Effect of content and spin state of iron on electronic properties and floatability of iron-bearing sphalerite: A DFT+U study
2024-01-07YaoFengZhuofanLiJianhuaChenYeChen
Yao Feng,Zhuofan Li,Jianhua Chen,Ye Chen,
a State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures,School of Chemistry & Chemical Engineering,Guangxi University,Nanning 530004,China
b MOE Key Laboratory of New Processing Technology for Nonferrous Metals and Materials,Guangxi University,Nanning 530004,China
c School of Resources,Environment and Materials,Guangxi Higher School Key Laboratory of Minerals Engineering,Guangxi University,Nanning 530004,China
Keywords:
ABSTRACT Iron is an impurity widely occurred in sphalerite,and its effect on sphalerite flotation is complex.In this work,the effects of iron content and spin state on electronic properties and floatability of iron-bearing sphalerite are comprehensively studied using density functional theory Hubbard U(DFT+U)calculations combined with coordination chemistry flotation.The band gap of ideal sphalerite is 3.723 eV,and thus electron transition is difficult to occur,resulting in poor floatability.The results suggest the band gap of sphalerite decreases with increasing iron content.For low iron content,the decreased band gap facilitates electron transition;at this case,Fe2+ in a high-spin state possesses one π electron pair,which can form a weak π-backbonding with xanthate,causing increasing floatability.However,for medium and high iron-bearing sphalerite,with the further decrease of band gap,Fe2+ is oxidized to Fe3+ due to electrochemical interaction,and hence π-backbonding is eliminated,leading to lower floatability of ironbearing sphalerite,which is consistent with the flotation experimental results.This work could give a deeper understanding of how sphalerite flotation behaviors are affected by iron content.
1.Introduction
Zinc,a non-ferrous metal widely used in batteries,metallurgy,medicine,chemical industry and other fields,plays a crucial role in modern industry.According to statistics,refined zinc production and zinc consumption reached 14.13 million tons and 14.09 million tons in 2021,respectively [1].Sphalerite (ZnS) is the primary source of zinc metal [2].In different deposits,sphalerite is often found associated with Cu,Fe,Ag,Sn,Sb,Sc and other elements[3,4].Generally,Fe,Cd,Mn and Cu atoms enter the sphalerite lattice by substituting Zn atoms [5,6].Among them,Fe2+,without changing the crystal structure,is the most common replacement in sphalerite [4].Iron content varies in sphalerite crystals [7];for instance,sphalerite with high iron content is called marmatite[8],of which proven deposits have an iron content of up to 26.2%[9].Previous studies have demonstrated that iron considerably influences the flotation behavior of sphalerite and copper activation [10,11].It is believed that iron has an adverse impact on copper activation and xanthate adsorption [12].Boulton et al.[13]studied the mechanism of the effect of iron content on sphalerite flotation and noted that iron impurity in the sphalerite lattice lowered the activation efficiency of copper,which is detrimental to the flotation of sphalerite.Pomianowski et al.[14]also concluded that the presence of iron would affect sphalerite flotation because the iron atoms occupy the copper activation sites;therefore,the higher the iron content,the weaker the copper activation effect.However,some scholars have different opinions.Yu et al.[15] studied ironbearing sphalerite with low iron content and the flotation results showed that the recovery increased with the increase of iron content.Harmer et al.[10]concluded that iron atoms on the surface of iron-bearing sphalerite were more active than zinc atoms,which facilitated the adsorption of xanthate on the mineral surface.Moreover,Gigowski et al.[16]investigated natural sphalerite with different iron contents and reported that xanthate would preferentially adsorb on the surface of sphalerite with high iron content.In summary,the discussion regarding the effect of iron content on sphalerite flotation remains controversial in past studies.Therefore,it is vital to study the effect of iron content on sphalerite.
Sphalerite has been proved a semi magnetic semiconductor or diluted magnetic semiconductor (DMS) [17,18].Unlike other impurities,iron itself has spin that will influence magnetism to a certain extent [19].In particular,the iron atoms of a certain content exhibit paramagnetism (PM),diamagnetism (DM),ferromagnetism (FM),ferrimagnetism (FIM),and antiferromagnetism(AFM) due to their differing spin states.It was reported as early as 1990 that the iron atoms exhibited magnetism to varying degrees after substituting zinc atoms in sphalerite [20].So far,there have been results that suggest the iron atoms of a high content exhibit ferromagnetism [21],as well as diamagnetism after substitution on specific sites [22].Studies have also demonstrated that the magnetism of iron-bearing sphalerite results from the degenerate ground state of iron,which is conducive to orbital spin and Jahn-Teller effect (a distortion of electron cloud of symmetric non-linear molecules)[19,23].In other words,spin and magnetism have effects on the d electron distribution of iron atoms in sphalerite.The coordination chemistry of mineral flotation put forward by Chen[24]for the interaction of reagent molecules with mineral surfaces shows that sulfydryl collectors are prone to form πbackbonding with low-spin metal ions,which are relatively stable.Consequently,the spin state and magnetism of iron in sphalerite can affect the electron distribution on the d orbital,and thus affecting the formation of π-backbonding with xanthate,which may contribute to the differences in flotation behavior.Therefore,it is necessary to investigate the spin state and magnetism of iron in iron-bearing sphalerite.
The studies on the effects of iron content on sphalerite flotation have seen industrial tests[13,15]and simulation research employed to a certain basis [8,12,25-27].Density functional theory (DFT),a convenient and accurate simulation approach,has been widely used in studies on sphalerite at the atomic level.However,previous studies on sphalerite largely put their focus on surface or interface interactions instead of bulk,which is the basis of surface simulation and of great significance for accurate calculations.To date,there has been little research on iron-bearing sphalerite bulk.The effects of iron content on crystal structure and electronic properties of sphalerite(iron-bearing sphalerite) have only been investigated by Deng et al.[7] using DFT.It is noteworthy that the band gap is a physical parameter of significance for a semiconductor,that is sphalerite.In previous studies,the sphalerite simulation set the band gap at 2.13 eV,far less than the experimental value of 3.723 eV [28].A smaller band gap contributes to electron transition,leading to overestimated electron activity and reactivity.DFT+U,as an effective and efficient approach,has been used for correcting underestimated band gap with a negligible increase in computational cost [29].In this paper,a DFT+U study was carried out in an attempt to approach the problem of lowered band gap with Hubbard U tested.
The spin property of iron in sphalerite has not been clarified and the problem of the underestimated band gap of sphalerite bulk remains to be resolved,which causes inaccuracy to research on sphalerite surface reactivity and iron-bearing sphalerite.Hence,it is necessary to optimize the calculations regarding band gap and the spin property of bulk iron in simulation research on sphalerite.The purpose of this paper is to accurately compute the band gap of sphalerite and investigate the spin property of iron in sphalerite bulk using DFT+U based on previous studies.Furthermore,the effects of iron content and spin state (magnetism) in iron-bearing sphalerite on the band structure and electromagnetic properties of sphalerite will be systematically investigated.From the perspective of coordination chemistry and electrochemistry,the interaction of iron-bearing sphalerite with xanthate is discussed.
2.Computational methods and models
2.1.Computational methods
Sphalerite,sulfide mineral,of which the main chemical composition is ZnS and space group belongs to isometric system.Fig.1 displays the lattice constant of ideal sphalerite.There are four Zn atoms and four S atoms in every unit cell.Experimental tests have shown that the(Zn,Fe)atom and S atom are located at(0,0,0)site and (0.25,0.25,0.25) site respectively.
In this paper,density functional theory calculations were performed with program [30].The exchange-correlation function applied was the generalized gradient approach (GGA) of Perdew Burke Ernzerhof (PBE) [31,32].The interactions between valence electrons and ionic core represented by ultrasoft pseudopotentials were used in this study [33,34].Valence electrons configuration considered in the study included Fe 3d64s2,Zn 3d104s2,and S 3s23p4states.The plane-wave cutoff energy of 380 eV was set,and the Brillouin zone was sampled with Monkhorst and Pack special of a 4×4×4 grid for the surface calculations [35].For the selfconsistent electronic minimization,the Pulay Density Mixing method was employed with a convergence tolerance of 5.0×10-7eV/atom.The convergence criteria for structure optimization and energy calculation were set as follows:5.0×10-6eV/atom for maximum energy tolerance,0.01 eV/Å for maximum force tolerance,0.02 GPa for maximum stress tolerance and 5.0×10-4Å for maximum displacement tolerance.
In previous studies,the calculated value was far less than the experimental value of band gap of ideal sphalerite.Therefore,the Hubbard U correction was necessary.Different Hubbard U values are defined to the 2p orbitals of the sulfur atoms in sphalerite and the results are presented in Table 1.Analysis of Table 1 data suggests that Hubbard U correction has little effect on unit cell parameters but the Hubbard U value is an important influencing factor for band gap,which increases with the Hubbard U value.With Hubbard U value of 5.7 eV,the calculated value of band gap is 3.719 eV,close to the experimental value of 3.723 eV.The Hubbard U value is thus set as 5.7 eV.

Table 1 Unit cell parameters and band gap after Hubbard U correction.
2.2.Computational models
In Fig.2,a 2×2×1 sphalerite supercell model was established and zinc was substituted with iron to investigate the effects of iron content,position and spin on the crystal structure and electronic properties of sphalerite.Different quantities of Fe atoms substitute for Zn atoms to indicate varying iron content,with yellow balls representing S atoms,blue Zn atoms and red Fe atoms.One Fe atom was substituted for one Zn atom to obtain the supercell of ZnFeLS with iron content of 3.6%,which is a low-iron sphalerite model,as shown in Fig.2a.Two Fe atoms were substituted for two Zn atoms to obtain the supercell of ZnFeMS with iron content of 7.3%,which is medium-iron sphalerite model of two relative positions of iron(medium-iron sphalerite refers to sphalerite with iron content of over 6%).Fig.2b shows the model of para-position of iron,the distance between iron atoms being 5.429 Å.Fig.2c shows the model of ortho-position of iron,the distance between iron atoms being 3.844 Å.In Fig.2d,three Zn atoms substituted by Fe atoms are denoted as ZnFeHS,and the iron content of 11%can be approximated as high-iron sphalerite (high-iron sphalerite refers to sphalerite with iron content of over 12%).In the 2×2×1 sphalerite supercell model,only four saturated zinc atoms coordinate with four sulfur atoms simultaneously,while the others are unsaturated.Based on this,regardless of position of the three substituted iron atoms,their relative positions remain the same,and therefore only one case exists for the positions of the three iron atoms.

Fig.2.The supercell of iron-bearing sphalerite.
The spin states of iron atoms in low-iron sphalerite were taken into account,which were high-spin and low-spin states.The spin direction was also considered when there were more than two iron atoms in sphalerite model.In ZnFeMS,when two iron atoms had spin in the same/opposite direction,sphalerite exhibited ferromagnetism/antiferromagnetism.In ZnFeHS,when three iron atoms had spins in the same direction,sphalerite exhibited ferromagnetism;when two iron atoms had spins in the same direction and the other in the opposite direction,sphalerite exhibited ferrimagnetism.
3.Results and discussion
3.1.Effect of iron content on the crystal structure of sphalerite
The lattice constant,properties and magnetic moments of ironbearing sphalerite with different iron content and spin states are presented in Table 2.It is shown that the unit cell volume increases with iron content.Low-iron sphalerite (ZnFeLS) has larger lattice constants ina,bandcdirections.It is also found that the substitution of either high-spin or low-spin of iron atoms has similar effects on the lattice constants and energy of sphalerite.In addition,the magnetic moment of iron atoms in low-iron sphalerite is 3.63 μBregardless of the set spin state.The unchanged lattice constants and energy show that only one spin state of iron exists in iron-bearing sphalerite,while relatively high magnetic moment further demonstrates that only high-spin states of iron are possible.
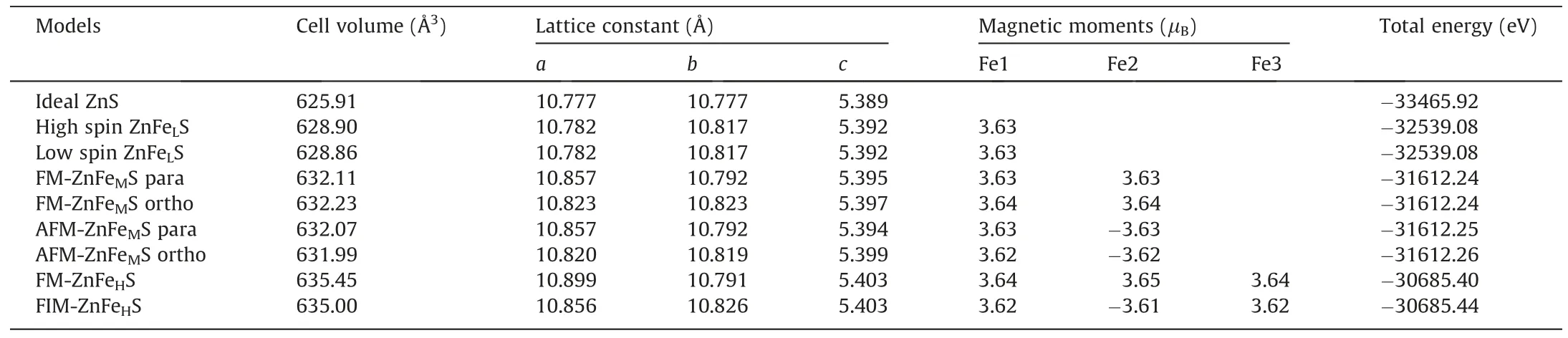
Table 2 Total energy,lattice structure,and magnetic moments of iron-bearing sphalerite with iron in different spin states and spin directions.
Four structure models of medium-iron sphalerite (ZnFeMS)were constructed considering differing relative positions and spin directions of iron atoms.The lattice experiences variation particularly in theaandbdirections.The two substituted iron atoms in the para-position have lattice constants growing by 0.08 and 0.015 Å in theaandbdirections respectively,whereas those in the ortho-position have the same increase in lattice constants.FM-ZnFeMS has high magnetic properties because it has two iron atoms with the same spin direction.In contrast,AFMZnFeMS is less magnetic because the iron atoms have opposite spin directions and the net magnetic moment in the system is zero,which will not generate a magnetic field.The analysis of system energy demonstrates that AFM-ZnFeMS has lower total energy than FM-ZnFeMS,regardless of the relative positions between iron atoms.This suggests that,in nature,the spin directions between iron atoms might be opposite.It has been shown that in nature FeS (sulfur-iron compound) mostly occur in the form of pyrrhotite,and the most two common crystal structures of pyrrhotite are monoclinic and hexagonal,with monoclinic pyrrhotite being FM and hexagonal pyrrhotite being AFM.The crystal structure of the iron substituted sphalerite is closer to the hexagonal structures of pyrrhotite.Therefore,iron-bearing sphalerite displays the same magnetic properties as the hexagonal structures of pyrrhotite,i.e.iron-bearing sphalerite presents AFM at this situation [11,38,39].This coincides with the conclusions of this study.
In high-iron sphalerite(ZnFeHS),FIM has a smaller volume than that of FM,which is possibly due to the stronger interaction in the FIM system.Meanwhile,the FIM system with lower total energy than the FM system has a more stable structure,also demonstrating the opposite spin direction between iron atoms.FM-ZnFeHS has three iron atoms in a high-spin state with the same direction while FIM-ZnFeHS has one iron atom,of which spin is in a different direction from the other two iron atoms,and thus its magnetic moment can approximately offset one magnetic moment of iron with the opposite spin direction.This is why the former mineral has stronger magnetism than the latter.Therefore,with the same iron content,FM-sphalerite has the strongest magnetism,followed by FIMsphalerite,and AFM-sphalerite has the weakest.
Iron-bearing sphalerite exists magnetic coupling between iron atoms.For example,the magnetic moments of iron atoms in FMZnFeMS in ortho-position are 3.64 μBwhile those in AFM-ZnFeMS in ortho-position are 3.62 μB(Fe1)and-3.62 μB(Fe2).It is obvious that altering the spin direction of one iron atom leads to changes in the magnetic moments of both iron atoms in the optimized sphalerite lattice constant,indicating the occurrence of magnetic coupling.For FM-ZnFeHS,the magnetic moments of its three iron atoms are 3.64 μBof Fe1,3.65 μBof Fe2,and 3.64 μBof Fe3.For FIM-ZnFeHS that differs from the former in the spin direction of Fe2,the magnetic moments are 3.62 μBof Fe1,-3.61 μBof Fe2,and 3.62 μBof Fe3.Change in the spin direction of Fe2 results in a difference between magnetic moments of iron atoms in FIMZnFeHS and FM-ZnFeHS.Therefore,altering the spin direction of one iron atom leads to changes in magnetic moments of all three iron atoms,that is,magnetic coupling.
Table 3 shows the bond lengths and the Mulliken population of iron-bearing sphalerite with different spin states and contents of iron.After iron substitution,the Mulliken populations of Zn(n)-S bonds near iron atoms and Zn(f)-S bonds away from iron atoms in ZnFeLS are 0.52 and 0.53,respectively,which are larger than that in ideal sphalerite of 0.43,indicating that Zn-S bonds have more stable covalent bonding in the presence of iron.This is not favorable for the flotation of sphalerite.On the other side,the Mulliken population for Fe-S bonds is smaller compared to Zn-S bonds,indi-cating that Fe-S bonds are less covalent,more prone to bond breakage and appear more active,thus hindering zinc separation.For ZnFeMS,the iron atoms exhibiting either FM or AFM have little impact on bond population.In ZnFeMS,the Zn(n)-S bond near the iron atoms and the Zn(f)-S bond far from the iron atoms have larger Mulliken population of 0.50 and 0.52 respectively than the Zn-S bond in ideal sphalerite of 0.43.This indicates that the farther the Zn-S bond is from iron atoms,the more covalent it is,and that iron substitution causes the Zn-S bond to become more covalent and sphalerite to exist more stably.The Fe-S bond has smaller population of 0.48 than the Zn-S bonds in ZnFeMS,suggesting the former is less covalent than the latter.For ZnFeHS,the Fe-S bond has a larger bond length and smaller population than Zn-S bond,indicating the former is less covalent and prone to bond breaking.The Zn(n)-S bond has smaller Mulliken population of around 0.48 than Zn(f)-S bond of 0.51,which means the Zn-S bond near iron atoms is less covalent and subject to bond breaking.
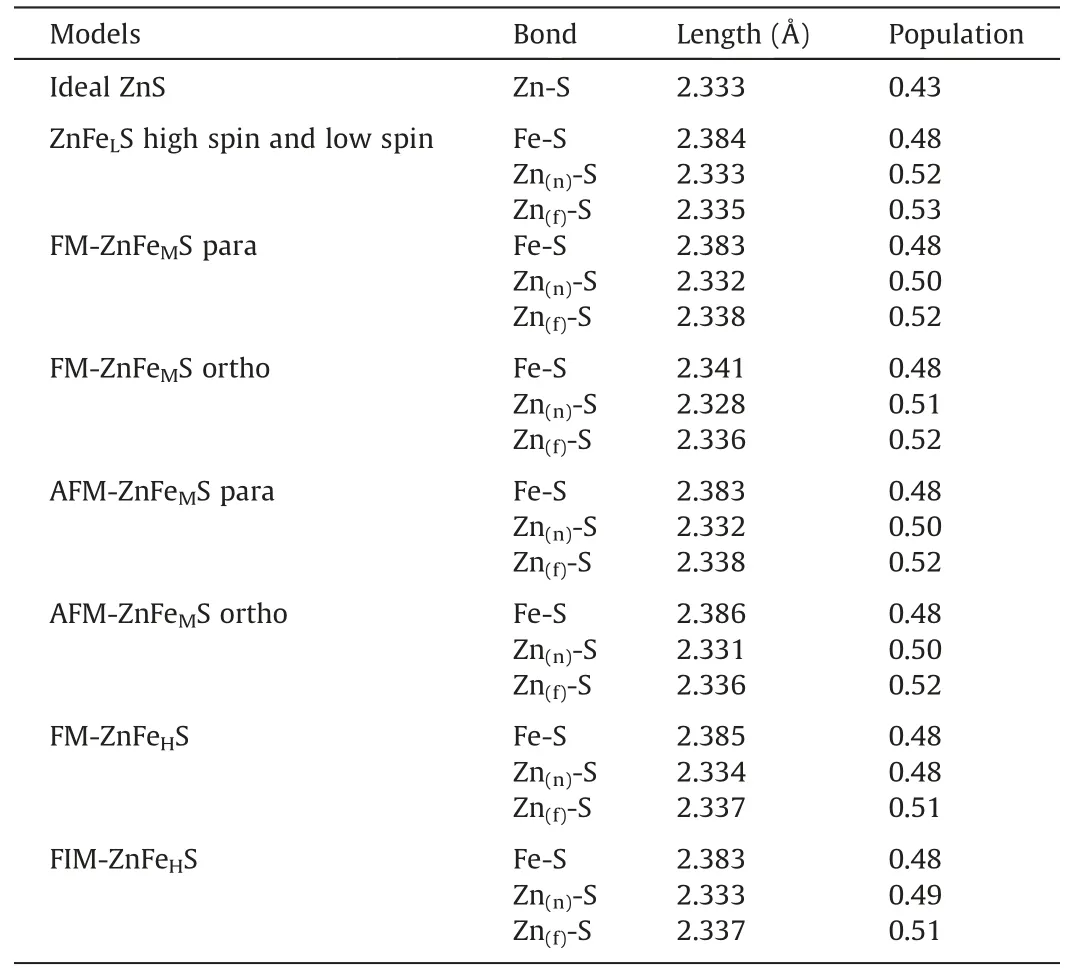
Table 3 Bond lengths and Mulliken population in sphalerite.
To sum up,the Fe-S bond and Zn-S bonds have larger population than the Zn-S bond in ideal sphalerite does,regardless of iron content,suggesting iron impurity causes sphalerite to turn more covalent and more stable,and increases difficulties in sphalerite flotation separation.The Fe-S bond has smaller population and larger bond length than Zn-S bonds,indicating that the former is less covalent,less stable and prone to bond breaking,and that iron impurity,more active and less stable than zinc,impedes zinc separation.
3.2.Band structure of iron-bearing sphalerite
Fig.3 shows the band structures of sphalerite with differing iron content.The band structure of ideal sphalerite is shown in Fig.3a.The optimized sphalerite band gap of ideal sphalerite is 3.719 eV,implying a low electronic activity since ideal sphalerite is an insulator.The ideal sphalerite has valence band maximum (VBM) and conduction band minimum (CBM) both located at the Gamma point,a high symmetry point in the Brillouin zone.

Fig.3.Energy band structures of ideal sphalerite and iron-bearing sphalerite.
Fig.3b and c represents the band structure of sphalerite doped with one iron atom set as high-spin and one iron atom set as lowspin respectively,of which band gaps are the same of 2.014 eV.After iron substitution,the crystal splits at the Fermi level with a new energy level generated and VBM almost overlapping with the Fermi level,indicating the hole density increases,electron density decreases and iron-bearing sphalerite is a p-type semiconductor.The iron-bearing sphalerite band gap becomes narrowed,conducive to electron transition from valence band to conduction band and decreasing resistivity and increasing conductivity of sphalerite.Furthermore,the Alpha energy bands (black) and Beta ones (red) do not completely overlap,revealing the presence of spin polarization;on this basis,the same energy band graphs of high-spin sphalerite and low-spin sphalerite are observed,indicating the iron in sphalerite can only occur in a high-spin state.
Fig.3-g shows the ZnFeMS band structure of FM in paraposition,of FM in ortho-position,of AFM in para-position,and of AFM in ortho-position,respectively.The band gaps of the four cases are 1.978,1.943,2.026 and 1.986 eV respectively.The four cases all see a new energy level of impurity occur after iron substitution with band structure narrower than those in the case of sphalerite substituted with one iron atom,suggesting that electron transition from valence band to conduction band is more likely to happen,increasing conductivity of sphalerite.It is found that ortho-position sphalerite has a lower band gap than paraposition sphalerite,indicating the former is more active and more likely to allow electron transition to happen than the latter.The FM-ZnFeMS has a lower band gap than AFM-ZnFeMS,suggesting the former is more active than the latter.Therefore,the iron atoms of FM in ortho-position are the most active in ZnFeMS.It is also demonstrated that the magnetism is greater when the iron atoms are ordered in a FM manner,as the α and β energy bands could not completely overlap,showing spin polarization is present.
Fig.3h and i represents the band structures of FM-ZnFeHS and FIM-ZnFeHS with band gaps of 1.941 and 1.924 eV respectively.The figures show that the Alpha and Beta energy bands of FMZnFeHS overlap to a smaller extent than those of FIM-ZnFeHS do,especially in the circled area,indicating the spin polarization and magnetism of FM sphalerite are stronger than those of FIM one.Notably,the band gap of FIM sphalerite is smaller and its electrons,in closer proximity to the Fermi level,appear more active.
In summary,it is observed that with increasing iron content,the sphalerite band gap tends to decrease,which is in favor of electron transition.
The smaller the band gap,the more likely the occurrence of electron transition.During the flotation of sulfide minerals,there are electrons transferred at the sulfide mineral-liquid interface,resulting in an electrochemical interaction between sulfide minerals and xanthate collectors.The transfer of electrons in this process is closely related to the band gap of minerals.The electrochemical interaction between sphalerite and xanthate can be expressed as follows: the collector (xanthate) loses electrons to generate a hydrophobic product at the anode,while oxygen accepts electrons and is reduced on the mineral surface at the cathode.
Reduction of oxygen at the cathode:
Oxidation of xanthate at the anode:
As aforementioned,ideal sphalerite,with a band gap of 3.723 eV,is an insulator,which has an extremely small carrier concentration,barely allows electron transfer and is not conductive.This means ideal sphalerite is difficult to produce an electric couple with xanthate,which is the reason why the xanthate is difficult to effectively recover sphalerite.As the iron content increases,the band gap decreases,making the conductivity of iron-bearing sphalerite increases,therefore resulting in the iron-bearing sphalerite transforming into a semiconductor that can better interact with xanthate.The iron-bearing sphalerite,therefore,exhibits better floatability.Although the presence of iron serves as an active site for the oxidation of iron-bearing sphalerite with xanthate and reduces the band gap of iron-bearing sphalerite.But the interaction between iron and xanthate is weak,which is related to the spin and magnetism of iron in sphalerite.
3.3.Electromagnetic property of iron-bearing sphalerite
Fig.4 shows the density of states(DOS)of sphalerite.The DOS of sphalerite substituted with the iron atoms set as high-spin and low-spin is represented in Fig.4a.The DOS of sphalerite substituted with the iron atoms set as high-spin shows the spin-up DOS and spin-down DOS are not symmetrical with total spin values not being zero to the right of the Fermi level,indicating the occurrence of spin polarization.The same works for the DOS of sphalerite substituted with the iron atoms set as low-spin.Therefore,the iron atoms in iron-bearing sphalerite,regardless of iron content,should be in a high-spin state,rather than in a low-spin state,which is why the lattice constant parameters of sphalerite substituted with either high-spin or low-spin iron atoms remain the same.Fig.4b presents the DOS of sphalerite substituted with the two iron atoms of FM in para-position,of FM in orthoposition,of AFM in para-position,and of AFM in ortho-position.It is observed that the DOS diagrams of sphalerite substituted with the iron atoms in differing relative positions and spin directions do not differ much,possibly due to little change in relative distance between iron atoms.The interaction between iron atoms in different relative positions are further clarified from the perspective of electron density below.Fig.4c shows the DOS of ZnFeHS exhibiting FM and FIM.It is seen that the spin state of iron has little effect on its DOS,and the peak of FIM Fe 3d orbital slightly moves to deeper energy levels,indicating FIM-ZnFeHS is more stable,in agreement with the results obtained by total energy.Fig.4d represents the DOS of ideal sphalerite and iron-bearing sphalerite with differing iron content.It is shown that with increasing iron content,the peak of Zn 3d orbital moves to deeper energy levels,suggesting Zn exists more stably in the system.To the right of the Fermi level,the height and width of the peak of iron atoms grow,conducive to electron transition and improving conductivity of sphalerite.
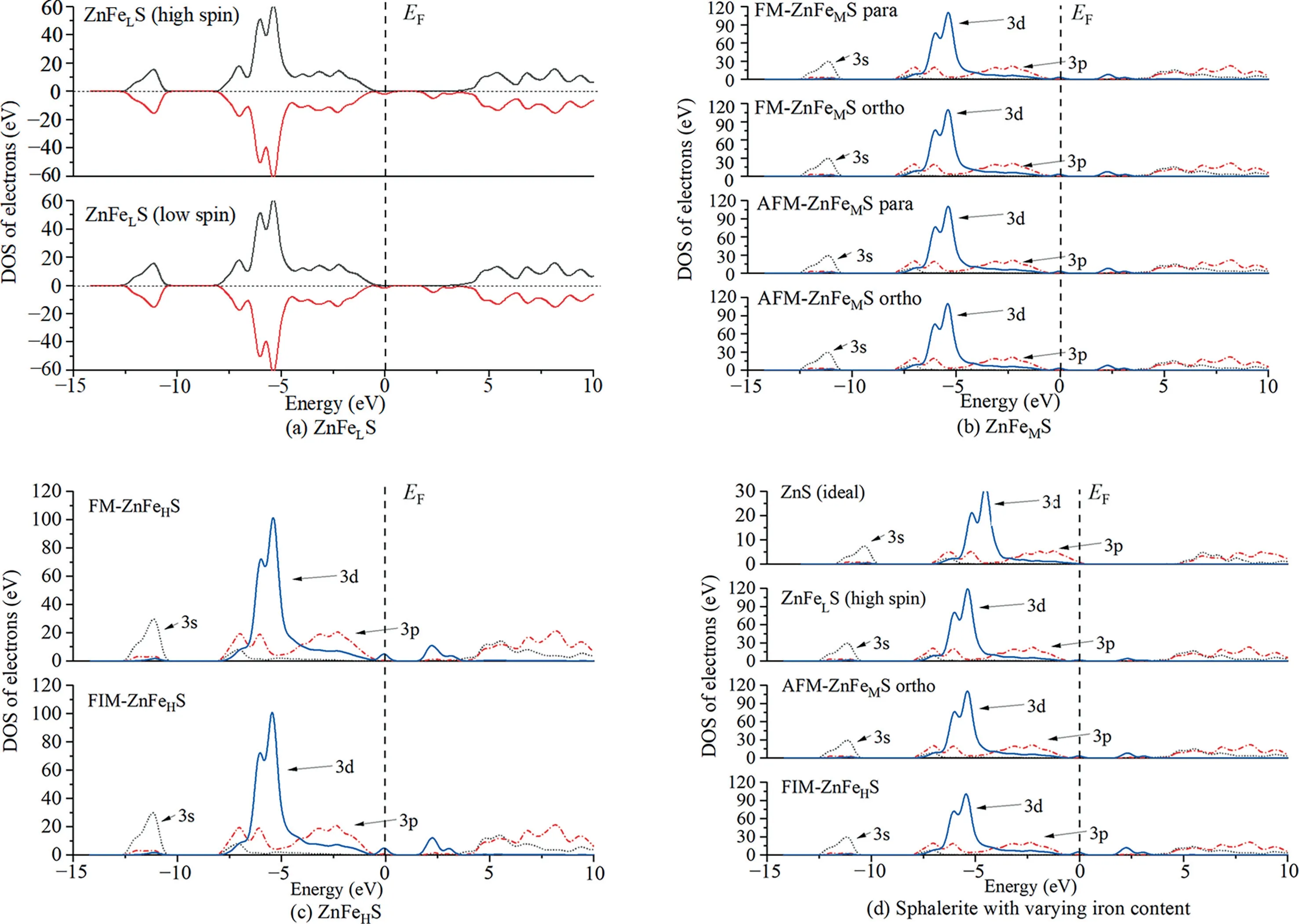
Fig.4.DOS diagrams of sphalerite.Note: EF represents Fermi level.
The electron densities of sphalerite are represented in Fig.5.In order to investigate the effect of distance between iron atoms on sphalerite,we compared the electron densities of iron atoms with the same spin at differing relative distances,as shown in Fig.5a and b.In the figure,the red color gradually turns into blue color,suggesting the electron density ranges from 0 to 0.2289 C/m2.It is seen that the iron atoms in ortho-position and para-position have overlapping electron densities of around 0.0168 and 0 C/m2,respectively,indicating there are interactions between iron atoms in ortho-position while there are not between iron atoms in paraposition.
Fig.5a and c represent the electron densities of AFM-ZnFeMS in ortho-position and FM-ZnFeMS in ortho-position.It is observed that both the AFM and FM iron atoms in ortho-position have a similar color in the bonding region,which means electron clouds have a similar density in this region.While the latter cloud((0.1681±0.0035765)C/m2)color is slightly lighter than the former((0.16095±0.0035766) C/m2).This suggests that altering the spin direction of one iron atom in ZnFeMS results in changes in electrons of iron atoms,which means galvanic couples are formed.
Fig.5d and e display the electron densities of FM-ZnFeHS and FIM-ZnFeHS.The circled areas show FIM-ZnFeHS((0.1681±0.0035766) C/m2) has darker cloud color than FMZnFeHS ((0.16095±0.0035766) C/m2),indicating the iron atoms in the former have more overlapping cloud areas and stronger interactions than in the latter,consistent with the conclusion in Section 3.1.The galvanic couples are also present among iron atoms of ZnFeHS because altering the spin direction of one iron atom in ZnFeHS results in changes in electron densities of all three iron atoms.
The spin state of iron affects the distribution of its electrons in 3d orbitals and the number of π electron pairs.The coordination chemistry of mineral flotation proposed by Chen et al.[21,40]suggests that there are two main interactions between the flotation reagent and the mineral surface,the one that provides electron pairs from the reagent molecules,and the other that a metal ion on the surface provides π electron pair to the unoccupied π orbital of the flotation reagent,forming π-backbonding.And the number of π electron pairs will directly affect the number of π-backbonding.Meanwhile,the most commonly used flotation reagent is xanthate for iron-bearing sphalerite.Therefore,the spin state significantly affects the interaction of iron-bearing sphalerite with xanthate.
In sphalerite crystal,of which the ligand field is a tetrahedral field,Zn2+has a coordination number of 4.The iron atom,after substituting for the zinc atom,bonds with four sulfur atoms to form a tetrahedral field,in which electrons are generally in a high-spin state,in agreement with what have been proved in previous sections.
The 3d electron distribution of Fe2+is shown in Fig.6.It is seen that Fe2+has 6 electrons that are in a high-spin state and tend to occupy empty orbitals rather than become pairs in 3d orbitals,and thus there is only one π electron pair.In iron-bearing sphalerite,Fe2+merely possesses one π electron pair,which forms a weak π-backbonding with xanthate,and therefore,xanthate is ineffective in collecting iron-bearing sphalerite.This is why the interaction of iron-bearing sphalerite with xanthate is still weak,despite the fact,as aforementioned,that iron can reduce the sphalerite band gap and provide an active site for interaction.

Fig.6.The π-backbonding formed between ferrous and iron ions with sulfydryl collector.
With increasing iron content,iron-bearing sphalerite,of which band gap decreases,is more prone to oxidation.The Fe2+is thus oxidized to Fe3+,with d electron distribution turning into,as shown in Fig.6.It is obvious that Fe3+has no π electron pair in 3d orbital to form π-backbonding with xanthate,and thus unfavorable for the interaction of iron-bearing sphalerite with xanthate.
3.4.Flotation behaviors of iron-bearing sphalerite
The theoretical inference of how sphalerite flotation behaviors are affected by iron content could be confirmed by the following flotation experiments and the experimental results are shown in Fig.7.
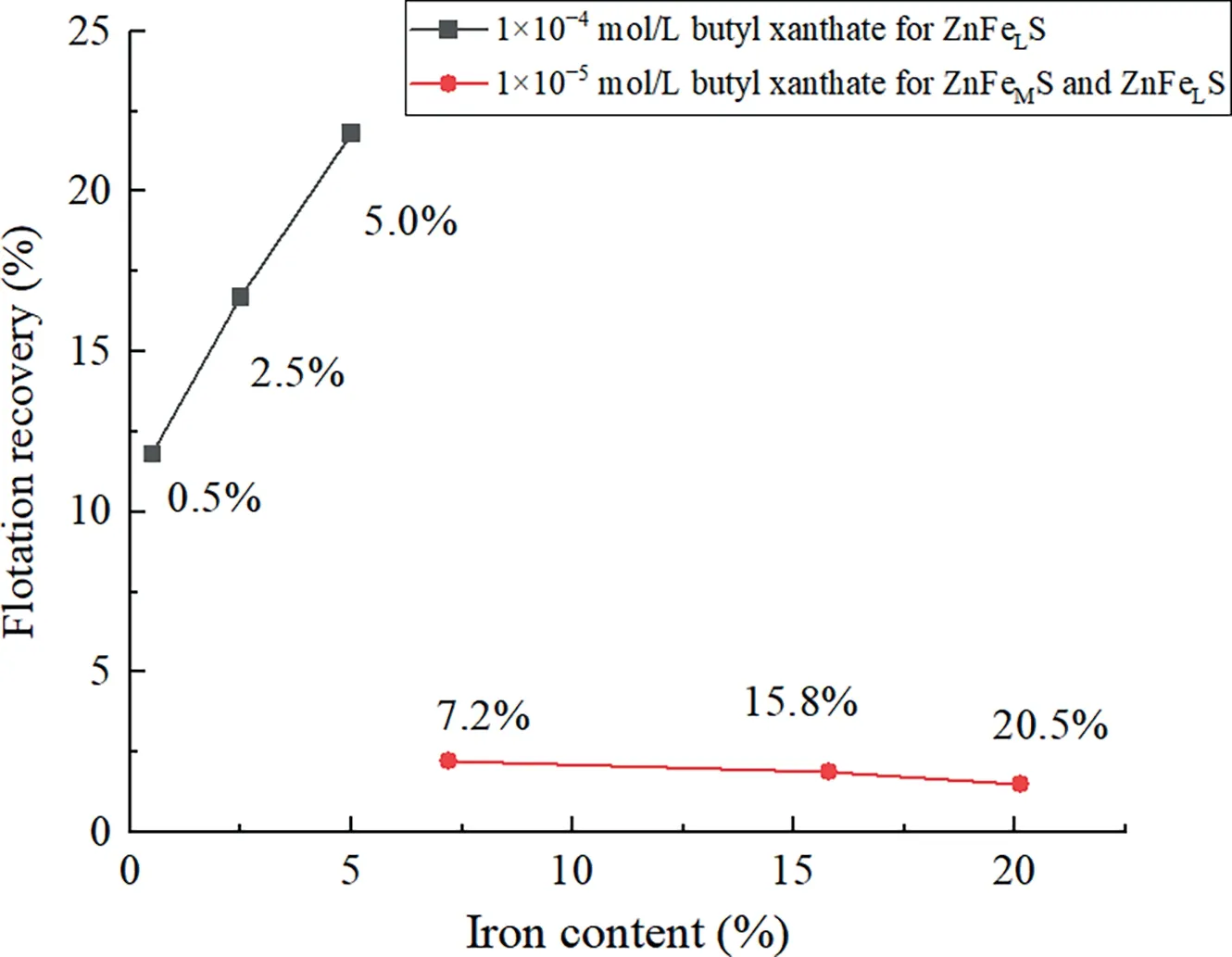
Fig.7.Flotation recovery of iron-bearing sphalerite without copper activation[41,42].
As shown in Fig.7,it is composed of the results of two experiments,where Zeng[41]and Xie[42]employed sphalerite samples with different iron contents and origins.By using a relatively high dosage of 1×10-4mol/L butyl xanthate as collector,Zeng[41]conducted flotation experiments adopting synthesized ZnFeLS with iron contents of 0.5%,2.5%,and 5.0%,and the corresponding recoveries of ZnFeLS were 11.8%,16.7%,and 21.8%.In the study of Xie[42],by using only 1×10-5mol/L of butyl xanthate,the recoveries of ZnFeMS and ZnFeHS with 7.2%,15.8% and 20.5% iron content were 2.2%,1.9% and 1.5%,respectively.The results indicated that the floatability of iron-bearing sphalerite increased with increasing iron content for ZnFeLS,whereas the opposite trend is observed for ZnFeMS and ZnFeHS.
4.Conclusions
In this paper,the effects of the content and spin state of iron on the electronic properties and floatability of iron-bearing sphalerite were investigated by using DFT+U simulations,electrochemistry,and coordination chemistry of mineral flotation.
It was shown that in the absence of iron,ideal sphalerite,with the band gap of about 3.719 eV,was an insulator with very weak electron activity,and electrons could not be transferred between the mineral surface and xanthate.Therefore,the electrochemical reaction could not occur between xanthate and ideal sphalerite,exhibiting poor floatability of ideal shpalerite.
The band gap of iron-bearing sphalerite decreased with increasing Fe content,implying that electrons could be transferred between the iron-bearing sphalerite surface and xanthate.Hence,compared with ideal sphalerite owning greater band gap,the floatability of low iron-bearing sphalerite is better.Meanwhile,the iron in low iron-bearing sphalerite was present in a high-spin state and thus possessed one π electron pair,showing weak interaction between xanthate and low iron-bearing sphalerite.Consequently,the low iron-bearing sphalerite exhibited weak floatability.
When the iron content increased further,Fe2+oxidized to Fe3+that had no π electron pairs and was not capable of forming πbackbonding with xanthate,leading to weaker floatability of medium and high iron-bearing sphalerite.
The flotation experiment results of iron-bearing sphalerite also confirm the conclusions above.For low iron content,the recovery of iron-bearing sphalerite increases with increasing iron content.However,for medium and high iron content,the recovery of iron-bearing sphalerite decreases with increasing iron content.
Acknowledgements
This work was supported by the National Natural Science Foundation of People’s Republic of China (No.NSFC52174246),and the Interdisciplinary Scientific Research Foundation of Guangxi University (No.2022JCC016).
杂志排行
矿业科学技术学报的其它文章
- Extraction and imaging of indicator elements for non-destructive,in-situ,fast identification of adverse geology in tunnels
- Study on damage-stress loss coupling model of rock and prestressed anchor cable in dry-wet environment
- Development of an improved three-dimensional rough discrete fracture network model: Method and application
- Mechanical response and microscopic damage mechanism of pre-flawed sandstone subjected to monotonic and multilevel cyclic loading:A laboratory-scale investigation
- Mechanism of gas pressure action during the initial failure of coal containing gas and its application for an outburst inoculation
- Morphological evolution and flow conduction characteristics of fracture channels in fractured sandstone under cyclic loading and unloading
