parameters about flower and fruit reproductions showed high colinearity.However,the beneficiary cover ratio was independent on either sex or study site(Fig.3).Supporting above results,when the first axis of the PCA of flower and fruit reproduction parameters was separately extracted,we found that hermaphrodites produced much more flowers than females in most populations except for PY population(P<0.001;Fig.4a).However,females inversely produced much more fruits than hermaphrodites in all study populations(P<0.001;Fig.4b).
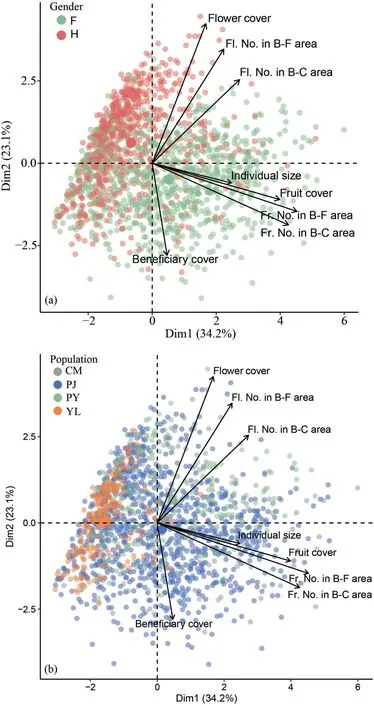
Fig.3.Results of the Principal Component Analysis with sex (a) and study site (b) as the predictor variables.Note: Fl.No.=Flower number;Fr.No.=Fruit number;B-F=Beneficiary-free;B-C=Beneficiary-covered.
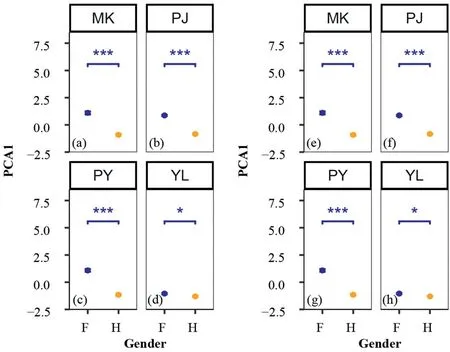
Fig.4.Flowering (a-d) and fruiting (f-h) ratios of different sex morphs in each study populations.* indicates significance at P <0.05,*** at P <0.001.
Average number of flowers and fruits per unit area (cm-2) in beneficiary-covered surface area was significantly lower than that in beneficiary-free surface area (P<0.001;Fig.5d,f),suggesting that covered by beneficiary plants indeed can reduce the reproductive functions of cushion individuals.Furthermore,we detected strong negative correlations between beneficiary cover ratio and flower cover ratio of cushion individuals for both females (r=0.49,P<0.001;Figs.5a and S2)and hermaphrodites(r=0.45,P<0.001;Figs.5b and S2).However,the coefficients were similar between female and hermaphrodite (0.49 for femalesvs.0.45 for hermaphrodites,respectively),implying the strength of negative effects were similar for both females and hermaphrodites.Although a negative correlation between beneficiary cover ratio and fruit cover ratio was detected for females(r=0.14,P<0.001;Figs.5b and S3),the coefficient is quite small(0.14),suggesting that beneficiary species may slightly reduce the fruit reproduction of female cushion individuals.While,the correlation between beneficiary cover ratio and fruit cover ratio was not significant for hermaphrodites (r <0.001,P=0.29;Figs.5b and S3),suggesting that beneficiary plants have no effects on fruit reproduction of hermaphroditic cushion individuals.
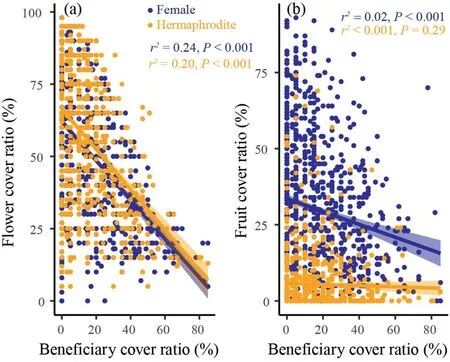
Fig.5.Correlations between beneficiary cover ratio and flower (a) and fruit (b) cover ratios of females (darkblue) and hermaphrodites (orange).
4.Discussion
Up to date,only a few studies have tested the beneficiary feedback effects on cushion reproductive functions (e.g.,Sch¨ob et al.,2014a,b,c;Michalet et al.,2016).Especially,the feedback effects of beneficiaries on different sex morphs of cushion plants still remain unclear (Cranston et al.,2012).The findings in this study,therefore,added important information on the sex-specific reproductive strategies and facilitation of alpine cushion plant.Such evidence can shed light on the future cushion population dynamics.
Consistent with previous studies (e.g.,Cranston et al.,2012;Chen et al.,2017a,b),our results showed that hermaphrodites of cushionArenaria polytrichoidesproduce much more flowers than females(Fig.2c and d).However,females can produce much more fruits than hermaphrodites (Fig.2e and f).Such results supported the general conclusion that females achieve fitness through fruit and/or seed production,while hermaphrodites achieve fitness through pollen production (e.g.,Charlesworth and Morgan,1991;Sakai et al.,1997;Williams et al.,2000;Chen et al.,2017a,b).In addition,such results imply that the population recruitment of cushion populations are mainly relied on female reproductive functions.Interestingly,the female : hermaphrodite ratios are generally closed to one except for a relative higher value in CM1 population and a lower value in YL population (Table 1),meaning that similar number of individuals of different sex morphs have been previously established in most of the study populations.Since females produce much more seeds than hermaphrodites,it is a reasonable task to make clear whether female mothers produce only female offspring or offspring of both sexes.
Cushions’facilitation may be size-dependent.For instance,Yang et al.(2017) found that individual size could affect the facilitation intensity of cushionArenaria polytrichoides,possibly because larger individuals own higher ability of ameliorating micro-environmental conditions.Similar findings were also confirmed in other nurse plants(e.g.,Bruno and Kennedy,2000;Tewksbury and Lloyd,2001;Zhao and An,2021).In this study,the individual size is not differed between females and hermaphrodites(Fig.2a).Thus,it makes sense for us to ignore the size-effects when comparing the difference in facilitation intensity between sex morphs.We found that the total beneficiary cover ratio within females was similar with that within hermaphrodites (Fig.2b),suggesting that the facilitation intensity may be similar between these two sex morphs.Such conclusion is contrary with Cranston et al.(2012) that hermaphrodites are stronger facilitator than females.Cranston et al.(2012) attributed the stronger facilitation of hermaphrodites to its greater allocational cost of producing pollen which could reduce the competitive effect of hermaphrodites on the related beneficiaries.Hermaphroditic cushionA.polytrichoidesproduces much more flowers than females(Chen et al.,2017a;also see results in this study);meanwhile,hermaphroditic flowers have to invest extra resources into pollen production.Considering that resources are limited,it might be true for our target cushion species that more allocation to flower and pollen production might result in less allocation to build competitiveness.However,contrary with Cranston et al.(2012),our results showed that compared with females,the more allocational cost in hermaphrodites do not constrain their facilitation intensity.This could be due to the fact that cushion plants facilitate other beneficiaries by offering suitable micro-environmental conditions (e.g.,Callaway and Pugnaire,1999;Cavieres et al.,2007;Chen et al.,2015b).Females and hermaphrodites of cushionA.polytrichoidesown similar architectural structures(authors'personal observation),possibly resulting in same capacity of modifying the severe alpine environments and hence same facilitation intensity.However,we have to acknowledge that we only considered total beneficiary cover ratio as the predictor of facilitation intensity,without assessing other indicators like species composition,abundance and/or richness.Some beneficiary plants may own different stress-tolerance,making them species-specifically associated with facilitators (e.g.,Badano and Marquet,2008;Chen et al.,2015b).
Our results showed that the reproductive outputs,including flowers and fruits,could be significantly separated into groups between sex morphs (Fig.3a) or between study sites (Fig.3b),confirming that the reproductive functions indeed change with sex morphs.However,such trend might be influenced by specific micro-environmental conditions in local communities.For example,the correlation between fruit cover ratio and beneficiary cover ratio was significantly negative in a few populations(PJ2 and CM2),while it was neutral in most other populations (Fig.S3).In addition,the differences in flower and fruit production between females and hermaphrodites were relatively smaller in the YL population than that in other populations (Fig.4).Based on the current available data,it is difficult for us to clearly specify the underlying mechanisms.However,a series ecological factors,including abiotic and biotic,can act in a concert to affect the resource uptake,reallocation and hence reproductive patterns of plants (e.g.,Weiner,2004;Reekie and Bazzaz,2005;Buckley and Avila-Sakar,2013).For instance,the low soil nutrients can decrease the fruit production of femaleCyananthus delavayi(Campanulaceae) but has no effect on the hermaphroditic individuals (Chen et al.,2017b).Inter-specific interactions can also affect the reproductive functions of plants.For instance,strong competition may lead plants allocate more resources to growth and/or defenses and hence reduce reproductive functions (e.g.,Sch¨ob et al.,2014c).The abiotic factors like soil nutrients,moisture and temperature,and biotic factors like species composition,population density and vegetation cover all change between our study sites(Chen et al.,2023).Such variations in ecological factors might partly explain the variations in reproductive functions between study populations.However,further tests are still necessary for specifying how those factors take effects.
Recently,studies confirmed that in plant-plant facilitation systems,to be the benefactor may simultaneously become to be a sufferer,especially with the increasing of facilitation intensity.For example,Sch¨ob et al.(2014c)found that when the cushionArenaria tetraquetrassp.Amabilisfacilitates other forb plants by improving their water status,it simultaneously suffers poorer water status and reduced flower density and seed set.Similar conclusion was further confirmed at the global scale that cushion reproductive outputs decline with the increasing in diversity and cover of cushionassociated species (e.g.,Sch¨ob et al.,2014a,b;Michalet et al.,2016).Consistent with Sch¨ob et al.(2014a,b,c) and Michalet et al.(2016),our results suggested that beneficiary plants can negatively affect the flower and fruit production of cushionA.polytrichoides,and the negative effects became intenser with the increasing of beneficiary cover(Figs.5,S2 and S3).All those findings imply that to facilitate other beneficiary plants could induce disadvantages in cushion’s reproductive functions.Actually,besides reproductive functions,beneficiary plants can also exert strong competition on cushion plants which can further accelerate the degeneration process of adult cushion individuals (Chen et al.,2023).
However,even so some studies have noted that the sex-specific attributes of benefactors can influence their facilitation effects(e.g.,Verdú et al.,2004;Montesinos et al.,2007;Lortie et al.,2012;Ridenour et al.,2012),very few studies have tested the feedback effects of beneficiary plants on different sex morphs of benefactor plants (but see Cranston et al.,2012).Cranston et al.(2012) found that hermaphroditicSileneindividuals supported a greater number of plant species and individual plants than female individuals,and females cost much more than hermaphrodites.Our results partly supported Cranston et al.(2012).We detected strong negative correlations between beneficiary cover and cushion flower production,but the coefficients were not differed too much between females and hermaphrodites(Fig.5a),indicating that these two sex morphs may suffer similar negative feedback effect of beneficiaries on their flower reproductions.However,we indeed detected negative correlations between fruit reproduction and beneficiary cover for females,but neutral correlations for hermaphrodites(Fig.5b).We indeed observed positive correlations between flower and fruit cover for both females (significant) and hermaphrodites(insignificant)(Fig.S1).Such findings might imply pollen limitation exist,especially for hermaphrodites,regardless of beneficiary cover ratio.However,given it is true,the females should suffer stronger pollen limitation because they do not produce any pollen.Accordingly,we argued that the strength of negative feedback effects were overall stronger for females.
Considering the higher reproductive costs of females in facilitating beneficiaries,Cranston et al.(2012) predicted that females might be displaced more easily by beneficiaries than hermaphrodites,and this could result in lower female frequency in future population dynamics.In our study,except for the YL population,female frequencies are relatively higher than one(Table 1),implying the cushion populations still are currently dominated by females.Furthermore,although females suffer much more than hermaphrodites,they actually can produce higher absolute number of fruits(meaning higher number of seeds) than hermaphrodites (Figs.2e and f and 4e-h).Given the seeds from female and hermaphroditic mothers have same quality in terms of germination,seedling survival and hence recruitment probability in the field,the female frequency may not decrease or even increase in the future.However,we still have no idea about the performance of offspring from different sex morphs.Moreover,the beneficiary plants could accelerate the degeneration process of adult cushion individuals(Chen et al.,2023),how they affect the processes of different sex morphs,however,still remains totally unclear.Accordingly,to further predict the cushion population dynamics,on the one hand,we should take the sexspecific effects into account on facilitation and the relevant costs;on the other hand,it is urgently necessary to reveal the differences in performance of offspring from different sex morphs,and to reveal the degeneration processes of adult individuals of different sex morphs as well as the underlying driving mechanisms.
Availability of data and material
All data and reproducible codes used in this study will be offered on reasonable request.
Author contributions
J.G.C.and H.S.conceptualized the idea,J.G.C.and X.F.C.designed methodology,X.F.C.,J.G.C.,L.S.Q.and R.Y.Z.conducted field investigations and collected data,Y.Z.Z.and J.G.C.performed the statistical analyses.J.G.C.and X.F.C.wrote the draft of manuscript.All authors contributed critically to the improvement of the current manuscript and gave final approval for publication.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by the Second Tibetan Plateau Scientific Expedition and Research Program(2019QZKK0502 to H.S.),the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050203 to H.S.),the Yunnan Applied Basic Research Project(202001AT070060 to J.G.C.),the National Natural Science Foundation of China(31271552 to J.G.C.),the CAS“Light of West China” Program (J.G.C.),and the Young Academic and Technical Leader Raising Foundation of Yunnan Province(202205AC160053 to J.G.C.).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.07.002.
杂志排行
植物多样性的其它文章
- Global patterns and ecological drivers of taxonomic and phylogenetic endemism in angiosperm genera
- Patterns and drivers of plant sexual systems in the dry-hot valley region of southwestern China
- Cryptic divergences and repeated hybridizations within the endangered “living fossil” dove tree (Davidia involucrata) revealed by whole genome resequencing
- Enhanced and asymmetric signatures of hybridization at climatic margins: Evidence from closely related dioecious fig species
- Cryptic diversity and rampant hybridization in annual gentians on the Qinghai-Tibet Plateau revealed by population genomic analysis
- Circumscription of the East Asia clade(Apiaceae subfamily Apioideae)and the taxonomic placements of several problematic genera
