A comprehensive review on the chemical constituents,sesquiterpenoid biosynthesis and biological activities of Sarcandra glabra
2023-12-29JinNingChuPremanandKrishnanandKuanHonLim
Jin-Ning Chu,Premanand Krishnan and Kuan-Hon Lim*
Abstract Sarcandra glabra (Thunb.) Nakai is a perennial evergreen herb categorised within the Sarcandra Gardner genus under the Chloranthaceae family.Indigenous to tropical and subtropical regions of East Asia and India,this species is extensively distributed across China,particularly in the southern regions (Sichuan,Yunnan,and Jiangxi).In addition to its high ornamental value,S. glabra has a rich history of use in traditional Chinese medicine,evident through its empirical prescriptions for various ailments like pneumonia,dysentery,fractures,bruises,numbness,amenorrhea,rheumatism,and other diseases.Besides,modern pharmacological studies have revealed various biological activities,such as antitumour,anti-bacterial,anti-viral anti-inflammatory and immunomodulatory effects.The diverse chemical constituents of S. glabra have fascinated natural product researchers since the 1900s.To date,over 400 compounds including terpenoids,coumarins,lignans,flavonoids,sterols,anthraquinones,organic acids,and organic esters have been isolated and characterised,some featuring unprecedented structures.This review comprehensively examines the current understanding of S. glabra’s phytochemistry and pharmacology,with emphasis on the chemistry and biosynthesis of its unique chemotaxonomic marker,the lindenane-type sesquiterpenoids.
Keywords Sarcandra glabra,CaoShanHu,Traditional Chinese medicine,Lindenane-type sesquiterpenoids,Biosynthetic pathway,Biological activities
1 Introduction
Sarcandra(SarcandraGardner) is a genus under the family of Chloranthaceae.The genus name,Sarcandra,is integrated from ‘Sarkos’ and ‘andrus’,which means fleshy anthers in Greek,while the epithet ‘glabra’ translates to‘hairless’ from Latin [1].Sarcandra glabra(Thunb.) Nakai(syn.Chloranthus glaber(Thunb.) Makino) orSarcandra glabrain short,represents an extensively researched species of the genusSarcandra.The plant is an evergreen shrub that grows up to 2 m tall and has glossy green leaves with a distinctive aroma.The plant is occasionally planted for ornamental purposes,otherwise used to prepare medicinal tea.
Also referred to as 草珊瑚 (CaoShanHu) in Chinese,S.glabrais valued in Traditional Chinese Medicine for its immunomodulatory [2],anti-inflammatory [3],and anti-tumour properties [4],and used to treat a variety of health conditions,including arthritis,bronchitis,and cancer [5].The distribution of the subshrub ranges from temperate East Asia to Southeast China,specifically in provinces such as Anhui,Fujian,Guangdong,Guangxi,Guizhou,Hainan,Zhejiang,and Sichuan [1].Ecologically,S.glabrathrives in geographic locations that are approximately 2000 m above sea level and can be found plentiful in forests,thickets,valleys,ravines,trail sides,grasslands,and swamps [1].
Within theS.glabraspecies,two subspecies are offi-cially accepted,namelySarcandra glabrasubsp.glabraandSarcandra glabrasubsp.brachystachys[6].S.glabrasubsp.glabra,known as 原亚种 (YuanYaZhong) in Chinese,is a subspecies indigenous to continental East Asia,including North and Central China,Korea,Japan and the Ryukyu Islands.Sarcandra chloranthoidesGardner is treated as a synonym of this taxon and its distribution is centred in India and Sri Lanka [1].
The second subspecies,S.glabrasubsp.brachystachys(Blume) has a widespread distribution in Northeast India,Northern Vietnam,Southern China and throughout the Malesian region.Sarcandra hainanensis(Pei) Swamy & Bailey (海南草珊) andChloranthus brachystachysBlume are two synonyms of this taxon and are sometimes used interchangeably [1].This subspecies differs from subsp.glabraby the length of its anther being almost as equal as the whole male structure,while in subsp.glabrathe anther is much shorter and the non-antheriferous part is well-developed [7,8].Anatomically,the ventral vein in subsp.hainanensisis single,as opposed to the paired strands in subsp.glabra[9].Sarcandra glabravar.melanocarpa(Ridl.) Verdc.orChloranthus brachystachysvar.melanocarpusRidl.is a lesser-known variation ofS.glabrasubsp.brachystachys.It is an endemic plant found in the montane rainforests of North Sumatra and Malesia and is characterised by its unique black fruits [6].
Since the mid-twentieth century,the diverse chemical constituents ofS.glabrahave piqued the scientific curiosity of various researchers.The observed diversity in the chemical constituents ofS.glabramay be attributed to its proliferative nature and location-specific environmental factors influencing the plant’s growth and metabolism.To date,studies have reported nearly 400 compounds from this species,including terpenoids,coumarins,lignans,flavonoids,sterols,anthraquinones,organic acids,and organic esters,many of which have been found to possess interesting structures and/or significant pharmacological activities.These findings underscore the potential ofS.glabraas a vast resource for drug discovery and its development.
While several published review papers have covered the phytochemistry ofS.glabra[5,10,11],there remains a gap in individual and comprehensive reviews that specifically address its chemical constituents and the associated biogenetic pathways.As more recent work surfaced,this review aims to provide a categorical progress update(up to August 2023) on the isolation and structural elucidation of chemical constituents ofS.glabra,along with the proposed biosynthetic pathways of specific dimeric and oligomeric sesquiterpenoids,and the biogenetic relationship among these terpenoid skeletons.Furthermore,this review covers an overview of the pharmacological and clinical exploration of crude extracts,medicinal preparations,and the bioactive compounds ofS.glabra.
2 Chemical constituents
2.1 Isolation of terpenoids from S. glabra
As the most widely occurring and extensively studied family in natural products,terpenoids are generally distinguished by the number of isoprene (C5) units constituting their carbon skeleton [12].The term terpenoids refers to modified terpenes that have been chemically altered through oxidation or rearrangement,and is occasionally used interchangeably with terpenes [12].More than 200 terpenoids have been reported fromS.glabra,including triterpenoids (C30),diterpenoids (C20),sesquiterpenoids (C15),monoterpenoids (C10),as well as meroterpenoids.
2.1.1 Sesquiterpenoids
Among the isolates fromS.glabra,sesquiterpenoids constitute the largest proportion with more than 180 members.The structures of sesquiterpenoids fromS.glabraare rather diverse and accompanied by complex stereochemistry.The structures of these sesquiterpenoids can be divided into eight main skeletal types,namely,eudesmane,lindenane,germacrane,eremophilane,aromadendrane,elemane,guaiane,and cadinene.Herein,the structures of all sesquiterpenoids isolated up to August 2023 are presented;however,only those that were first isolated fromS.glabraare highlighted.
2.1.1.1 Eudesmane and eudesmane dimersKnown as selinanes in the early literature,the basic skeleton of eudesmane features an isopropyl-bicyclodecane with four asymmetric centres at C-4,C-5,C-7,and C-10 [13].The formation of a lactone moiety involving C-7,C-8,C-11,and C-12 gives rise to eudesmane-type sesquiterpene lactones known as eudesmanolides,which account for the majority of the eudesmane-type sesquiterpenoids obtained fromS.glabra.Currently,a total of 30 eudesmane compounds (1-30) have been reported (Table 1,Fig.1),the majority of which were monomers isolated from the whole plants and leaves ofS.glabra(1-27).
Sarcaglabosides A and B (2and3) were the first examples of hepatoprotective compounds reported from the whole plants ofS.glabra[14].Zhu et al.[15] reported compound5as the 9α-hydroxy eudesmanolide derivative of4,while Hu et al.[16] reported compound6as a new eudesmanolide glycoside from the whole plant ofS.glabra.Sancandralactone B (9) was reported as a new compound fromS.glabraby He et al.[17] but it was originally characterised as serralactone A fromChloranthus serratus[18].Glabranol B (12) is a new eudesmanetype sesquiterpenoid separated from the aerial parts of a VietnameseS.glabraspecimen [19].Wang et al.[20]reported the trihydroxyeudesmanolide derivative13,while Hu et al.[21] reported the 4,15-glycol derivative14from the whole plant ofS.glabra.Designated as the aglycone of sarcaglaboside B (3),sarcandralactone E (15) was obtained from the whole plant ofS.glabraof Guangxi origin [22].The presence of three eudesmane compounds(16-18) was detected in the seeds ofS.glabra[23],with16obtained as a racemic mixture and17characterised as the C-8 epimer of14.TheS.glabra(whole plant) collected from Jiangxi gave four eudesmane-type sesquiterpenoids (19-22) [24],among which compounds19and20represent eudesmanolides incorporating a rare 1,4-epoxy bridge.The same plant also yielded compounds28and29,which were the first heterodimer representatives isolated from aSarcandraplant that incorporate eudesmane and eremophilane sesquiterpenoid halves,along with compound30,which is a symmetric homodimer featuring a different dimerisation pattern from the former dimeric compounds [24].
A chemical investigation of the leaves ofS.glabrafrom Guangxi province led to the discovery of compounds23-27,with compounds23and24being unusual γ-lactamcontaining eudesmane-type sesquiterpenoids [25].Unfortunately,while compounds28-30,19and20were given the trivial names sarglanoids A-E,respectively,compounds23-27were coincidentally given the same names as both sets of compounds were published around the same time.
2.1.1.2 Lindenane and lindenane oligomersDespite the limited distribution of lindenane-type sesquiterpenoids in natural sources,their presence is exceptionally prominent inS.glabra.The occurrences of lindenane-type sesquiterpenoids as oligomers,including homodimers,heterodimers,and trimers inS.glabrahave gained much research interest due to their intriguing structures.Among them,sarcanolides,sarcandralactones,sarglabolides,sarglalactones,sarcaglabrins and sarcaglabosides could serve as the characteristic components and important chemotaxonomic markers ofS.glabradue to their species-wide exclusivity [5].

Fig. 1 Eudesmane-type sesquiterpenoids and eudesmane-type dimers (1-30)
In monomeric form,lindenane sesquiterpenoids possess a common skeleton comprising a unique linear 3/5/6 tricyclic ring.The system is embedded with a chiral carbon (C-10),an atypicaltrans-5/6 junction,and a sterically congested cyclopentane with an angular C-14 methyl group [28].The structural variation of lindenane oligomers is attributed to the combination of monomeric units that make up the backbone,which could be assembled by different linkage patterns.Generally,most dimeric lindenanes fromS.glabrawere proposed to be constructed fromendo-Diels-Alder reactions [29].
At present,30 lindenane-type sesquiterpenoids(31-60) fromS.glabrawere reported,among which lindenane-type sesquiterpene lactones (lindenanolides)account for a significant proportion (Table 2,Fig.2).The first lindenane-type sesquiterpenes (31-32) were isolated by Uchida et al.[30] fromChloranthus glaber(synonym ofS.glabra).Subsequently,the presence of several known lindenane compounds (33-39) was reported from the same plant [14,15,26,31,32].The whole plants ofS.glabrafrom Xiushui,Jiangxi yielded two new lindenane glycosides,sarcaglabosides F and G (41and42),together with a known compound (40) [16].In search for cytotoxic sesquiterpenoids from a HainaneseS.glabraplant,He et al.[17] reported sarcandralactone A (43),which is a C-5 hydroxylated analogue of shizukanolide A (34).The butanolic extract ofS.glabra(whole plant) provided a new trihydoxylindenanolide,glabranol A (44)[19].Compounds47and48were reported as new compounds fromS.glabraby Ni et al.[22],and were named sarcandralactones C and D,respectively.A phytochemical investigation of the anti-inflammatory constituentsfromS.glabraled to the discovery of sarglabolide L (52),representing a rare lindenane derivative possessing an 18-membered macrocyclic triester,and shizukanolide 8-O-β-D glucopyranoside (53) [23].From the dichloromethane and petroleum ether fractions ofS.glabra,Chi et al.[33] reported the isolation of sarglalactones I-M(54-58),a group of unique 8,9-secolindenane derivatives featuring an opened lactone ring.
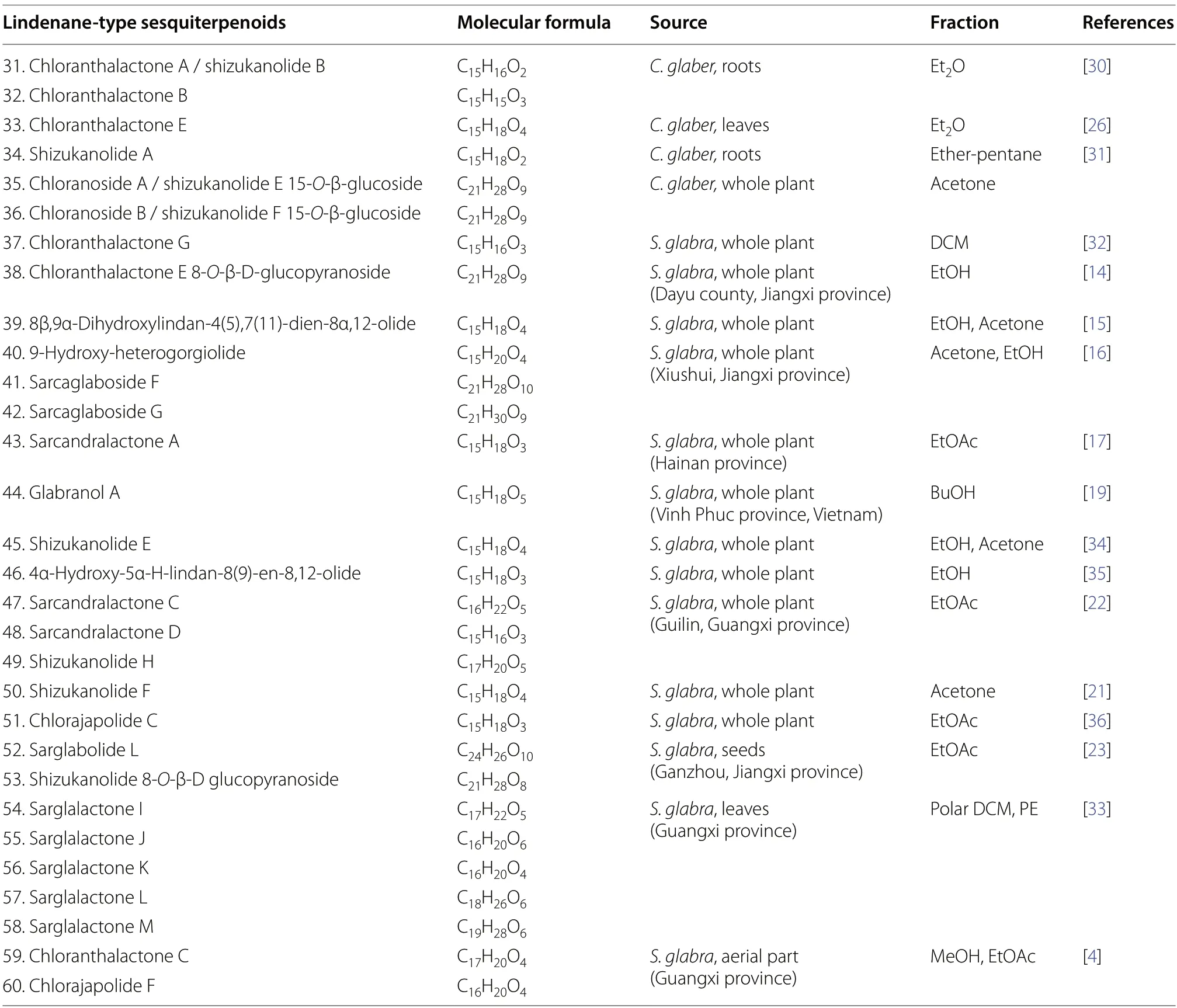
Table 2 Lindenane-type sesquiterpenoids from S. glabra
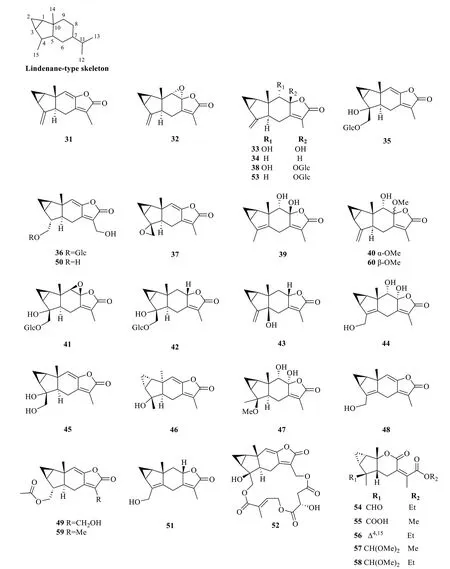
Fig. 2 Lindenane-type sesquiterpenoids (31-60)
Lindenane-type sesquiterpenoid oligomers and their derivatives are recognised as the characteristic taxonomic symbol ofS.glabra.Despite having complex structures and functionalities,86 lindenane-type sesquiterpenoid oligomers (61-146) were successfully characterised fromS.glabra(Table 3,Fig.3).
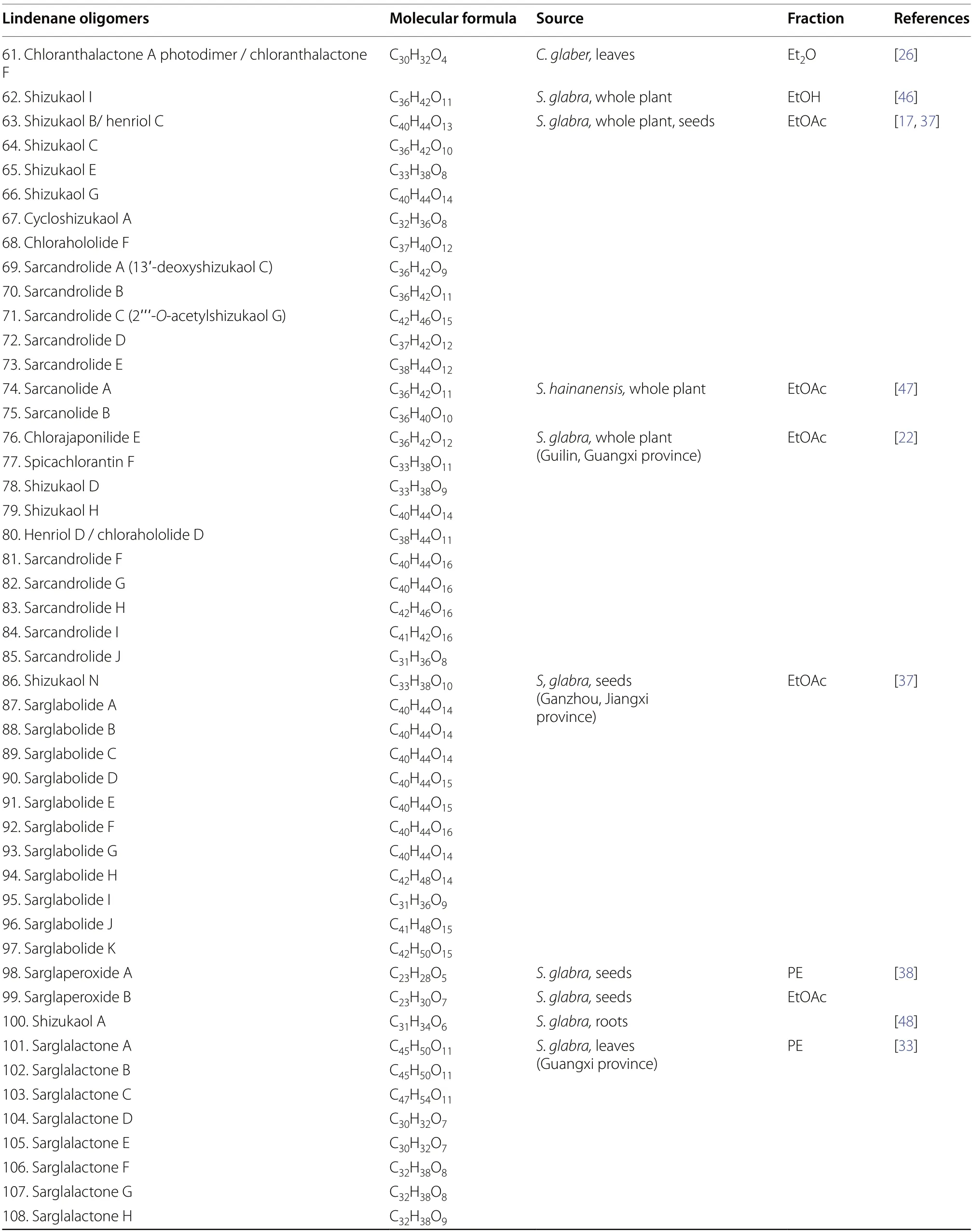
Table 3 Lindenane oligomers from S. glabra
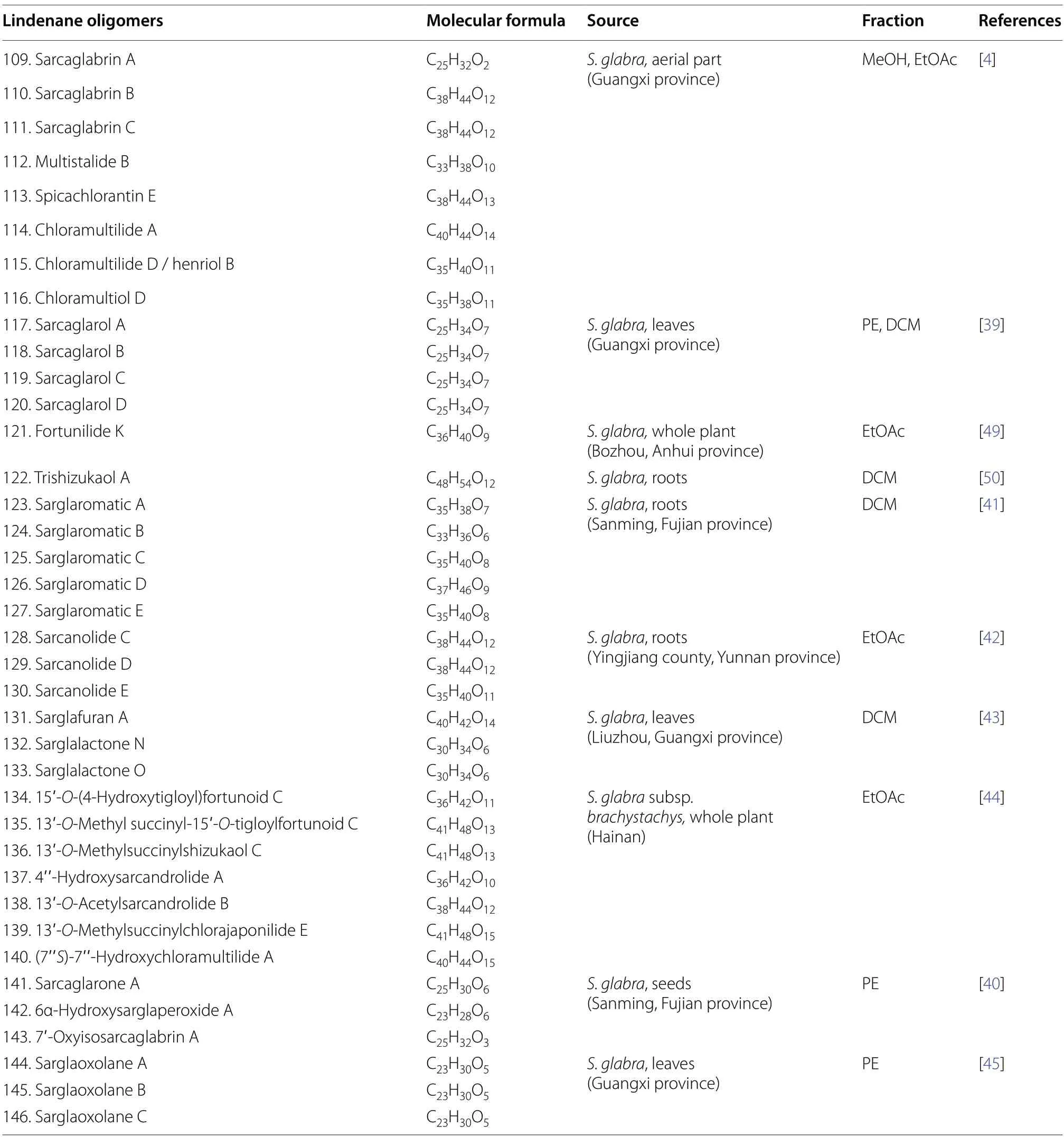
Table 3 (continued)
Although most of the dimeric lindenane sesquiterpenoids fromS.glabrawere presumed to be biosynthesised via Diels-Alder,several compounds displayed variations in the linkage between two constitutional units.Chloranthalactone F (61),later renamed as chloranthalactone A photodimer,is a representative dimeric sesquiterpenoid formed by a [2+2] cycloaddition [26].Being the only [6+6] lindenane cycloadduct isolated fromS.glabra,cycloshizukaol A (67) has an interesting C-2-symmetrical structure that incorporates a cyclododecatetraene ring [17].He et al.[17] isolated a group of structurally related lindenane dimers known as sarcandrolides A-E (69-73) from the whole plant ofS.glabra.The same group also isolated two new dimers,sarcanolides A and B (74and75),featuring an unprecedented nonacyclic scaffold fromS.hainanensis(subspecies ofS.glabra).
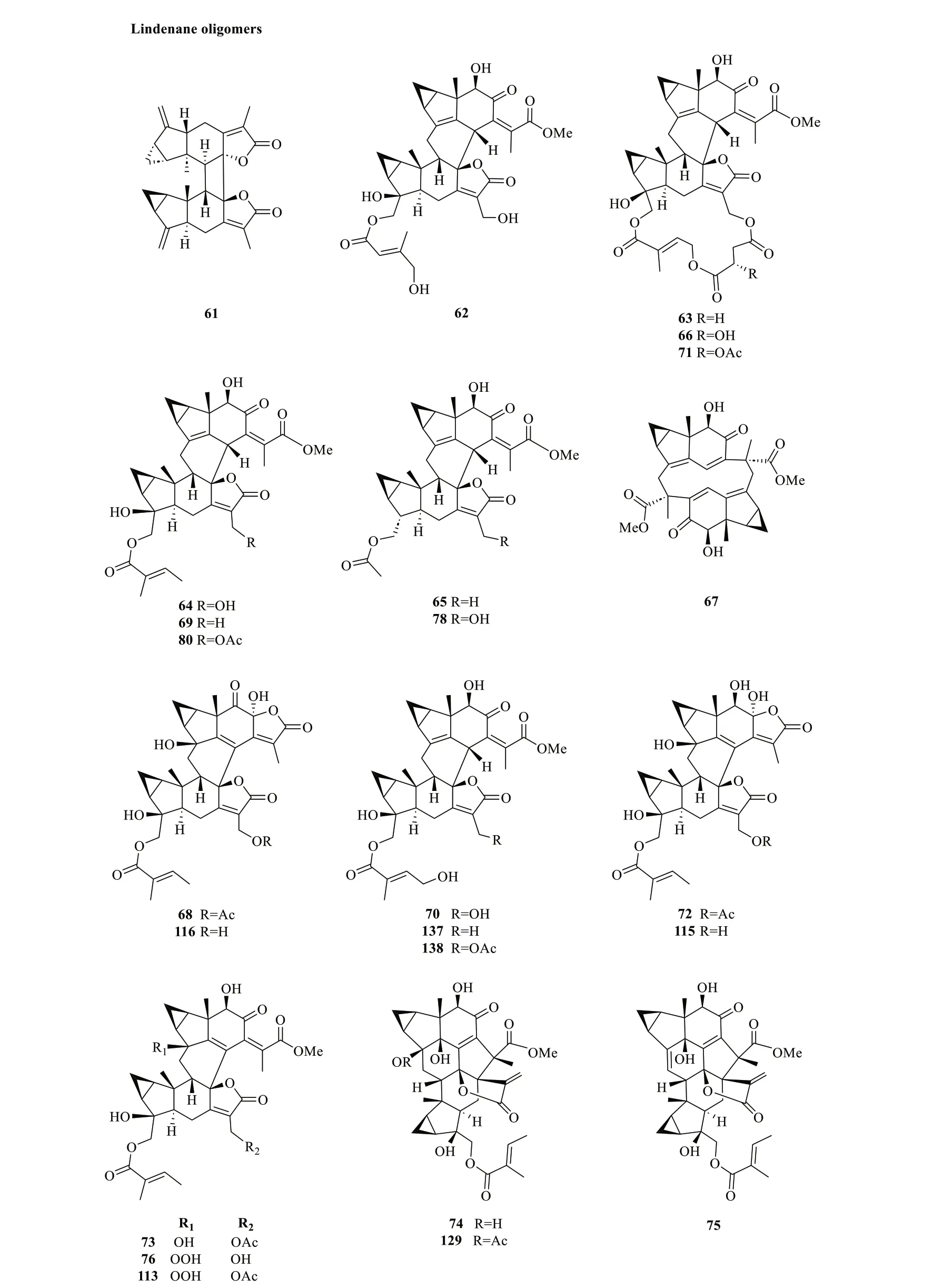
Fig. 3 Lindenane oligomers (61-146)

Fig.3 continued
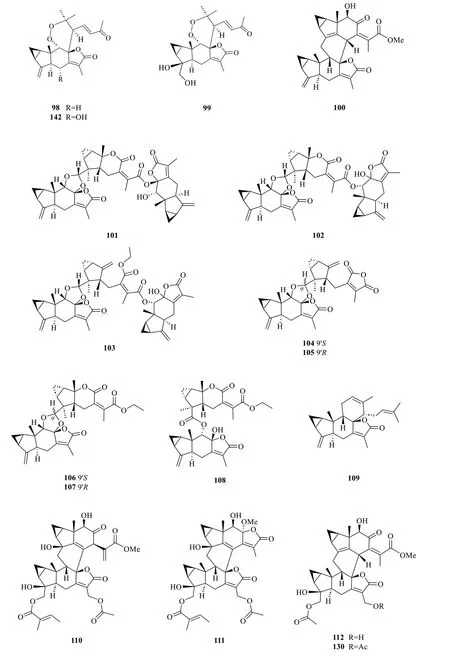
Fig.3 continued
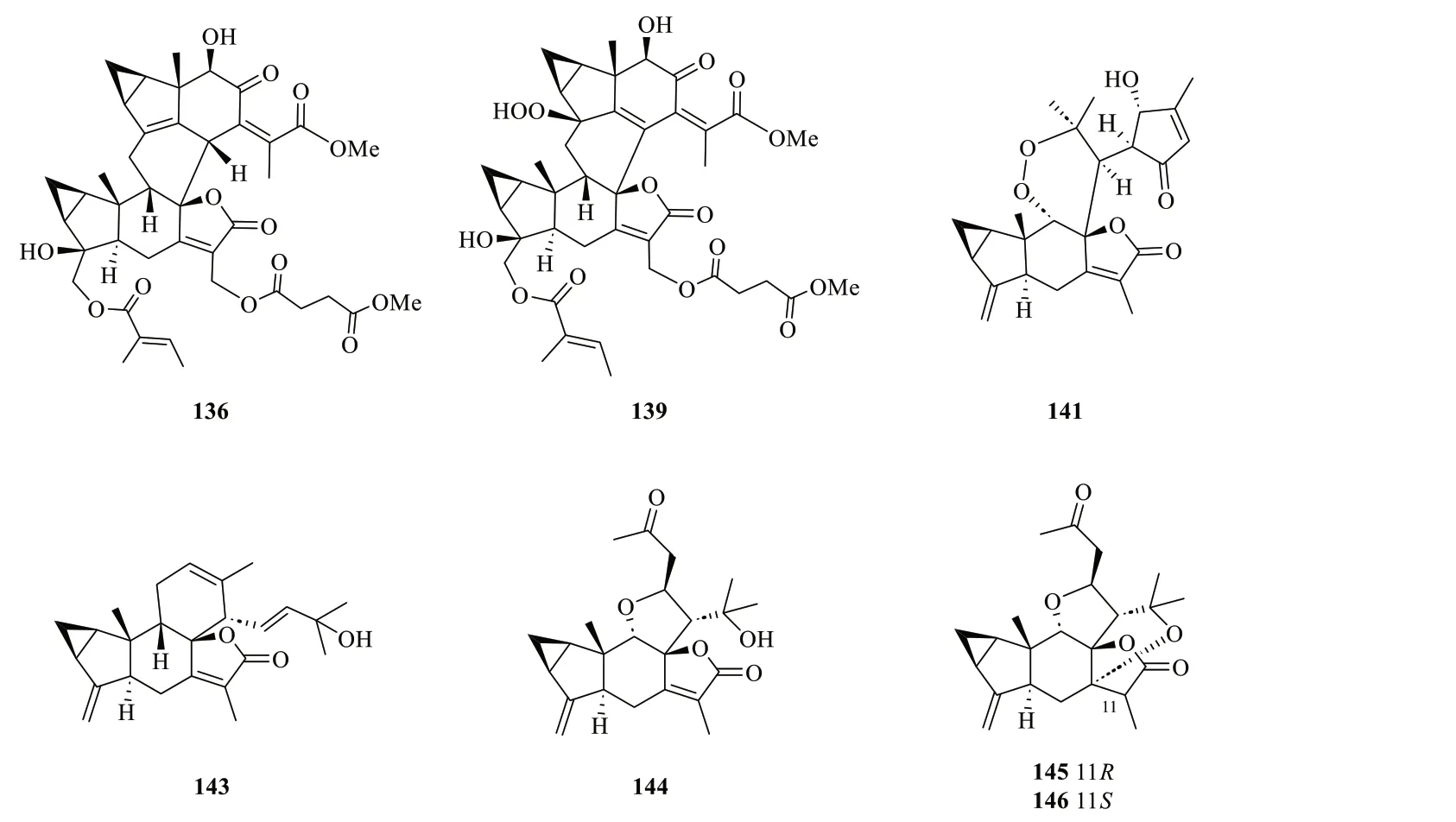
Fig.3 continued
Ni et al.[22] reported five new dimeric lindenanes,sarandrolides F-J (81-85),together with five known compounds (76-80) in the course of cytotoxicity screening ofS.glabrasesquiterpenoids.Sarcandrolide F (81) represents the first example of a lindenane-type dimer with a hydroperoxy group at C-5 [22].Wang et al.[37] investigated the seeds ofS.glabra,which led to the isolation of 11 new lindenane dimers,sarglabolide A-K (87-97).Among the isolates,compound87has a notable 17-membered macrocyclic ester ring that differs from the usual 18-membered system featured in other lindenane dimers.The continued endeavour of Wang’s group [38] resulted in the discovery of two uncommon heterodimers,sarglaperoxides A and B (98and99),featuring a lindenane and a normonoterpene unit assembled via a 1,2-dioxane ring,from the seeds ofS.glabra.With the guidance of MS/MS molecular networking,Wang et al.[39] reported the occurrence of four additional isomeric heterodimers,sarcaglarols A-D (117-120),whose skeletal structures resemble those of98and99,from the leaves ofS.glabra.Another structurally related heterodimer sarcaglarone A (141) was isolated by Sun et al.[40] from the seeds ofS.glabra,which incorporates a distinctive lactone in the monoterpene fragment.
An investigation by Chi et al.[33] onS.glabrafrom Guangxi afforded a series of unprecedented 8,9-secolindenane-type sesquiterpenoid oligomers,including three trimers,sarglalactones A-C (101-103),and five dimers,sarglalactones D-H (104-108).S.glabraspecimens from the Guangxi region were also found to contain a rare lindenane-monoterpene heterodimer sarcaglabrin A(109),two new lindenane dimers sarcaglabrins B and C(110-111),and five known compounds (112-116) [4].Sun et al.[41] reported the isolation of five norlindenane dimers,sarglaromatics A-E (123-127),bearing a naphthalene or a dihydronaphthalene core from the roots ofS.glabra,whereas Xiao et al.[42] reported the addition of three new sarcanolides (128-130) from the roots ofS.glabra.Compared to their congeners (74and75),sarcanolide C (128) bears a supplemental orthoformate ring at C-4 and C-5,whereas sarcanolide D (129) was elucidated as the 4-O-acetyl derivative of sarcanolide A (74).From the leaves ofS.glabra,Wang et al.[43] encountered an unprecedented [4+2]-type lindenane dimer,sarglafuran A (131),which represents the first dimer to contain a furan moiety in its lindenane monomer unit instead of an α,β-unsaturated lactone or an opened-ring lactone.Wang’s group [43] also reported the isolation of sarglalactones N and O (132and133),a pair of C-4 epimers that also possess a [2+2]-type dimeric skeleton and were identified as dihydroxy derivatives of61.

Table 4 Germacrane-type sesquiterpenoids from S. glabra
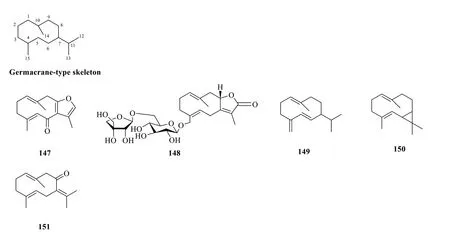
Fig. 4 Germacrane-type sesquiterpenoids (147-151)
Zhou et al.[44] revealed the presence of seven new dimeric lindenanes (135-140) with significant antimalarial activities and a known compound (134) from the roots ofS.glabrasubsp.brachystachys.15'-O-4-Hydroxytigloylfortunoid C (134) and 13'-O-methyl succinyl-15'-O-tigloylfortunoid C (135) share a common heterodimeric core comprising a lindenane and a eudesmane unit,whereas 4″-hydroxysarcandrolide A (137)and 13'-O-acetylsarcandrolide B (138) were identified as structural analogues of70.6α-Hydroxysarglaperoxide A(142) and 7'-oxyisosarcaglabrin A (143) are two new heterodimers isolated from the seeds ofS.glabra,and were determined as the derivatives of98and109,respectively [40].Guided by a single-node-based molecular networking approach,Cui et al.[45] discovered the first tetrahydrofuran-linked-lindenane-normonoterpene heterodimer sarglaoxolane A (144) from the leaves ofS.glabra,together with a pair of pseudonatural epimers sarglaoxolanes B and C (145-146),which were spontaneously formed from144.
2.1.1.3 GermacranesGermacranes are an elemental class of sesquiterpenoids containing a characteristic cyclodecane ring formed from the ring closure of C-1 and C-10 of farnesane [51].The carbon skeleton features an isopropyl group at C-7 and two methyl groups at C-4 and C-10 [52].Five germacrane-type sesquiterpenoids(147-151) have been isolated from the essential oils and ethanolic extract ofS.glabra(Table 4,Fig.4),with sarcaglaboside E (148) being identified as a new glucoside derivative [14].
2.1.1.4 EremophilanesEremophilanes belong to a family of sesquiterpenoids featuring a bicyclodecane skeleton similar to that of eudesmane-type sesquiterpenoids.The carbon skeleton of eremophilane is naturally derived from the rearrangement of eudesmane derivatives,exemplified by the migration of the methyl group from position C-10 to C-5 [51,54].The number of eremophilane-type sesquiterpenoids isolated fromS.glabraamounts to five so far (Table 5,Fig.5),four of which were first isolatedfrom other plants,i.e.,152-155.Sarglanoid F (156),the 8-ethoxy derivative of153,is the only eremophilane that was first reported fromS.glabra[25].

Table 5 Eremophilane-type sesquiterpenoids from S. glabra
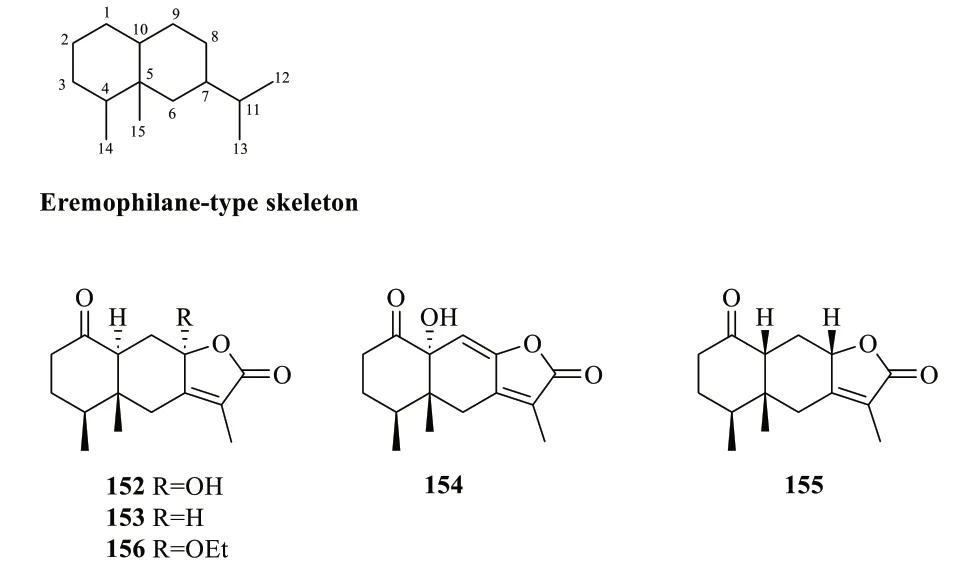
Fig. 5 Eremophilane-type sesquiterpenoids (152-156)
2.1.1.5 AromadendranesThe scaffold of aromadendranes usually incorporates agem-dimethylcyclopropane ring that is fused to a hydroazulene skeleton [57].The skeleton bears a strong resemblance to that of guaianetype sesquiterpenoids,differing only in having a cyclopropane ring formed by the bond linking C-6 and C-11 in aromadendranes.Hence,aromadendrane-type sesquiterpenoids are also known as 6,11-cycloguaianes [51].At present,eight aromadendrane-type sesquiterpenoids(157-164) fromS.glabrawere documented in the literature (Table 6,Fig.6).Sarglanoid G (164) was reported for the first time from the roots ofS.glabra[58].
2.1.1.6 ElemanesElemanes constitute a small group of sesquiterpenoids inS.glabraand are olefinic compounds with cyclohexane as their core [60].A total of eight sesquiterpenes of this class (165-172) have been reported fromS.glabra(Table 7,Fig.7),including two new elemanolide aglycons,sarcaglaboside C and D (165and166)[14],five known compounds (167-171),and a new furanbearing elemane-type sesquiterpenoid sarglanoid H (172)[58].
2.1.1.7 Guaiane,cadinane,and other sesquiterpenoidsCompound173is the only guaiane-type sesquiterpenoid isolated from the whole plant ofS.glabrafeaturing a distinctive epoxy bridge at C-4 and C-6,whereas compound174,a component of cade oil [62],is the only cadinane-type sesquiterpenoid isolated from the essential oil ofS.glabra(Table 8,Fig.8).
Apart from the aforementioned sesquiterpenoid subtypes,there are more than a dozen other sesquiterpenoids isolated fromS.glabra(Table 8,Fig.8).These include aliphatic farnesenes (175,179-181),cyclic farnesenes (177and178),megastigmane-type sesquiterpenoids (182and187),and their glucosides (176,183,186,188).Li et al.[63] reported the isolation of two new compounds,sarcabosides A and B (184and185),fromS.glabraspecimens collected from Sichuan province.Both compounds possess interesting,highly conjugated skeletons.
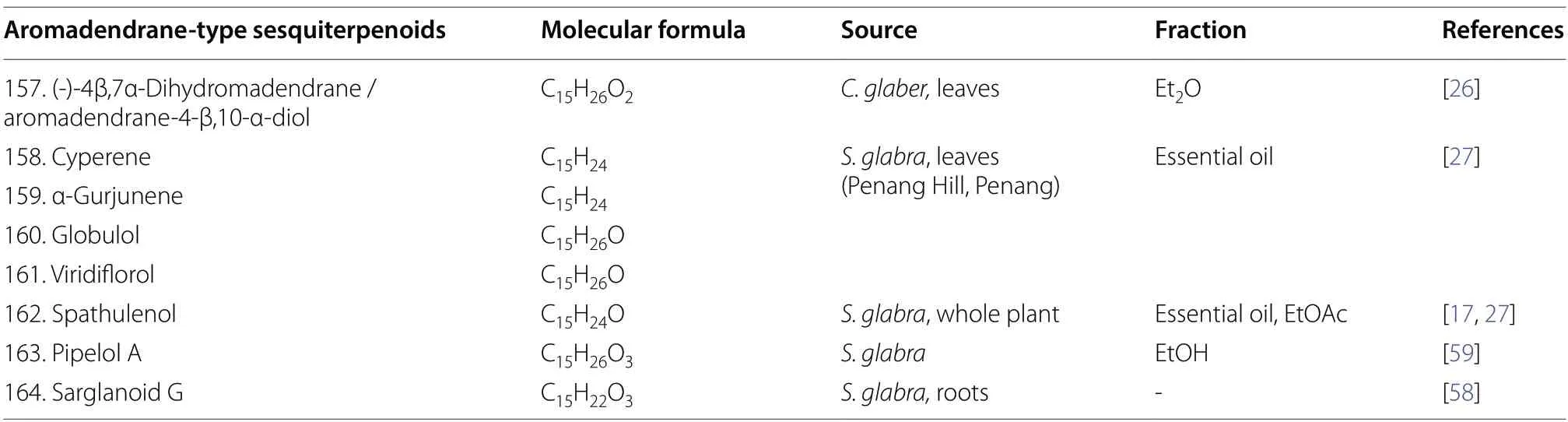
Table 6 Aromadendrane-type sesquiterpenoids from S. glabra
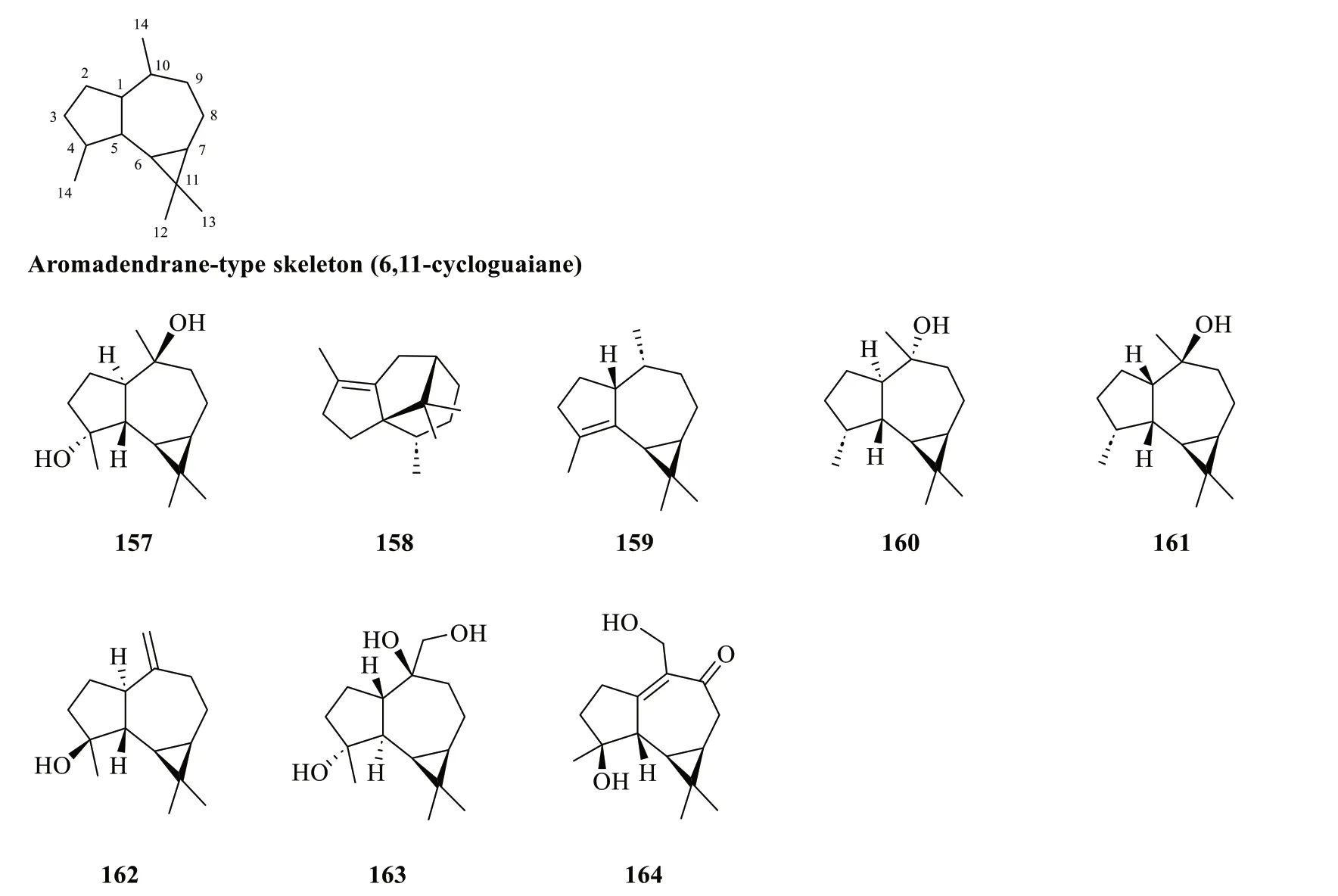
Fig. 6 Aromadendrane-type sesquiterpenoids (157-164)
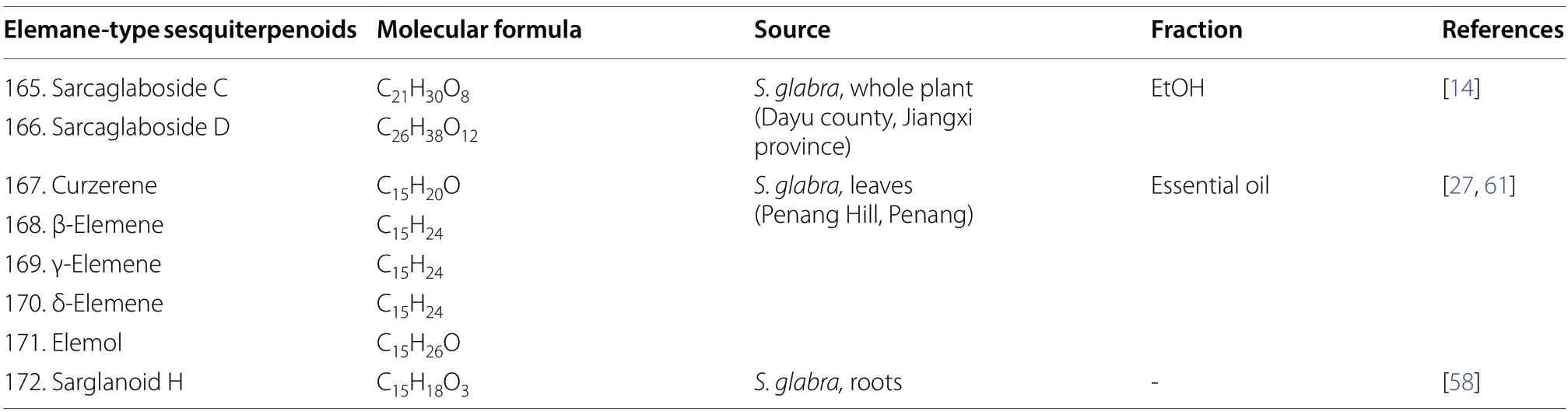
Table 7 Elemane-type sesquiterpenoids from S. glabra
2.1.2 Monoterpenoids
Monoterpenoids are a terpenoid class consisting of two isoprene units with the general molecular formula C10H16[65].They are derived from the putative precursor,geranyl diphosphate,and may exist in linear forms(acyclic) or comprise ring structures (cyclic).Acyclic monoterpenoids are formed by the head-to-tail polymerisation of isoprene monomers,whereas bicyclic monoterpenoids are formed from additional cyclisation and rearrangement via monoterpene synthases [66].To date,16 monoterpenoids (189-204) have been reported from the essential oils,whole plants,and seeds ofS.glabra(Table 9,Fig.9).Among them,seven are acyclic (192,197-199,201-202,204),six are monocyclic (193-196,200,203),and three have bicyclic structures (189-191).6-Hydroxy-2,6-dimethylhepta-2,4-dienal (204) is a ninecarbon normonoterpene newly isolated fromS.glabra[38].

Fig. 7 Elemane-type sesquiterpenoids (165-172)

Table 8 Guaiane,cadinane,and other sesquiterpenoids from S. glabra
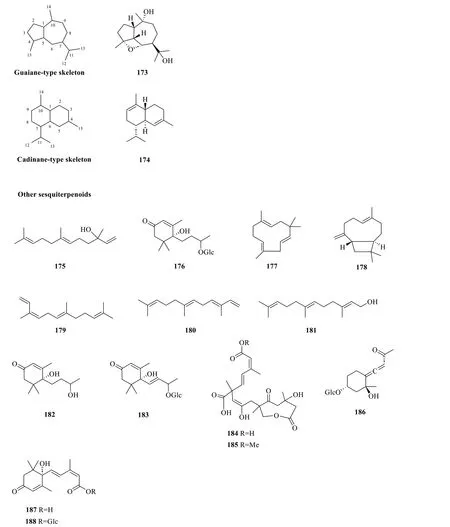
Fig. 8 Guaiane-type sesquiterpenoid (173),cadinane-type sesquiterpenoid (174),and other sesquiterpenoids (175-188)
2.1.3 Diterpenoids and triterpenoids
The molecular formula of diterpenoids (C20H32) is indicative of their 20-carbon skeleton derived from the condensation of four isoprene units [65].All diterpenoids originate from a common substrate,geranylgeranyl diphosphate,of which cyclisation into different scaffolds by diterpene synthase gives rise to their structural diversity [67].All four reported diterpenoids(205-208) fromS.glabraare labdane-type diterpenoids,three of which (206-208) were isolated as diastereomers (Table 10,Fig.10).

Table 9 Monoterpenoids from S. glabra

Fig. 9 Monoterpenoids (189-204)

Table 10 Diterpenoids and triterpenoids from S. glabra
Composed of six isoprene units (C30H48),triterpenoids have a common acyclic biosynthetic precursor,squalene [68].Overall,their skeletons may be categorised based on the number of rings present in the structure.Pentacyclic triterpenoids are mostly prevalent in the whole plants ofS.glabra(Table 10,Fig.10).Seven of these triterpenoid compounds (209-215),including two new triterpenoid saponins,sarcandrosides A and B(210and211),were isolated from the aerial parts and whole plants ofS.glabra.
2.1.4 Meroterpenoids
The term meroterpenoid was initially coined by Cornforth [71] and is used to define natural products of mixed biosynthetic origin that are partially derived from terpenoid pathways.Presently,a total of 13 meroterpenoids (216-228) have been isolated fromS.glabra(Table 11,Fig.11).Yang et al.[72] reported the discovery of three novel meroterpenoids,namely a chalcone-coupled monoterpenoid,glabralide A (216),and two geranylated meroterpenoids,glabralides B and C (217and218),from the whole plants ofS.glabra.Compound216contains a bicyclo[2.2.2]octene core,while compound218displays an unprecedented linearly fused 6/6/6 ring system.Subsequently,a study on aS.glabraspecies from Anhui province [49] led to the isolation of five new glabralide meroterpenoids,glabralides D-H (219-223),possessing structures resembling that of218.Sarglamides A-E (224-228)represent five unprecedented indolidinoid-monoterpenoid hybrids derived from toussaintine C and α-phellandrene isolated from the whole plant ofS.glabrasubp.brachystachys[73].
2.2 Isolation of phenylpropanoids from S. glabra
Phenylpropanoids are a vast and structurally diverse group of natural products in the plant kingdom.They play vital roles in plant development through their interaction with the environment and other living organisms.Phenylpropanoids are derived from the aromatic amino acids,phenylalanine and tyrosine,via the shikimate pathway [74].4-Coumaroyl-CoA represents a crucial enzyme in the metabolic network that leads to the biosynthesis of phenylpropanoid compounds such as coumarins,lignins,flavonoids,and phenolic acids [75].Diversification of the subsets of phenylpropanoids is achieved by an array of successive enzymatic transformations such as acylation,condensation,cyclisation,glycosylation,hydroxylation,methylation,and prenylation [76].
2.2.1 Coumarins
Coumarins represent a class of aromatic lactones derived from theortho-hydroxylatedcis-hydroxycinnamic acid in the phenylpropanoid pathway [77].Due to the versatility of the coumarin scaffold,various pharmacophores and functionalised coumarins could be generated via a series of transformations and substitutions,which explains the exceptional pharmacological activities displayed by this class of compounds [78-80].Currently,the identification of 26 coumarins (229-254) has been reported in various literature ofS.glabra(Table 12,Fig.12),which mostly consist of known compounds,particularly isofraxidin (230) and its dimers (233and 238),as well as its substituted analogues at C-6,C-7 and C-8 (231-232,234-237,239-241,243,245-246,254).This designates isofraxidin (230) as a representative compound of this class and establishes its inclusion as a quality control marker for medicinal preparations ofS.glabrain the Chinese Pharmacopoeia [81].Feng et al.[78] discovered a new coumarin,sarcandracoumarin (244),from the whole plant ofS.glabrabearing a 1-phenylethyl substituent at C-3.Unfortunately,the absolute configuration of this compound remains unclear.Wang et al.[82] reported a new coumarin,3,5-dihydroxycoumarin-7-O-α-Lrhamnopyranosyl-2H-chromen-2-one (247),possessing anti-inflammatory properties,fromS.glabra.From the stems ofS.glabra,Du et al.[83] uncovered the presence of five coumarins,namely,a pair of coumarin-phenylpropanoid enantiomers (7S,8S) and (7R,8R)-sarcacoumarin(250and251) with promising acetylcholinesterase inhibitory activities,and three known compounds (252-254).

Fig. 10 Diterpenoids (205-208) and triterpenoids (209-215)

Table 11 Meroterpenoids from S. glabra
2.2.2 Lignans and neolignans
Lignans and neolignans are dimeric structures commonly derived from the oxidative coupling of two lignol units.They may differ in the degree of oxidation of the threecarbon sidechain and the substitution on the aromatic ring [91].Up to the present,only two lignans (255and256) and an array of neolignans (257-263) were isolated from the whole plants ofS.glabra(Table 13,Fig.13).The isolation of a pair of dihydrobenzofuran neolignan enantiomers,(+) and (-)-sarcanan A (262and263),from the aerial parts ofS.glabrawas recently reported [79].
2.2.3 Flavonoids
Flavonoids,a diversified group of phytochemicals found in many natural sources,are the second most predominant secondary metabolites inS.glabra.Despite having a common structure,flavonoids inS.glabrahave intrigued the interest of numerous scientists due to their extensive pharmacological and biological activities that hold significant pharmaceutical importance [93,94].
Chemically,the general structure of flavonoids consists of a C6-C3-C6 diphenylpropane backbone [95].Flavonoids can be divided into a myriad of subgroups in terms of ring arrangements and functionalisation of the ring systems.Flavonoids in which ring B is attached to the C-3 position of ring C are called isoflavonoids [96],while those in which ring B is linked to C-2 of ring C are further categorised into different subtypes depending on the hydroxylation pattern and variations in the chromane ring or ring C [97].Some relevant examples of these subtypes include flavans,flavones,flavonols,flavanones,flavanols,flavanonols,and anthocyanins.Chalcone otherwise known as open-chain flavonoids are distinguished from other flavonoids by their two aromatic rings (A and B),which are linked by an α,βunsaturated carbonyl chain.
2.2.3.1 ChalconesFollowing the isolation of a series of dihydrochalcones (264-269) from the aerial parts ofS.glabra,subsequent studies have reported 21 more chalcones (270-272,276-293) fromS.glabraand an additional three (272-275) from its subspeciesS.hainanensis(Table 14,Fig.14).Interestingly,Liu et al.[98] isolated a rare class of uncommon monoterpene-chalcone conjugates with potential autophagy-inducing activities from the aerial parts ofS.glabra,some of which are connected via a dihydrofuran ring or an ether bridge.The compounds include four monoterpene-conjugated chalcones,glabratins A-D (279-282),seven monoterpene-conjugated dihydrochalcones,glabratins E-K (283-289),and four known analogues (290-293).
2.2.3.2 AnthocyanidinsAnthocyanidins are a class of flavonoids known for their roles as natural pigments that bestow fruits and flowers different colours.Their distinctive skeletal backbone is based on a flavylium cation that lacks the ketone group at C-4 [99] So far,only three anthocyanidin-type compounds (294-296) were characterised fromS.glabra(Table 14,Fig.15).Ishikura [100] was the first researcher to report the occurrence of two glycosides of anthocyanidins (294and295) from the fruits ofC.glaber(syn.S.glabra),whereas Li et al.[89] isolated a leucoanthocyanidin compound (296) from the whole plant ofS.glabra.

Fig. 11 Meroterpenoids (216-228)
2.2.3.3 Flaνones and flaνonolsThe backbones of flavones and their hydroxylated derivatives (flavonols) are distinguished by the presence of a double bond between C-2 and C-3,which extends the π-conjugation onto the carbonyl group in the pyranone ring [105].Collective studies onS.glabrahave reported a total of 15 flavone and flavonol-type compounds (297-311),including a number of quercetin and kaempferol derivatives (Table 15,Fig.16).
In a recent study,Qin et al.[106] revealed the presence of two new methylenedioxyflavones (297and298)and two known derivatives (299and300) fromS.glabra,among which compounds297,299and300showed pronounced cytotoxic effects.Ubiquitous in various naturalsources,the structure of quercetin (301) comprises a pentahydroxyflavone,which is oftentimes conjugated with residual sugars to form quercetin glycosides [107].Overall,all reported quercetin-type flavonoids fromS.glabrawere obtained from the whole plants and are derivatives substituted with glycoside groups at C-3,such as glucuronide (302-304),rhamnoside (305),rutinoside(306),galactose (307) and glucoside (308).Kaempferol(309) is a tetrahydroxyflavone in which the four hydroxyl groups are at C-3,C-5,C-7 and C-4' [108].So far,only three compounds of this class (309-311) have been isolated from the whole plants ofS.glabraandS.hainanensis(subsp.brachystachys).
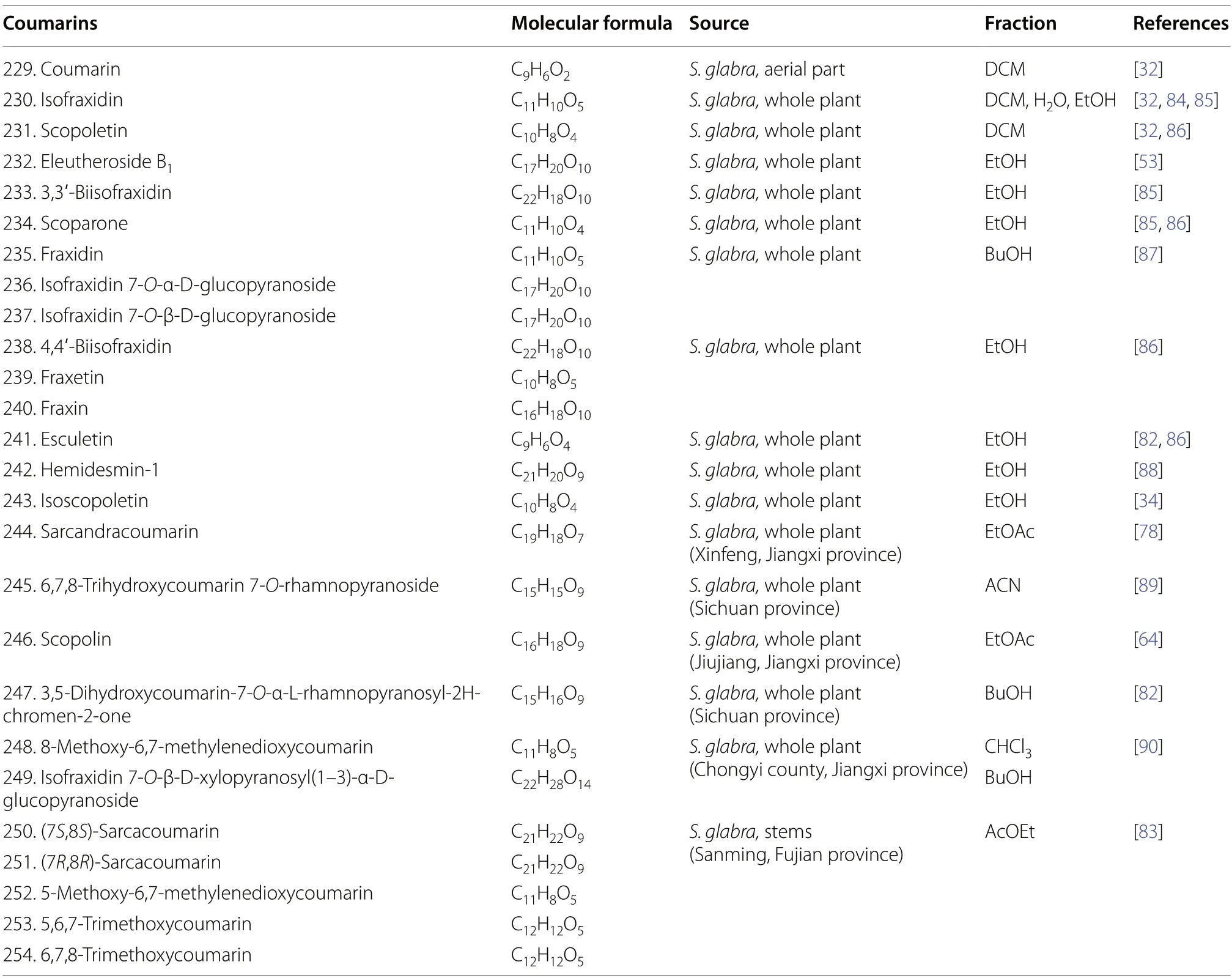
Table 12 Coumarins from S. glabra
2.2.3.4 Flaνanones and flaνanolsDerived from flavones,flavanones are important precursors and key intermediates in the flavonoid biosynthetic pathway.Flavanones and their hydroxylated derivatives (flavanols) differ from flavones and flavonols by two features: the presence of a C-2 chiral centre and the absence of a double bond between C-2 and C-3 [112].Up to now,a total of 29 flavanone and flavanol-type compounds (312-339),including naringenin and catechin derivatives,were reported fromS.glabra(Table 16,Fig.17).
Naringenin is a pertinent example of a flavanone aglycone having three hydroxyl groups at C-5,C-7,and C-4’[113].Most of the compounds belonging to this class occur as methoxy and glycosidic derivatives (312-314)and were isolated from the whole plants ofS.glabraandS.hainanensis(subsp.).Interestingly,some of the naringenin-type compounds isolated fromS.glabraare epimeric at C-2,as exemplified by315-318[89,114].
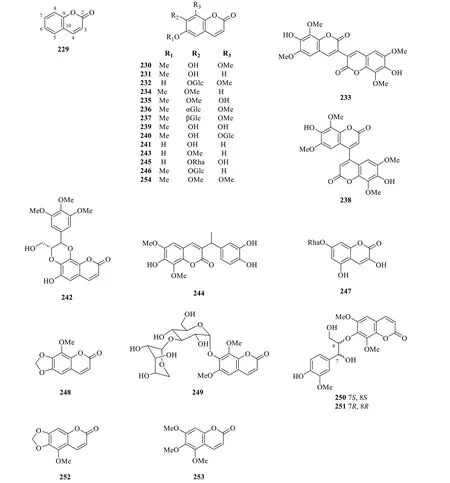
Fig. 12 Coumarins (229-254)
Catechins are secondary metabolites that contribute to the antioxidative activities in plants [115].They exist as minor constituents inS.glabraand are flavan-3-ols characterised by the absence of a ketone group at the C-4 position.To date,only six catechin-type flavanols (319-323) were documented in the literature.Li [59] reported the isolation of a rhamnopyranoside derivative (319) and two new epimeric phenylpropanoid-substituted catechin glycosides,glabraosides A and B (320and321),from the whole plant ofS.glabra.Subsequently,Wang et al.[20]expanded the family by reporting two new catechin glycosides,glabraosides C and D (322and323),with their phenylpropanoid fragments fused to ring A at C-5/C-6 and C-7/C-8,respectively.Liu et al.[98] reported glabratins L-N (337-339) as three monoterpene-flavanone conjugates from the aerial parts ofS.glabra.Compounds337and338were obtained as a pair of C-2 epimers,whereas the flavanone core of339is linked to the monoterpene unit via an ether bridge instead of a C-C bond.
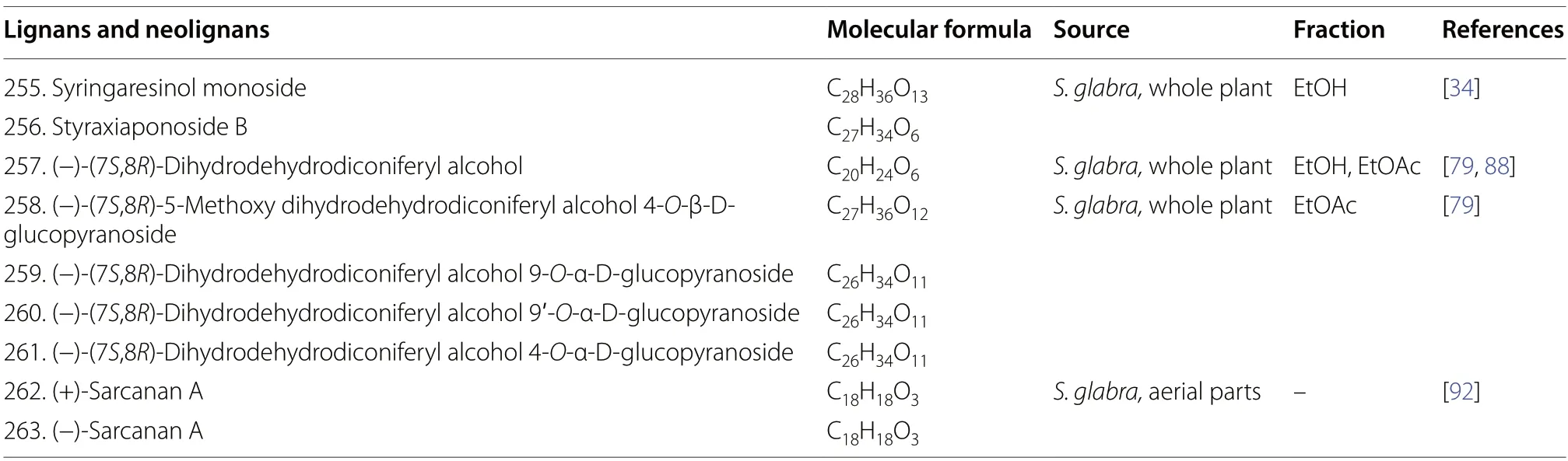
Table 13 Lignans and neolignans from S. glabra
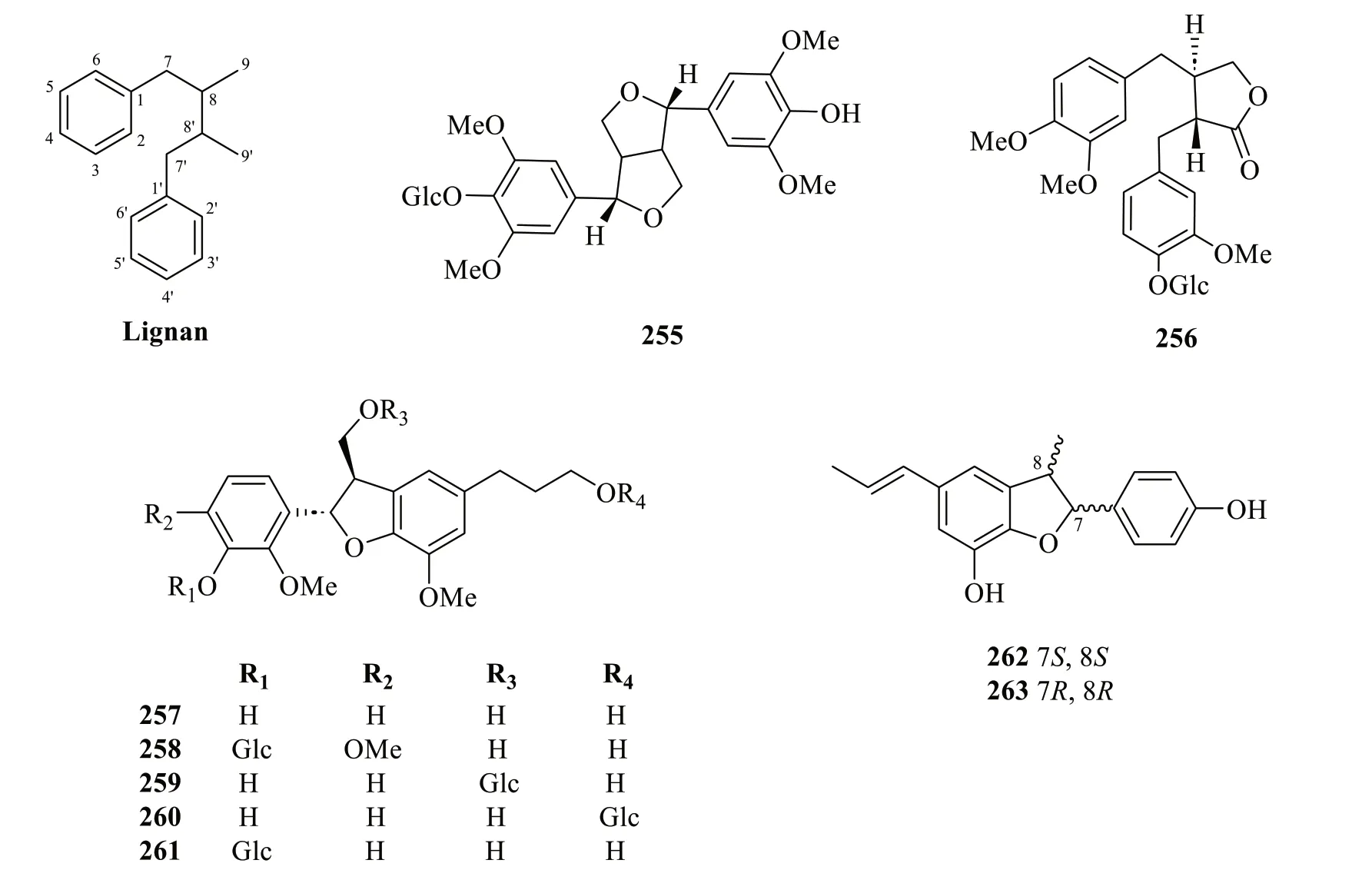
Fig. 13 Lignans (255 and 256) and neolignans (257-263)
2.2.3.5 Dimeric flaνonoidsThe occurrence of dimeric flavonoids was found to be exclusive to theS.hainanensissubspecies,as evidenced by the isolation of four compounds (340-343) from the dichloromethane extract of the whole plant (Table 16,Fig.18).Sarcandrones A and B (340and341) represent new hybrid flavan-chalcones that are linked from C-4 of the flavan moiety to C-3' of the chalcone moiety [116].On the other hand,sarcandrones C and D (342and343) were characterised as analogues that are linked from the flavan moiety to the flavanone moiety at C-4 to C-8' and C-4 to C-6',respectively [111].
2.3 Isolation of anthraquinones,organic acids,and organic esters from S. glabra
Anthraquinones,also referred to as anthracenediones or dioxoanthracenes,are associated with the quinone class under the polyketide family.A characteristic feature of anthraquinones is their polycyclic aromatic structure wherein two keto groups are located on the central ring[117].Current literature onS.glabrahas cumulatively reported five anthraquinone-type compounds (344-348),which mainly consist of hydroxy,methoxy or glycoside derivatives (Table 17,Fig.19).

Table 14 Chalcone and anthocyanidin-type flavonoids from S. glabra
As shown in Table 17 and Fig.20,S.glabraplants are rich sources of organic acids.At present,more than a dozen known organic acids have been isolated fromS.glabra(349,352-355,357-367),while three (350-351,356) were obtained from the subspeciesS.hainanensis.These include dicarboxylic acids (349and364),longchained fatty acids (350,351,354,356,361-363),and multi-substituted phenolic acids (352-353,355,357-360,364-367).
Organic esters (368-391) were particularly abundant inS.glabra.These compounds are distributed in different plant parts and their structures could be divided into several categories (Table 17,Fig.21).For instance,compounds386,387,and391were classified as caffeic acid derivatives,while compounds381,383,and384are generally known as caffeoylshikimic acids.Esters in which their quinic acid core is acylated with one or more caffeoyl groups are known as caffeoylquinic acids or chlorogenic acids.Eight compounds of this group (372,377-380,385,388-389) were isolated from the polar partitions ofS.glabra.From the whole plant ofS.glabra,Wu et al.[79] reported a new glycoside compound (390),benzyl 2-β-glucopyranosyloxybenzoate.Among all organic esters,rosmarinic acid (369) is specifically recognised for its pronounced pharmacological activities and its high content withinS.glabra[3,118].For this reason,rosmarinic acid is established as one of the key quality control markers in the Chinese Pharmacopoeia [81].
Interestingly,the distribution of organic esters differs between the plant parts ofS.glabra.This hypothesis was validated by Zhou et al.[114] who concluded that chlorogenic acids,caffeic acids,and 4-O-glucopyranosyl rosmarinic acid were mostly concentrated in the stems,whereas chlorogenic acids were dominant in the leaves.On the other hand,rosmarinic acids were found to be distributed in the aerial parts (stems and leaves) ofS.glabra.

Fig. 14 Chalcone-type flavonoids (264-293)
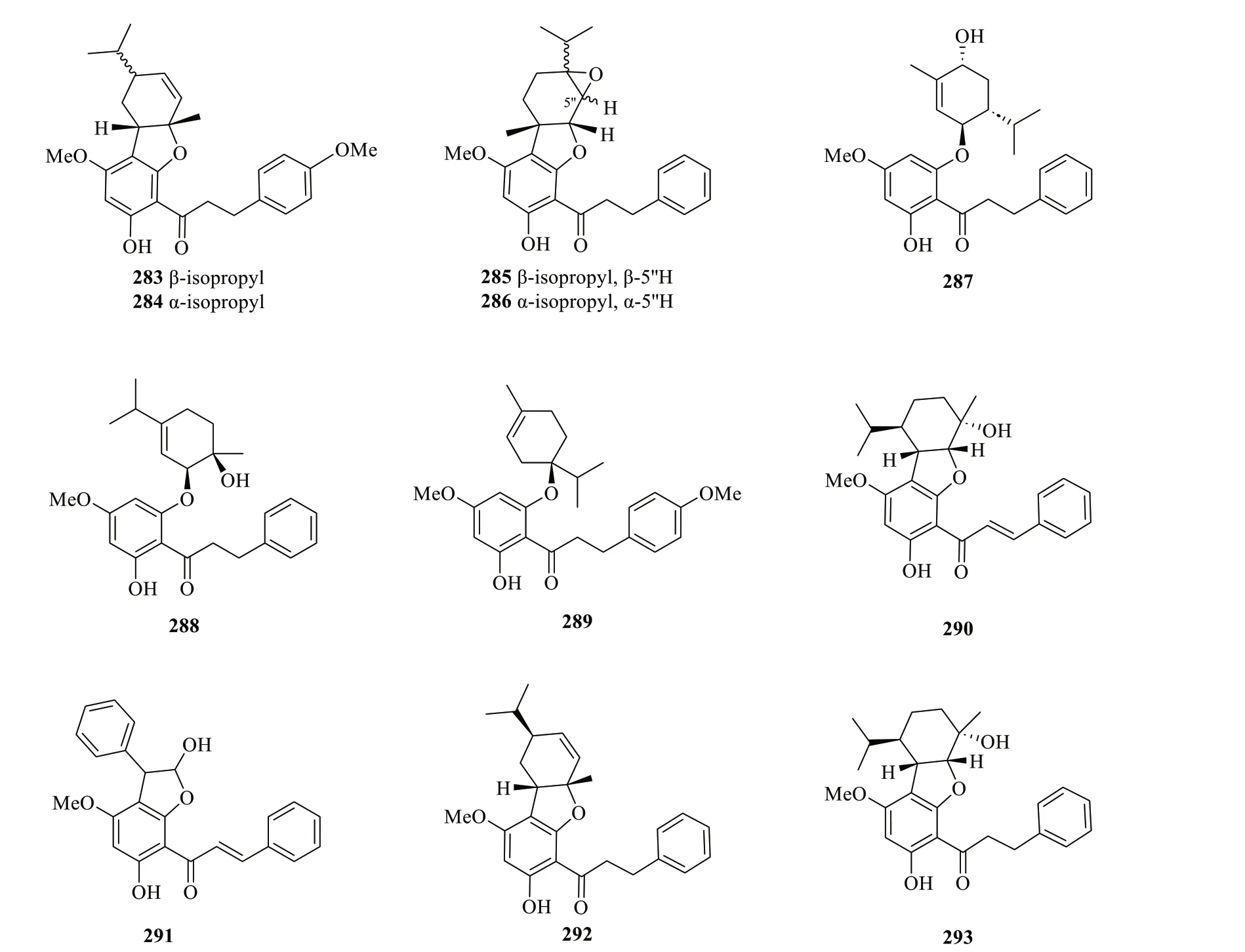
Fig.14 continued

Fig. 15 Anthocyanidin-type flavonoids (294-296)

Table 15 Flavones and flavonols from S. glabra
2.4 Isolation of alcohols and sterols from S. glabra
A total of ten alcoholic compounds (392-401) were reported from the whole plants ofS.glabra,including a saturated fatty alcohol (393),a sugar alcohol (394),and eight phenol derivatives (392,395-401) (Table 18,Fig.22).
Sterols are a subgroup of steroids distinguished by their hydroxyl group at C-3 of ring A [127].All four stigmastane-types phytosterols (402-405) reported in the literature were isolated from the whole plants ofS.glabra,except for compound402,whose occurrence was also reported from the subspeciesS.hainanensis.
2.5 Isolation of other compounds from S. glabra
Apart from the mentioned classes of secondary metabolites,the rare presence of a ketone (406),monosaccharide (407),alkaloids (408,412-413),alkane (410),and lactones (409and411) have also been reported during the isolation process (Table 18,Fig.23).
3 Biogenetic pathways of oligomeric sesquiterpenoids and meroterpenoids from S.glabra
The biosynthesis of oligomeric sesquiterpenoids and meroterpenoids fromS.glabrais a complex process involving a series of reactions.Generally,the majority of lindenane-type oligomers inS.glabrawere assembled via Diels-Alder reaction,facilitated by the presence of diene and dienophile elements within the monomeric units.Occasionally,the dimerisation or oligomerisation process also involves the integration of direct cyclisation,oxidative coupling,esterification,acetalisation,aldol reaction,and Michael-type reaction using various linkers [129].Herein,the proposed biogenetic routes for a selection of oligomeric sesquiterpenoids and meroterpenoids isolated fromS.glabraare summarised.A biogenetic relationship that connects the various key terpenoid skeletons obtained fromS.glabrais also presented.
3.1 Biosynthesis of shizukaol A and lindenatriene as a key biogenetic building block
The first dimeric lindenene sesquiterpene,shizukaol A,was reported by Kawabata et al.[130].Since then,numerous other heterodimeric sesquiterpenes have been reported with a carbon skeleton similar to that of shizukaol A.Shizukaol A was hypothesised to be biogenetically derived from a Diels-Alder reaction (Scheme 1)based on a sealed tube pyrolysis experiment of shizukaol A,yielding lindenatriene and chloranthalactone A as the retro-Diels-Alder adducts [130].However,since lindenatriene was highly unstable and rapidly decomposed in situ,it was only obtained in trace amounts and partially characterised by1H NMR.
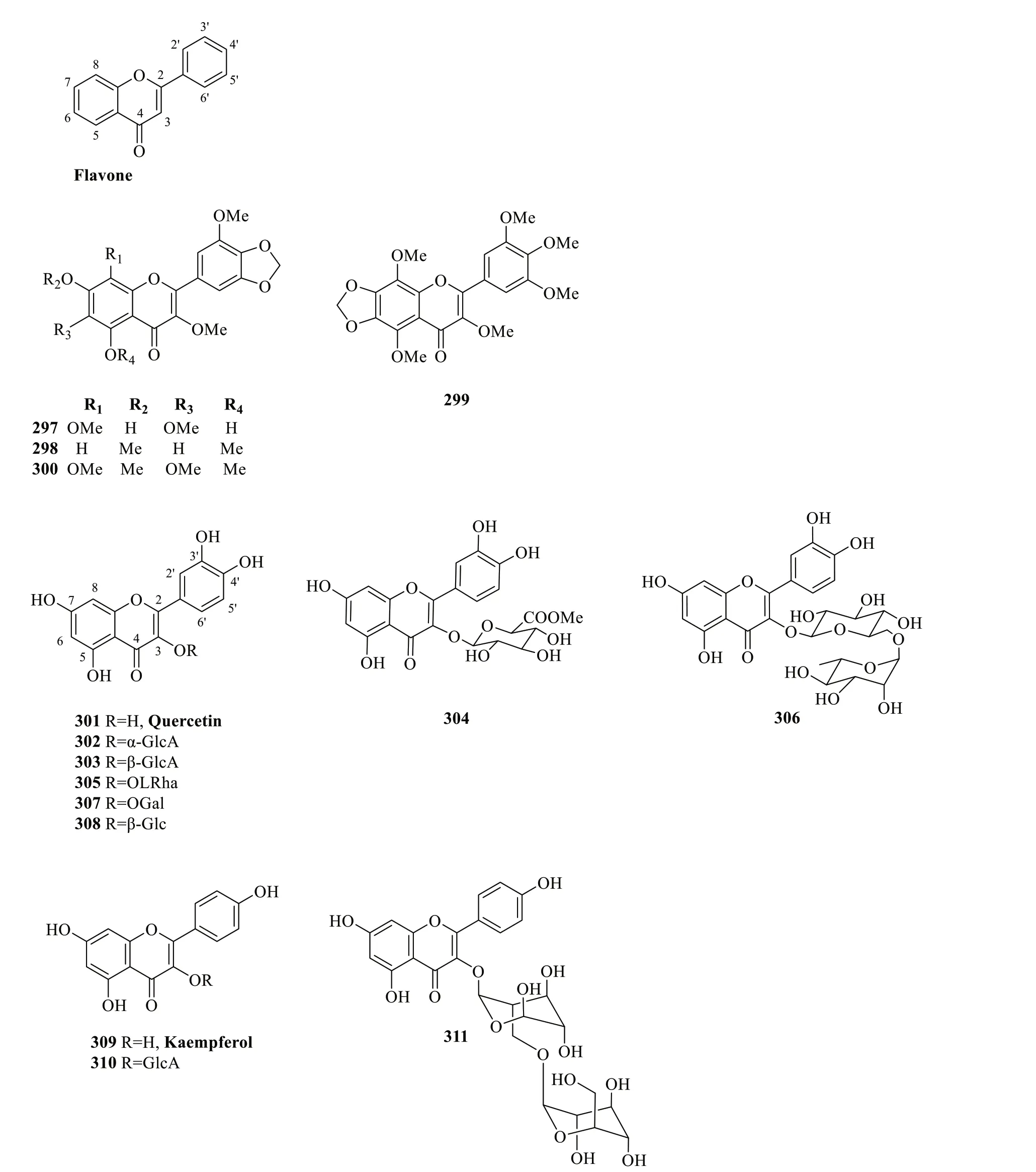
Fig. 16 Flavones and flavonols (297-311)
The structure proposed for lindenatriene by Kawabata’s group was finally confirmed almost two decades later through a synthesis by Eagan et al.[131].They showed that lindenatriene was unstable and readily isomerised to its more thermodynamically stable tautomer,iso-lindenatriene,under mildly basic conditions (Scheme 2).Due to the inherent instability of the triene moiety,Yuan et al.[132] encountered a setback in obtaining the anticipated Diels-Alder adduct in their model biomimetic reaction,in which they employed the equally unstabledes-hydroxy derivative of lindenatriene.Despite synthesising lindenatriene,theisolated and characterised compound was its tautomer,iso-lindenatriene.Consequently,the discrepancies between the1H NMR chemical shifts reported by Yuan et al.and those reported by Kawabata et al.raised questions regarding the validity of the proposed biogenetic mechanism.Finally,Martinez et al.[133] re-assessed the literature and NMR data,affirming lindenatriene’s continued relevance as a building block in Kawabata’s original biogenetic hypothesis.
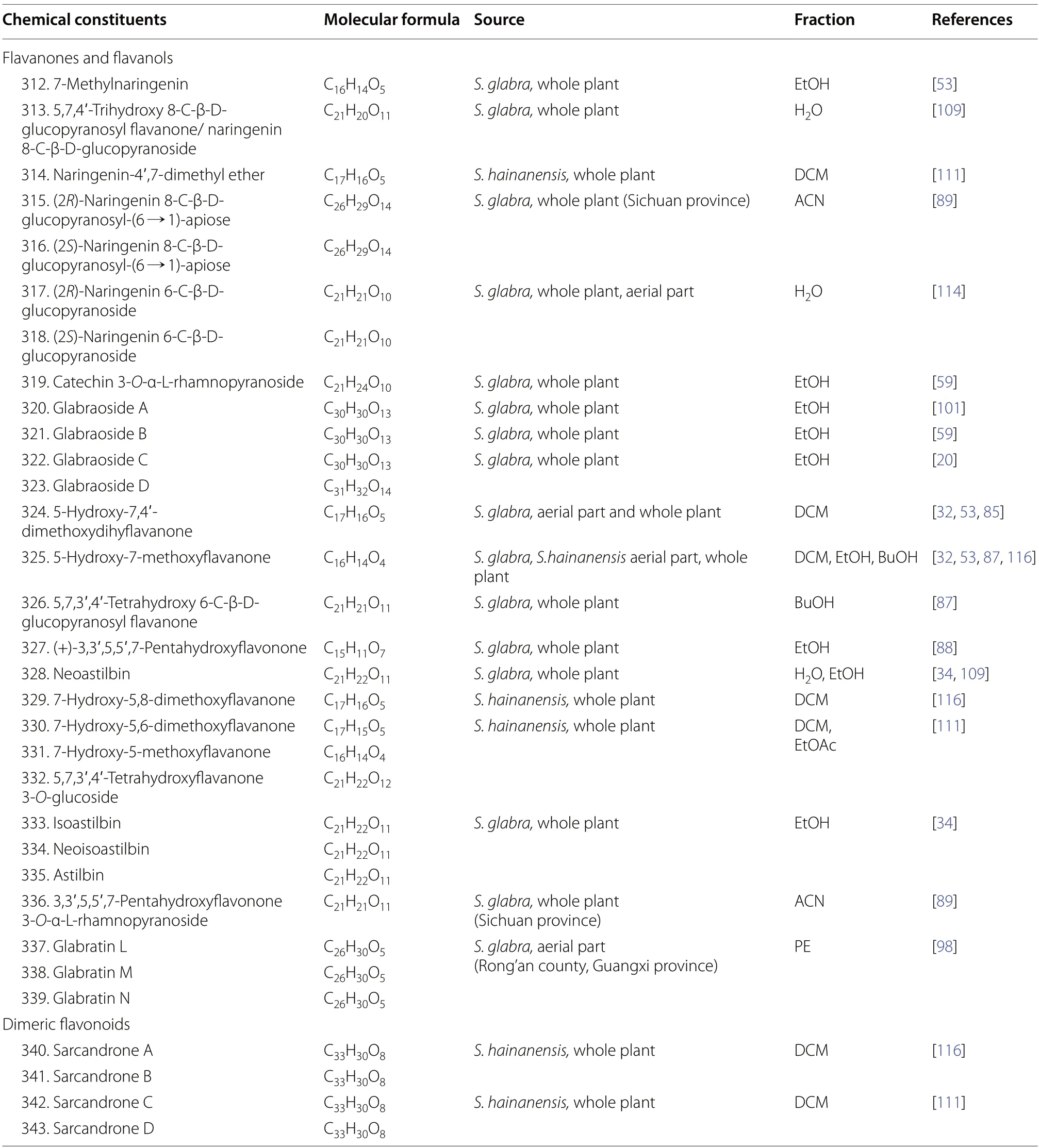
Table 16 Flavanones,flavanols,and dimeric flavonoids from S. glabra

Fig. 17 Flavanones and flavanols (312-339)

Fig.17 continued
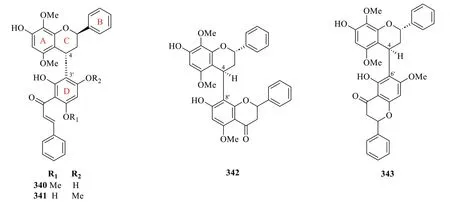
Fig. 18 Dimeric flavonoids (340-343)
3.2 Biosynthesis of sarglanoids A-C (28-30)
Li et al.[24] proposed that sarglanoids A-C (28-30)originate from a common precursor farnesyl diphosphate(FDP).A cascade of spontaneous cyclisation,oxidation,lactonisation,and double-bond migration was hypothesised to result in the formation of the eudesmane (i)and eremophilane (ii,iii) monomers (Scheme 3).Subsequently,the lactone moieties of the monomeric units are directly connected by a C-C bond via free-radical coupling reactions to form sarglanoids A-C.
3.3 Biosynthesis of sarcanolides A-D (74,75,128 and 129)
As depicted in Scheme 4,He et al.[47] proposed a biosynthetic route for sarcanolides A (74),B (75),C (128)and D (129),which involves a Diels-Alder reaction between lindenatriene and chloranthalactone A.The resulting cycloadduct then undergoes an acid-catalysed intramolecular cyclisation connecting C-11 to C-7',generating a β-oriented lactone.Sequential oxidation and acylation of the nonacyclic intermediate would ultimately form sarcanolide A (74),which could then be transformed into sarcanolides B,C and D (75,128and129) by dehydration,ortho-ester formation,and acetylation,respectively.
3.4 Biosynthesis of sarglaperoxides A and B (98 and 99),sarcaglarols A-D (117-120),sarcaglarone A (141),and 6α-hydroxysarglaperoxide A (142)
Wang et al.[39] proposed a common biosynthetic pathway for compounds98-99,117-120,141and142as shown in Scheme 5.It was inferred that geraniol and chloranthalactone A,two abundant components ofS.glabra,serve as biogenetic precursors for the formation of these dimers.The fundamental core of these dimers was hypothesised to be formed through a photocatalytic aerobic [2+2+2] cycloaddition step.Firstly,intermolecular cycloaddition between geraniol and chloranthalactone A in the presence of O2generates an intermediate that yields sarcaglarols A-D (117-120) via a series of oxidative reactions.The sarcaglarols undergo successive oxidative cleavage at the C-2',C-3'-diol fragment,leading to the formation of sarglaperoxide A (98)and its hydroxylated analogues,99and142.Finally,the formation of the lactone functionality in sarcaglarone A(141) involves oxidation of the sarcaglarols,followed by a free-radical-mediated intramolecular cyclisation that links C-1' and C-5'.
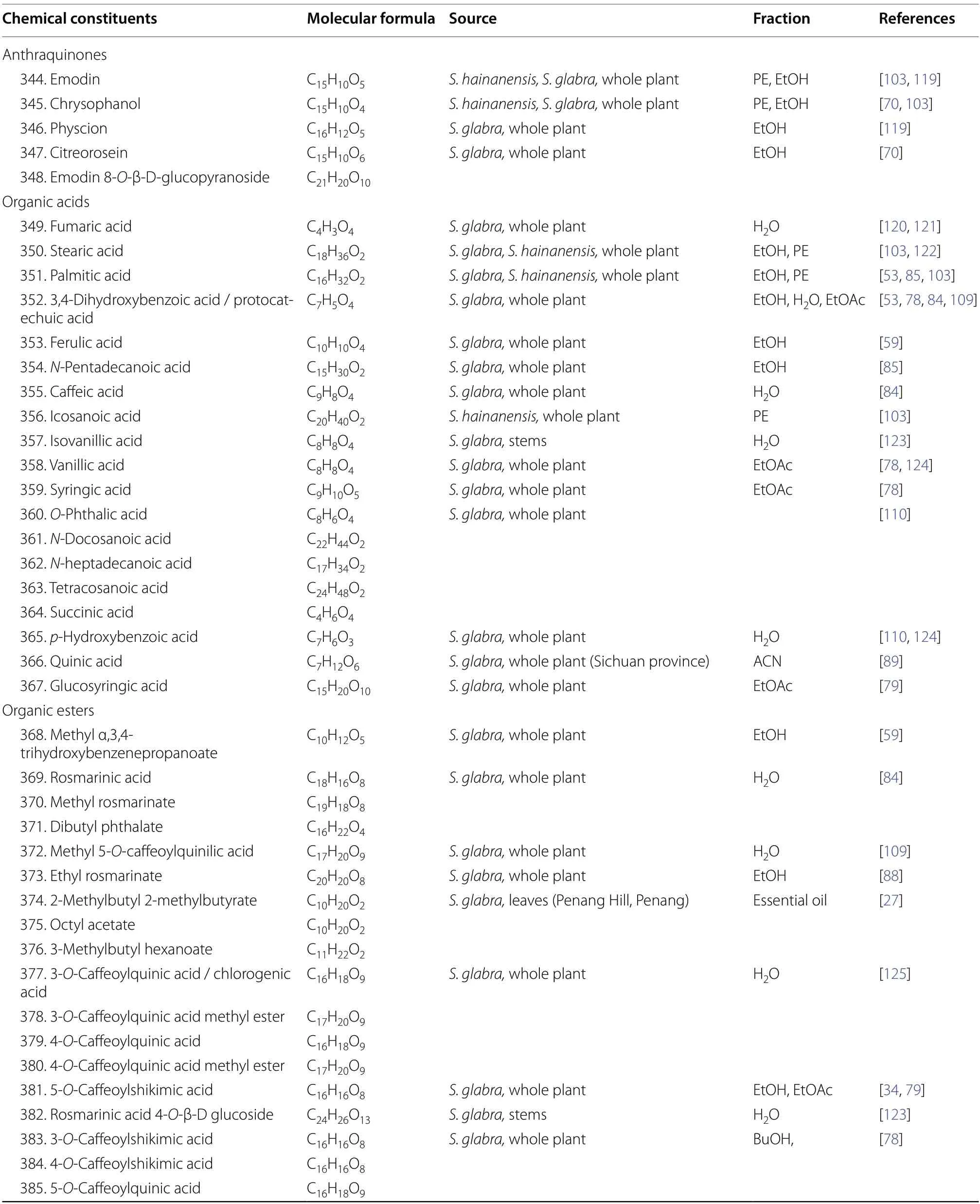
Table 17 Anthraquinones,organic acids,and organic esters from S. glabra
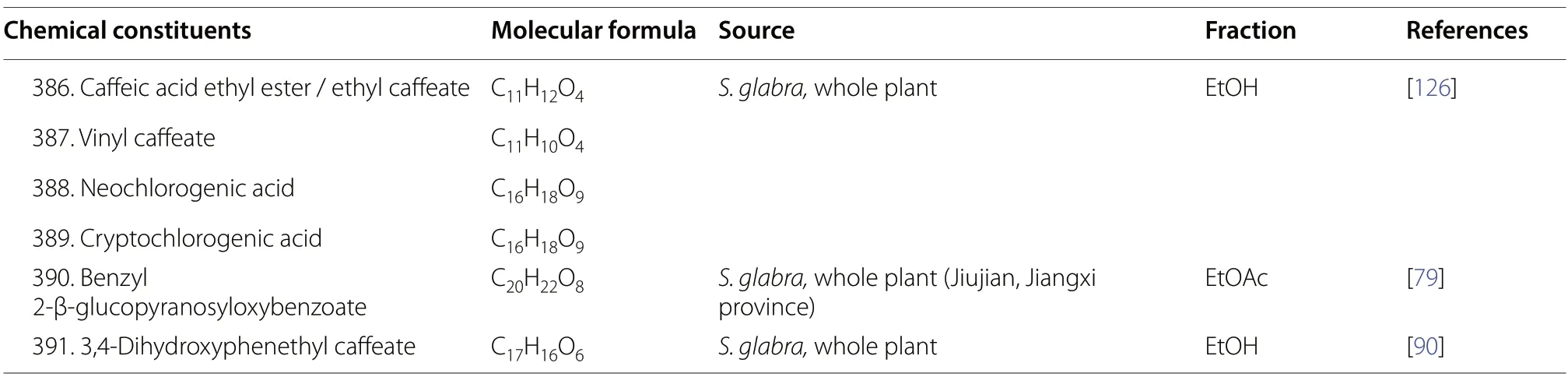
Table 17 (continued)

Fig. 19 Anthraquinones (344-348)
3.5 Biosynthesis of sarglalactones A-H (101-108)

Fig. 20 Organic acids (349-367)

Fig. 21 Organic esters (368-391)
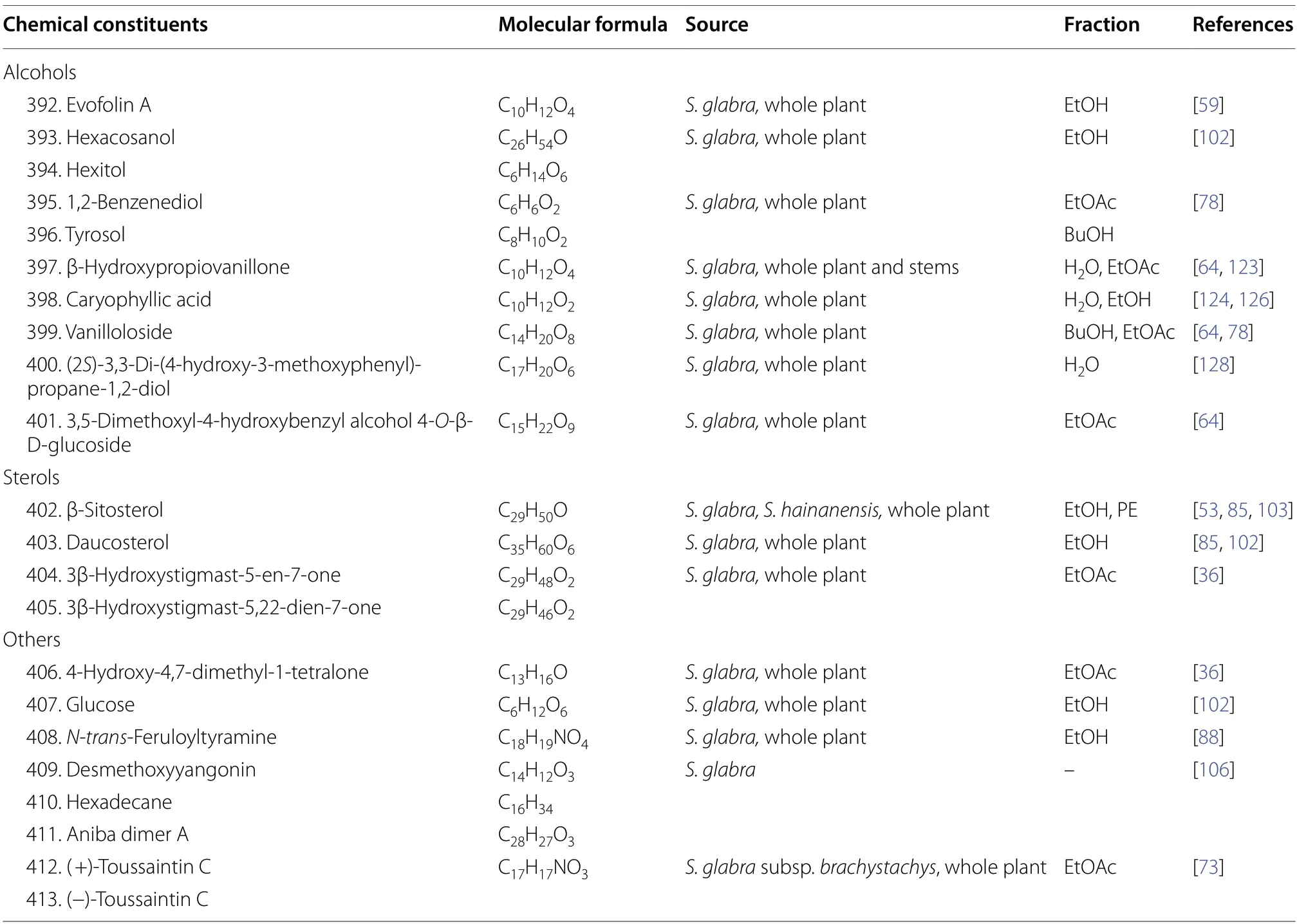
Table 18 Alcohols,sterols,and other compounds from S. glabra
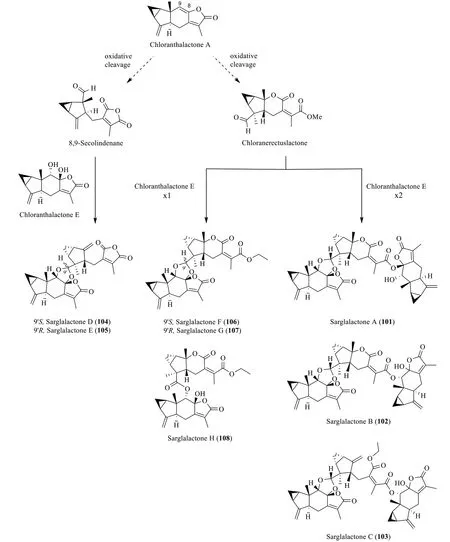
Based on the postulation by Chi et al.[33],the oligomers sarglalactones A-H (101-108) could originate from chloranthalactone A,whose highly conjugated lactone undergoes oxidative cleavage at the Δ8,9double bond to yield two presumptive precursors chloranerectuslactone and 8,9-secolindenane (Scheme 6).The skeleton of the trimers101-103arises from the condensation between a chloranerectuslactone core and two units of chloranthalactone E through acetalisation and transesterification,while the dimers106-108are assembled in the same manner,except that they lack a second unit of chloranthalactone E.On the other hand,epimers104and105are linked by the monomers,8,9-secolindenane and chloranthalactone E,through a cyclic acetal moiety.
3.6 Biosynthesis of sarcaglabrin A (109)and 7'-oxysarcaglabrin A (143)
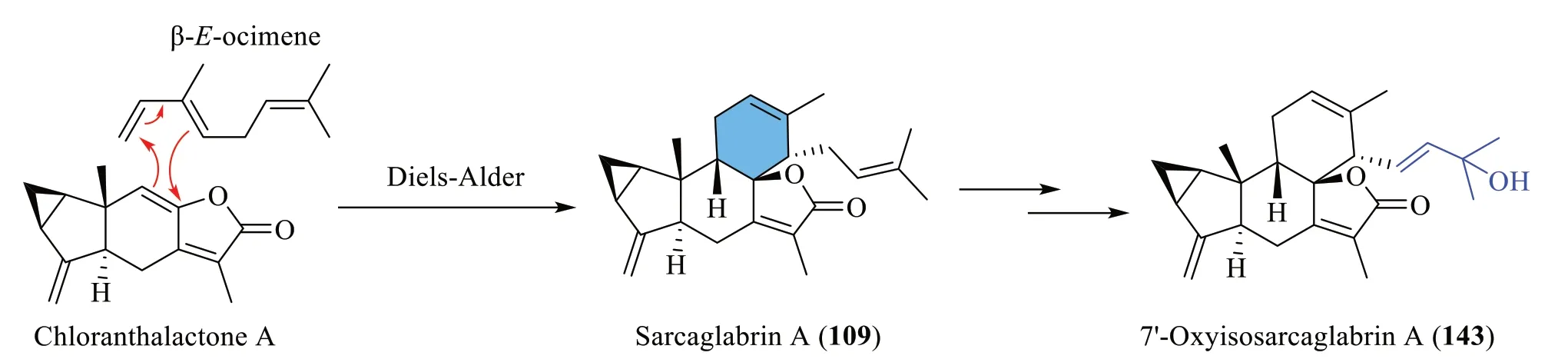
Sarcaglabrin A (109) was presumed to be the Diels-Alder adduct of chloranthalactone A and a naturally occurring geranyl diphosphate (GDP)-derived monoterpene,β-Eocimene (C10) [4].On the other hand,the hydroxylated analogue143isolated by Sun et al.[40] was deduced to adopt a similar biosynthetic route (Scheme 7).
3.7 Biosynthesis of sarglaromatics A-E (123-127)
According to Sun et al.[41],the unprecedented skeletons of sarglaromatics A-E (123-127) were presumed to derive from chlorahololide D (80) and sarcandrolide A (69),two abundant [4+2] lindenane sesquiterpenoid dimers fromS.glabra(Scheme 8).The removal of H-6 of the precursors consequently generates a C-11 free radical that performs an intramolecular attack onto C-11' of the lactone ring,thereby forming the key radical intermediate (iv).Subsequently,radical termination could occur in two possible ways.The first pathway (A) involves a decarboxylation process that gives rise to a dimer bearing an aromatic framework,which eventually transforms into sarglaromatics A-C (123-125) by way of a carbocation intermediate (v).Alternatively,the opening of the cyclopropane ring in the carbocation intermediate leads to the formation of sarglaromatic D (126).In the second pathway (B),the free radical at C-11' is terminated with the formation of an exocyclic bond.By way of a carbocation formation at C-4 (vi),the corresponding dimer,sarglaromatic E (127),is formed.

Fig. 22 Alcohols (392-401) and sterols (402-405)
3.8 Biosynthesis of sarglafuran A (131)
According to Wang et al.[43],sarglafuran A (131) was proposed to be biosynthesised through the hydroxylation of the C-13 methyl group in sarglabolide C (89),followed by an intramolecular nucleophilic addition to form a hemiketal intermediate.Finally,dehydration at C-8 furnishes the characteristic furan moiety in131(Scheme 9).
3.9 Biosynthesis of sarglaoxolanes A-C (144-146)
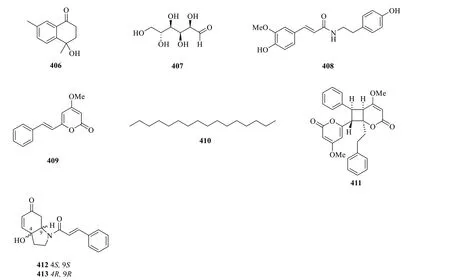
Fig. 23 Other compounds (406-413)

Scheme 1 Pyrolysis of shizukaol A and the biosynthetic hypothesis of dimeric lindenanes [130]

Scheme 2 Proposed tautomerisation of lindenatriene and iso-lindenatriene
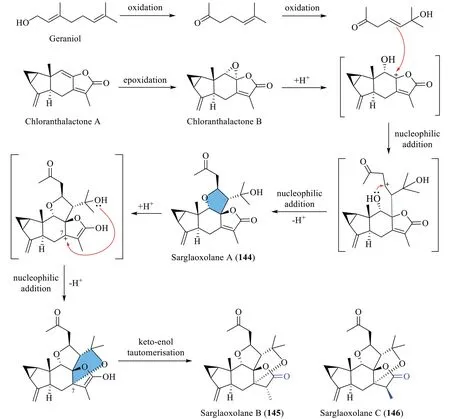
A possible biogenetic and transformational pathway of sarglaoxolanes A-C (144-146) is presented in Scheme 10[45].The biosynthesis commences with the epoxidation and oxidation of the precursors chloranthalactone A and geraniol,which results in a carbocation intermediate and a tertiary alcohol,respectively.Subsequently,the skeleton of heterodimer144is constructed through two consecutive nucleophilic additions.Meanwhile,epimers145and146could be spontaneously formed from144by nucleophilic addition of the tertiary alcohol to the carbocation C-7,followed by tautomerisation.
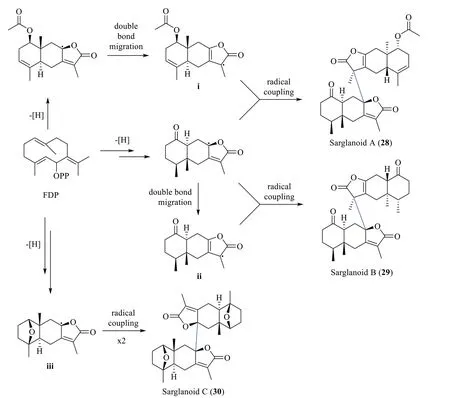
Scheme 3 The biosynthetic route of compounds 28-30
3.10 Biosynthesis of glabralides A-F (216-221)
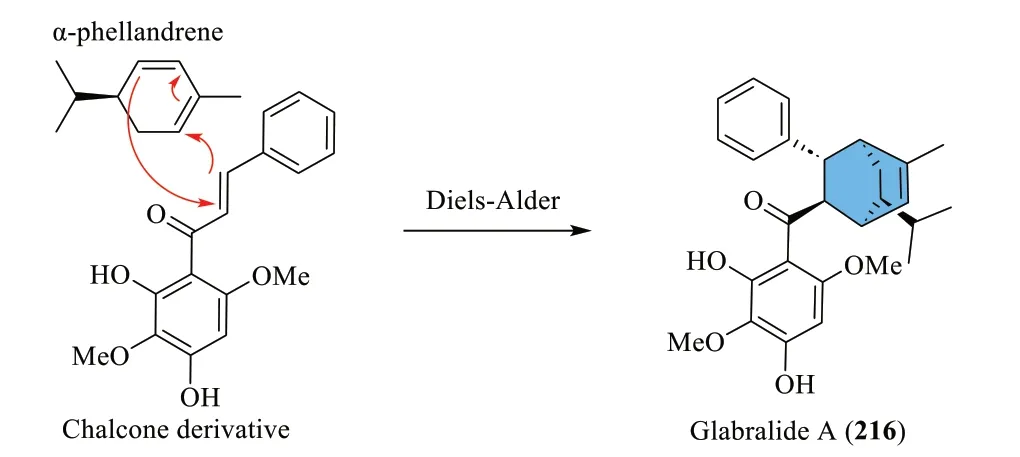
Yang et al.[72] proposed that the scaffold of glabralide A (216) is constructed via an enzyme-catalysed Diels-Alder reaction between α-phellandrene and a chalcone derivative (Scheme 11).On the other hand,glabralides B-F (217-221) were postulated to adopt a divergent route featuring a geranylated phenylacetic intermediate(Scheme 12).Oxidation of the phenylacetic intermediate forms a conjugated ketone moiety,which then undergoes radical lactonisation to give a lactone radical.Successive radical addition involving 2,2,3-trimethyloxirane,followed by an acetaldehyde elimination step eventually gives glabralide B (217).From the same phenylacetic intermediate,an alternative radical intermediate is formed through methyl esterification and oxidation,which,by radical addition and oxidation produces a tertiary carbocation intermediate.Subsequent cationic cyclisation and oxidation then give rise to a fused 6/6/6 ring system exhibited by glabralide C (218).Ultimately,hydroxylation of the ring system or the geranyl moiety would result in the formation of glabralides D-F(219-221).
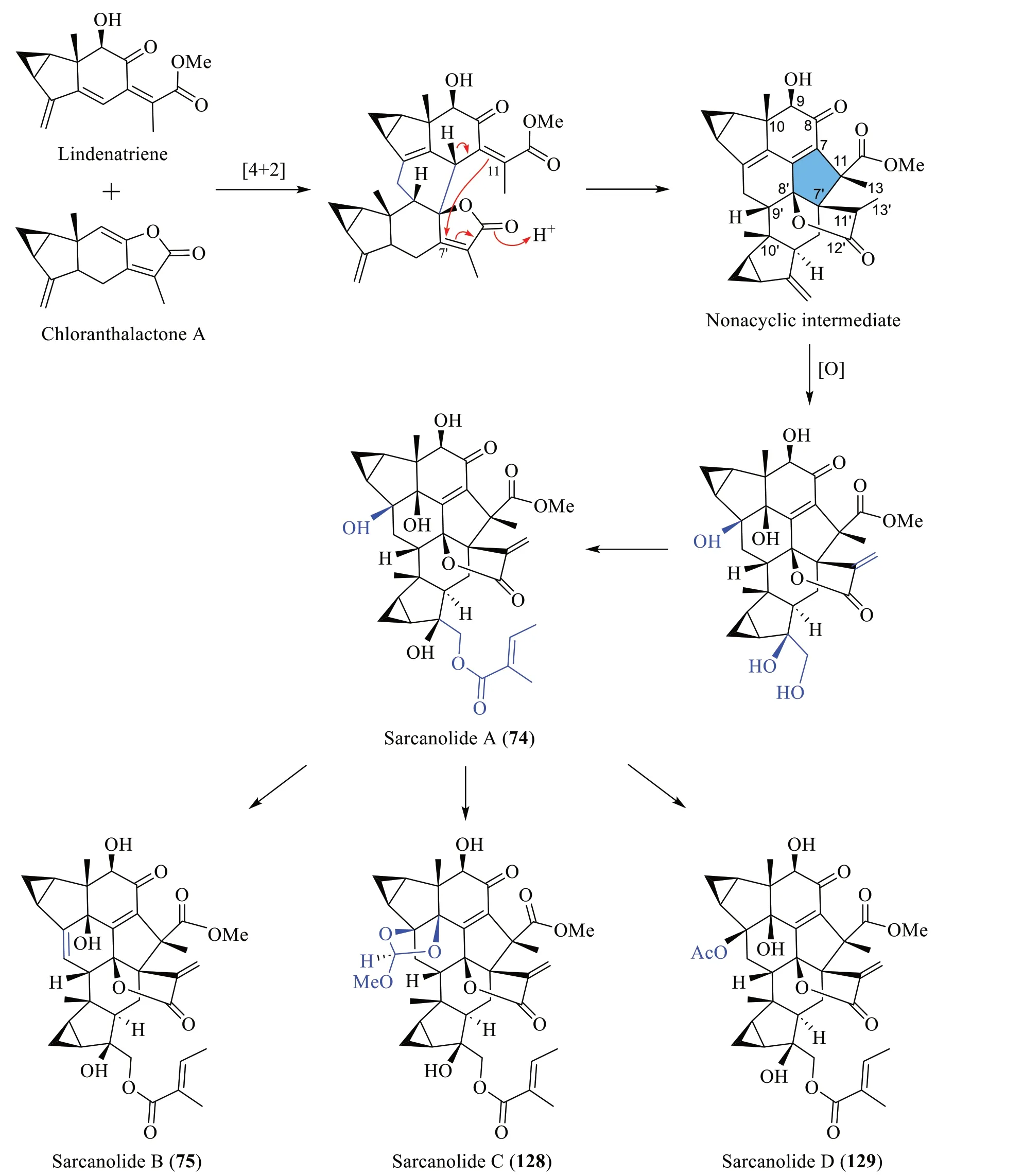
Scheme 4 The proposed biosynthetic route of compounds 74,75,128 and 129
3.11 Biosynthesis of sarglamides A-E (224-228)
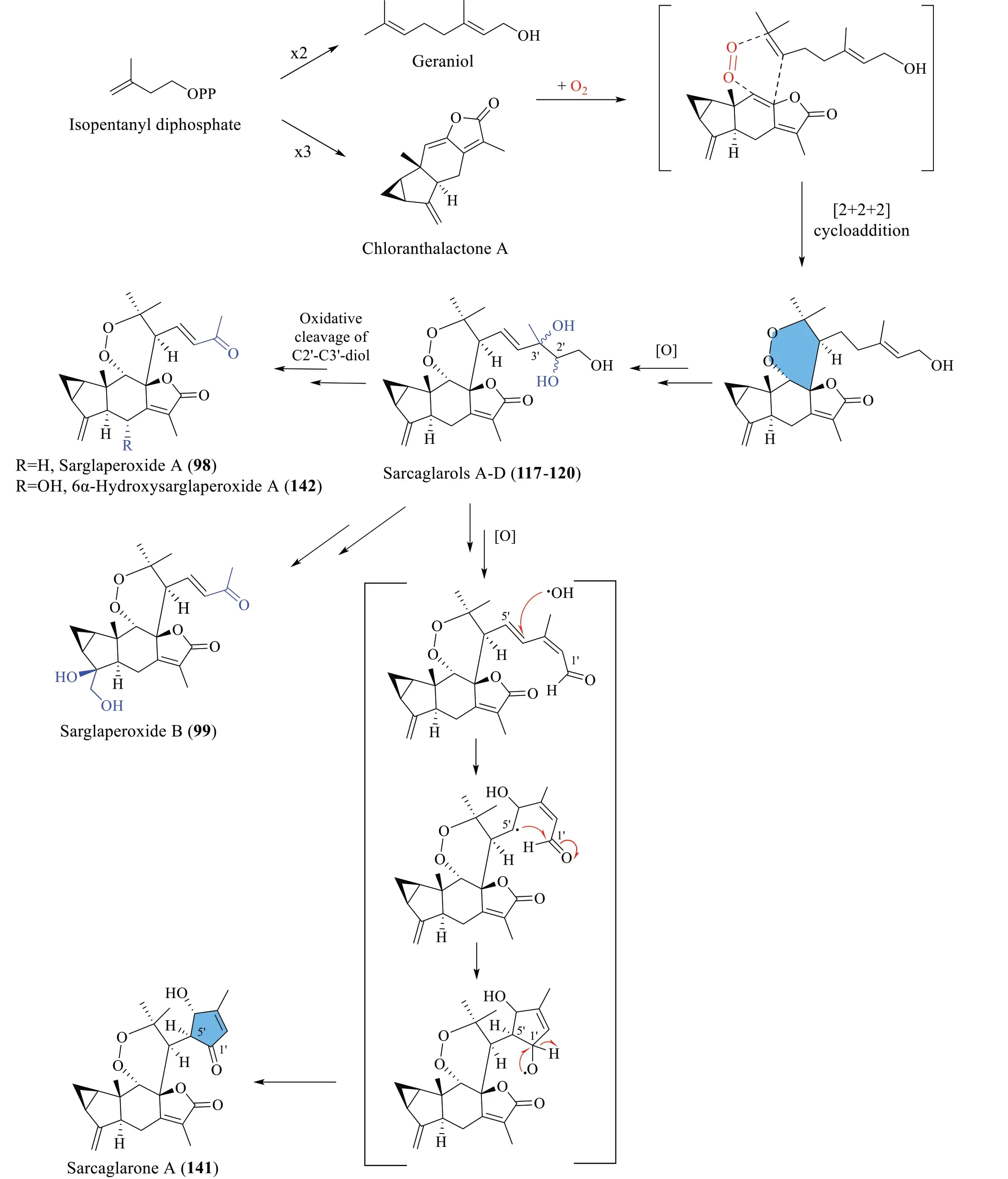
Scheme 5 The proposed biosynthetic route of compounds 98-99,117-120,141 and 142
Scheme 6 The proposed biosynthetic route of compounds 101-108
Scheme 7 The proposed biosynthetic route of compounds 109 and 143
The indolidinoid-monoterpene conjugates,sarglamides A-E (224-228),were proposed to derive from the precursors (S)-α-phellandrene and toussaintine C(Scheme 13) [73].The biosynthesis of sarglamides A and B (224and225) was assumed to involve a head-toheadendo-Diels Alder between (S)-α-phelladrene and(+)-toussaintine C.The two regioisomers differ in the approach direction of the (S)-α-phellandrene dieneophile during the reaction,with the isopropyl group assuming an α orientation for224and a β orientation for225.Conversely,sarglamide C (226) was postulated to form via anendo-Diels Alder between (S)-α-phellandrene and(-)-toussaintine C in a head-to-tail manner.Sarglamide D(227) could be derived from226via acetalisation at the C-7 carbonyl,followed by an electrophilic addition that links the C-7 oxygen to C-1″.On the other hand,an acidcatalysed electrophilic addition of the C-4 hydroxyl to the Δ1'',2''alkene in226would give rise to228.
3.12 Biogenetic relationship among various terpenoid skeletons of S. glabra
A biogenetic pathway linking the various terpenoid skeletons ofS.glabrais depicted in Scheme 14.The terpenoid constituents ofS.glabraare produced via a common mevalonate pathway.Within this pathway,geranyl diphosphate (GDP) and farnesyl diphosphate (FDP)serve as pivotal intermediates,directing the synthesis of monoterpenoids and sesquiterpenoids,respectively.Cyclisation of GDP yields the monoterpene α-phellandrene,a precursor of meroterpenoids fromS.glabra,e.g.,glabralides A-F (216-221) and sarglamides A-E (224-228).
On the other hand,the addition of an isopentenyl diphosphate (IDP) unit to GDP forms FDP,an acyclic precursor of different classes of cyclic sesquiterpenes.The process involves the formation of the highly reactive farnesyl carbocation intermediate,which undergoes cyclisation and rearrangements to construct the diverse ring systems exhibited by germacrane,elemane,guaiane,eudesmane,eremophilane and cadinene-type sesquiterpenoids [134].
Alternatively,lindenane-type sesquiterpenoids may be synthesised from FDP through isofuranodiene,a naturally occurring constituent discovered in plants of the Chloranthaceae family [135].Intramolecular cyclopropanation of isofuranodiene would generate lindenene,the biosynthetic precursor from which shizukanolide A and lindenatriene are derived.Through Michael addition and Diels-Alder reaction,lindenatriene could oligomerise into the trimer trishizukaol A (122),whereas modifications and functionalisation of shizukanolide A (34) at C-4,C-8,C-9,and C-15 enables its conversion into various lindenane-type sesquiterpenoids,including chloranthalactone A (31).
Chloranthalactone A (31) is a lindenane-type sesquiterpenoid with a highly unsaturated skeleton that exhibits versatility in undergoing dimerisation or oligomerisation via diverse pathways.The resulting array of dimeric and oligomeric sesquiterpenoids varies based on the number and positions of double bonds engaged in the reaction.For example,[2+2] and [6+6] cycloadditions between two units of chloranthalactone A (31) lead to the formation of the homodimers chloranthalactone F (61) and cycloshizukaol A (67),respectively.
Additionally,chloranthalactone A assumes a pivotal role as a building block in the biosynthesis of the[4+2]-type dimeric lindenanes,the chemotaxonomic constituents ofS.glabra.The basic skeleton of this class of compounds is depicted by shizukaol A (100),a cycloadduct of chloranthalactone A and lindenatriene.Through subsequent transformations such as acetylation,esterification,oxidation,glycosylation,epoxidation,and lactonisation,shizukaol A can be transformed into various [4+2]-type congeners.Chloranthalactone A is also the putative precursor of a series of sesquiterpene-normonoterpene heterodimers,exemplified by compounds98,99,141,142,117-120,and144-146.The biosynthetic pathway occurs through [2+2+2]cycloaddition or nucleophilic addition reactions with geraniol.On the other hand,the conjugation of chloranthalactone A with a different monoterpene moiety,β-E-ocimene,would yield compounds109and143.The conjugation of chloranthalactone A derivatives with chloranthalactone E through an atypical acetalisation and esterification process would result in the formation of 8,9-secolindenane-derived dimers and oligomers (101-108).

Scheme 8 The proposed biosynthetic route of compounds 123-127

Scheme 9 The proposed biosynthetic route of compound 131
4 Biological activities
The multifarious biological activities ofS.glabrarevealed by modern pharmacological studies include antioxidative,antibacterial,antifungal,antiviral,antimalarial,anti-thrombocytopenic,antitumour,antiinflammatory,immunomodulatory,hepatoprotective effects,etc.A compilation of the reported bioactivities exhibited by plant extracts,medicinal formulations,and isolates fromS.glabracan be found in Table 19.
4.1 Antioxidative
The antioxidative properties ofS.glabraextract (SGE)have been widely investigated in several animal models.Preliminary and in vivo studies reported that SGE can ameliorate radiation-induced reactive oxygen species (ROS) injury and aid post-radiation recovery in guinea pig and rat models [136-138],besides moderating stress-attenuated immune response in restrained mice [139].In miniature pigs,S.glabrapowder exhibited prominent scavenging activities against radiationinduced ROS in the parotid gland [140].
The antioxidant properties of SGE exhibited concentration-dependent behaviour,as demonstrated by in vitro free radical scavenging assays [141,142].The most pronounced antioxidant efficacy was observed in the 75% ethanolic stem extract and 95% ethanolic leaf extract as determined by a comprehensive assessment employing 2,2-diphenyl-1-picrylhydrazyl (DPPH),2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)(ABTS),and ferric reducing antioxidant power (FRAP)assays [143].The antioxidative potential ofS.glabracould be ascribable to the presence of phytochemical constituents such as phenolic acids,flavonoids,and polysaccharides,which possess intrinsic redox properties that help stabilise ROS due to their structural conformations [125,144-146].For example,the primary polyphenols and flavonoids ofS.glabra,namely rosmarinic acid (369) and astilbin (335),possess the ability to scavenge ROS via hydrogen/electron transfer or Fe2+chelation [147].Rosmarinic acid (369) was also found to attenuate ROS by regulating PGC1-a/NOX4 signalling [118].
Scheme 10 The proposed biosynthetic route of compounds 144-146
Scheme 11 The biosynthetic route of compound 216
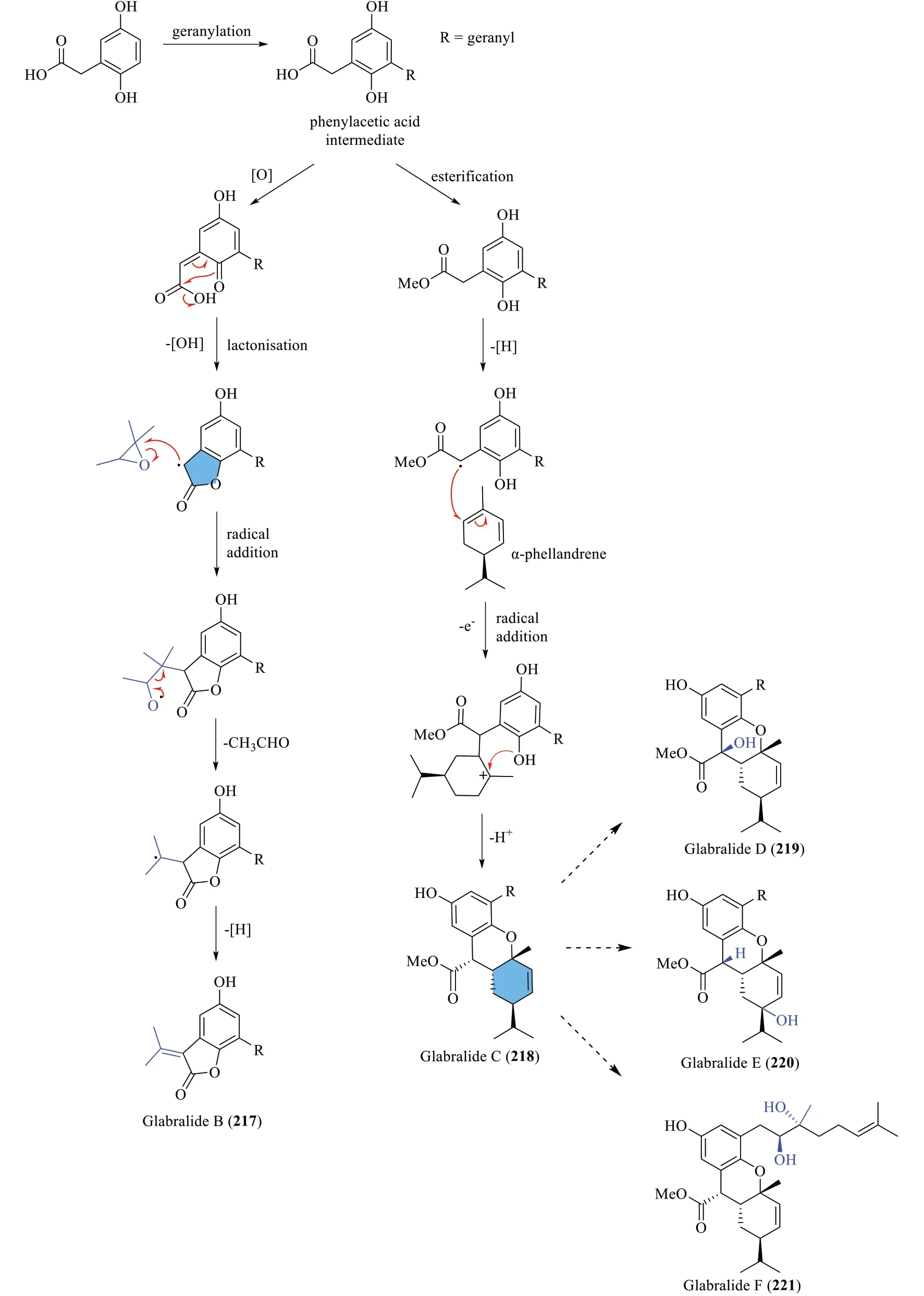
Scheme 12 The biosynthetic route of compounds 217-221

Scheme 13 The biosynthetic route of compounds 224-228
It was also established that the phenolic-rich ethyl acetate fraction of SGE manifested a strong antioxidative activity (IC50=6.84 ± 0.45 μg/mL) and displayed neuroprotective effects by enhancing cholinergic signalling in cognitive deficit mice [142].In a bioactive structural basis study,it was unveiled that the antioxidant efficacy of proteoglycans fromS.glabrawas attributed to the presence of monosaccharides,namely xylose,glucosamine,and glucuronic acid [148].
4.2 Antibacterial,antifungal and antiviral

Scheme 14 The biogenetic relationship among various terpenoid skeletons of S. glabra
Preliminary studies on SGE have shown promising results regarding its antimicrobial activity,among which ethyl acetate and n-butanol fractions in particular exhibited excellent inhibition against pathogenic bacterial strains in the disk diffusion test [87,149].The n-butanol extract ofS.glabrawas also recently reported to show minimum inhibitory concentrations of 15 mg/mL and 10 mg/mL againstStaphylococcus aureusandEscherichiacoli,respectively [150].In another study,sarglaperoxide A (98),an isolate from the seeds ofS.glabra,displayed 64.5% inhibition againstS.aureusat a concentration of 25 μg/mL [38].In addition to demonstrating bacteriostatic effects againstPropionibacterium acnes[151],the ethanolic extract ofS.glabradisplayed notable antifungal activities againstPhomopsis mangiferaeat 2.5 mg/mL.Over a period of seven days post-treatment,the maximum inhibition rate reached a notable 50.29% [152].
The antiviral properties of SGE have also been wellcharacterised and extensively studied,particularly on influenza viruses [80,153-157].Rosmarinic acid 4-O-β-D-glucoside (382) was reported to mitigate pulmonary oedema and post-influenza infection by impeding virus proliferation [154],while eleutheroside B1(232) displayed broad-spectrum antiviral activities in vitro,with IC50ranging from 64 to 125 μg/mL [155].Subsequent investigations into the structure-activity relationship of eleutheroside B1(232) confirmed the significance of its aglycone moiety in mediating the observed viral nucleic acid suppression.Jin et al.[80] reported that isofraxidin(230) effectively inhibited platelet aggregation through the PI3K/AKT and mitogen-activated protein kinase(MAPK) pathways,thereby alleviating lung inflammation induced by influenza A.In the work by Pan et al.[158],SGE was found to inhibit HIV-1 protease and cathepsin L with IC50values ranging from 0.003 to 0.07 mg/mL and 0.11 to 0.26 mg/mL,respectively.Notably,chlorogenic acid (377),a prominent constituent of the extract,displayed the most potent inhibitory activity against the two viral proteases.The unique dual-inhibitory function of SGE and its active components against viral proteases could be useful in discovering lead compounds for developing new antiviral agents.
4.3 Antimalarial
An assessment of antimalarial properties has been conducted on several compounds derived fromS.glabra[44].Among the compounds,13'-O-methylsuccinylshizukaol C (136) exhibited remarkable efficacy against chloroquine-resistantPlasmodium falciparum,with an EC50value of 4.3 pM.This potency surpassed that of artemisinin by approximately 1,000-fold.Furthermore,the compound demonstrated exceptional selectivity against the malaria parasite,as confirmed through a toxicity evaluation (IC50=39.0 ± 1.3 μM) conducted on a mammalian embryonic cell line.
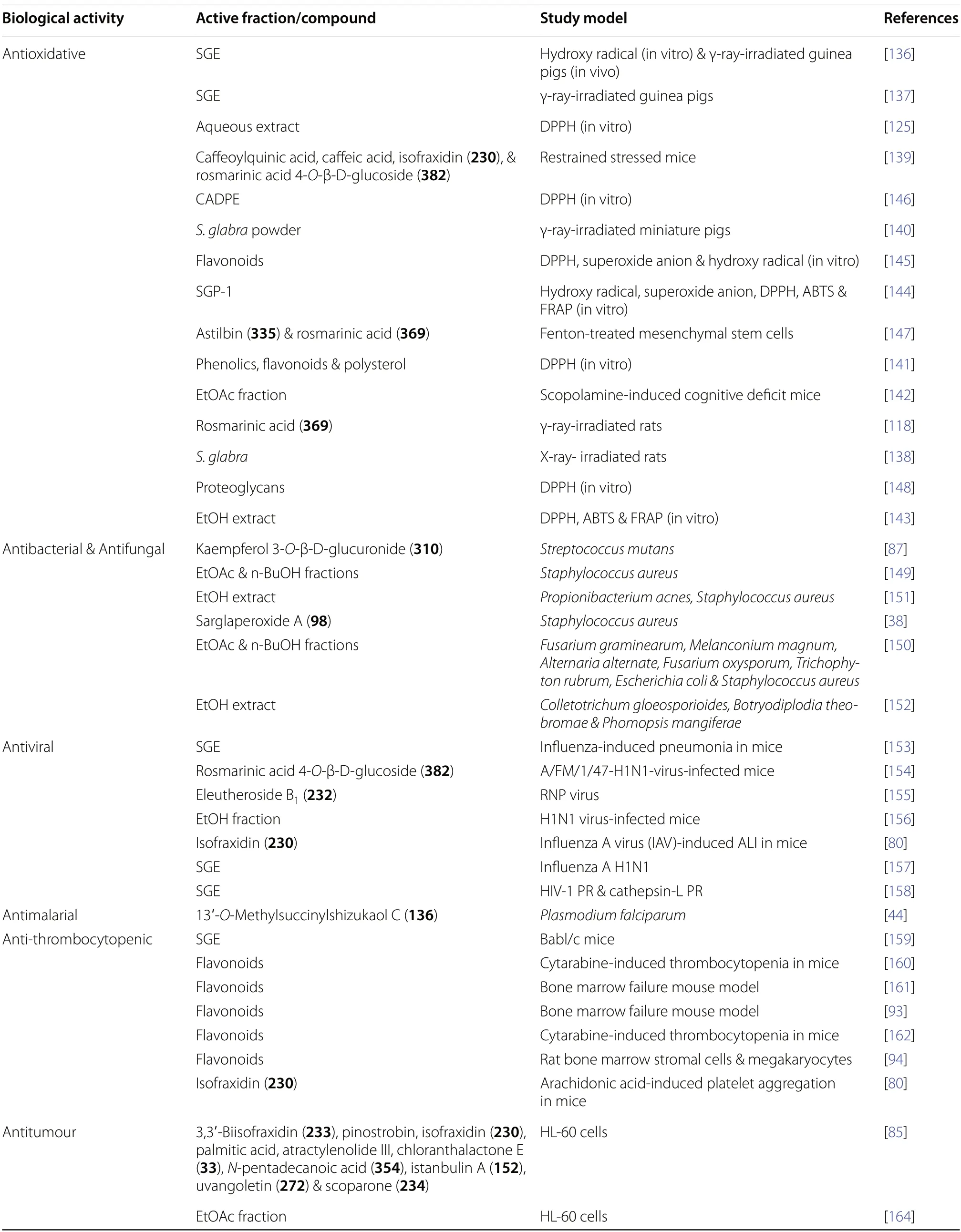
Table 19 Biological activities of S. glabra
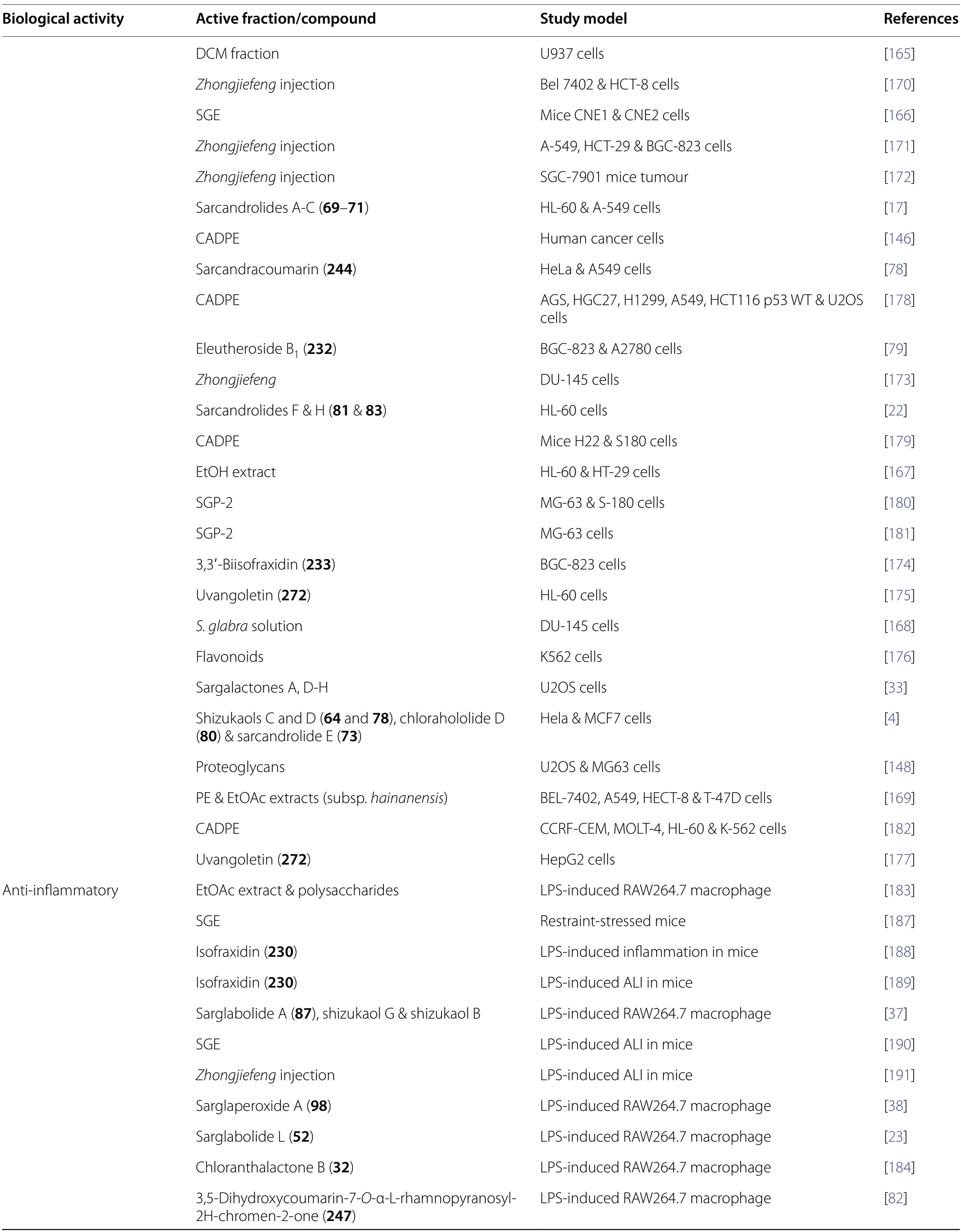
Table 19 (continued)
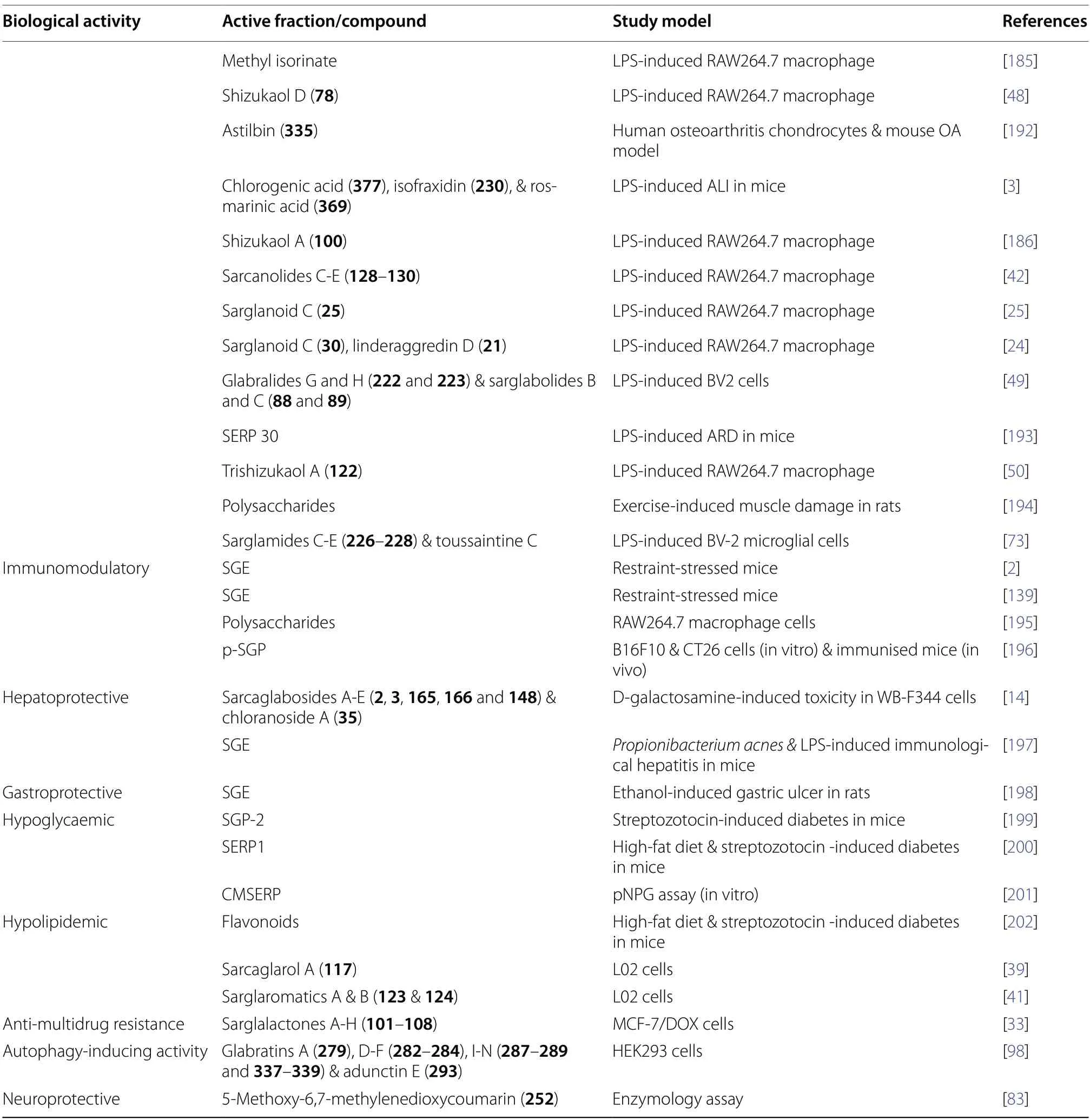
Table 19 (continued)
4.4 Anti-thrombocytopenic
The anti-thrombocytopenic properties of SGE have been extensively studied on mice models [80,93,94,159-163].It was unveiled that SGE promoted platelet activation and prevented platelet apoptosis via the mitochondrial pathway.Its effective constituents,specifically flavonoids,played a role in regulating mitochondrial transmembrane potential,externalisation of phosphatidylserine,and expressions of pro-apoptotic markers on circulating thrombocytes [93,161].At 63 mg/kg and 94.5 mg/kg,S.glabraflavonoids significantly increased the number of peripheral platelets and the polyploid ratio of megakaryocytes (p < 0.01)in thrombocytopenic mice [162].The mechanism was associated with the elevation of thrombopoietin levels,which triggered megakaryocyte differentiation via the TPO-C-mpl pathway [162,163].
4.5 Antitumour
S.glabrahas gained recognition for its significant cytotoxic effects.Extensive in vitro and in vivo investigations have been conducted over the past decades on various cell lines,focusing on plant extracts [164-169],medicinal formulations [170-173],and chemical constituents.The main cytotoxic chemical components identified fromS.glabrainclude sesquiterpenoids [4,17,22,33],coumarins[78,79,85,174],flavonoids [175-177],and polysaccharides [146,148,178-182].
A number of isolates were reported to exhibit selective and potent activities against a panel of cell lines.For example,sarcandrolides A-C (69-71) significantly inhibited HL-60 leukocyte cell line with IC50< 10 μM [17],whereas sarcandracoumarin (244) demonstrated moderate activity against human cervical carcinoma (HeLa)with an IC50value of 49.3 μg/mL [78].In a combination therapy,sarglalactones A-H (101-108) and doxorubicin exhibited exceptional synergistic cytotoxic effects on human osteosarcoma epithelial cells (U2OS) [33].
Caffeic acid 3,4-dihydroxyphenethylester (CADPE),a natural polyphenol derived from the aqueous extract ofS.glabra,exhibits potent anticancer attributes.Its proposed mode of action involves the initiation of tumour senescence via the Twist1-mediated signalling pathway[178].In a pharmacokinetic investigation,CADPE rapidly underwent hydrolysis into its anticancer metabolites,hydroxytyrosol and caffeic acid [179].Additionally,CADPE regulated glycogen synthase kinase-3β (GSK3β),prompting the ubiquitin-dependent degradation of the proto-oncogene c-Myc [182].Consequently,this modulates cell cycle regulators and anti-apoptotic proteins,resulting in tumour cell cycle arrest and apoptosis.
Malignant tumours frequently exhibit increased expression of eukaryotic initiation factor 4F (eIF4F),a protein primarily modulated by mitogen-activated protein kinase (MAPK).An acidic polysaccharide derived fromS.glabra(SGP-2) employs this mechanism to trigger a MAPK-mediated intrinsic apoptosis pathway,effectively impeding tumour growth in both human and murine models [180].Furthermore,the polysaccharide demonstrates excellent anti-proliferative effects on human osteosarcoma MG-63 cells,achieved through regulation of apoptotic cell population and activation of caspase-3 [181].
Validated through an in vivo xenograft assay,a preliminary study by Zheng et al.[175] validated the antitumour effects of uvangoletin (272) on HL60 cells.The proposed mechanism involves the interaction between mitochondria-mediated apoptotic proteins,leading to cytochrome C release into the cytosol and subsequent activation of apoptosis-executing caspases.Building upon this finding,Shen et al.[177] reported the promising cytotoxic activities of uvangoletin (272) on hepatocellular carcinoma cells (HepG2).This was evidenced by detected autophagy and apoptosis both in vivo and in vitro,coupled with metastasis suppression.The likely underlying mechanism involves the modulation of MAPK,AKT/mTOR,and TGF-β/smad2 signalling pathways.
4.6 Anti-inflammatory
S.glabrais renowned for its remarkable anti-inflammatory activities,substantiated by multiple studies reporting its bioactivities in vitro [23-25,37,38,42,48-50,73,82,183-186] and in vivo [3,187-194].The anti-inflammatory properties were found to be imparted by the presence of polysaccharides [183,193,194],phenolics [3,185],coumarins [3,82,188,189],and flavonoids [192].
Among the compounds identified fromS.glabra,lindenane-type sesquiterpenoids exhibit prominent potential as anti-inflammatory agents,with extensive studies conducted on their effectiveness against lipopolysaccharide(LPS)-induced RAW264.7 macrophage [23-25,37,38,42,48-50,73,184,186].Three newly characterised dimeric lindenanes,namely sarcanolides C-E (128-130),showed greater inhibition of LPS-induced nitric oxide (NO) production compared to the positive control (L-NMMA) at a concentration of 25 μM.The observed IC50values for these dimers ranged from 13.4 to 17.2 μM [42].Additionally,sarglanoid C (25) was recently revealed to display anti-inflammatory effects on LPS-induced RAW 264.7 cells.The IC50value recorded was 20.00 ± 1.30 μM,indicating a twofold potency compared to L-NMMA(IC50=41.40 ± 2.30 μM) [25].
In another study,Li et al.[24] reported the promising anti-inflammatory properties of linderaggredin D (21) and sarglanoid C (30).Their IC50values were 25.7 ± 0.2 μM and 11.5 ± 0.3 μM,respectively,which were comparable to that of the positive control,dexamethasone (IC50=9.3 ± 0.2 μM).Bioinformatics and transcription factor analysis revealed that the anti-inflammatory activity of linderaggredin D (21) was associated with multiple pathways related to transcription factor NF-κB,a key component in inflammation progression.
A neuroinflammatory assay utilising the Griess reaction was employed to evaluate the anti-neuroinflammatory potential of meroterpenoids and sesquiterpenoid dimers derived fromS.glabra[49].These compounds exhibited significant inhibitory effects comparable to dexamethasone at concentrations < 5 μM.Utilising protein-protein interaction (PPI) network analysis and molecular docking,the anti-inflammatory mechanisms of the meroterpenoids glabralides G and H (222and223)were predicted.The PPI analysis indicated a prominent interaction with Hsp90AA1,a heat shock protein associated with neuroinflammation.
Regarding mechanistic action,Wei et al.[48] proposed that shizukaol D (78) elicits its anti-inflammatory effects by activating the AKT/Nrf2/HO-1 signalling cascade,which subsequently enhances the activity of antioxidant enzymes such as superoxide dismutase (SOD),glutathione (GSH),and glutathione peroxidase (GSH-px).Additionally,shizukaol A’s potent inhibitory effect on NO(IC50=13.79 ± 1.11 μM) was attributed to its capacity to trigger antioxidant genes and regulate oxidative stress via the HMGB1/Nrf2/HO-1 pathway [186].
The anti-inflammatory activities of aS.glabrapolysaccharide,SERP-30,were reported for the first time by Feng et al.[193].Corroborated by western blotting results,the postulated mechanism of SERP 30 involves the inhibition of LPS-induced phosphorylation of p38 and p65 via NF-κB and MAPK signalling pathways,leading to the protection of endothelial glycocalyx in LPS induced-acute respiratory distress syndrome (ARDS) in mice.In addition,the polysaccharides sourced fromS.glabraexhibited the capacity to mitigate muscular injury in rats subjected to prolonged high-intensity exercise.This effect was achieved by elevating the levels of recuperative enzymes and cytokines such as creatine kinase(CK),lactate dehydrogenase (LDH),and tumour necrosis factor (TNF-α) [194].
The anti-neuroinflammatory activities of three indolidinoid-monoterpene compounds isolated fromS.glabra,sarglamides C-E (226-228),along with toussaintine C were recently reported [73].These compounds were evaluated for their impact on NO production in BV-2 microglial cells induced by LPS.Notably,sarglamides C-E (226-228) exhibited considerable inhibitory activities (> 30%) at concentrations < 20 μM,while showing no cytotoxicity on BV-2 cells (cell viability > 80%).On the other hand,the enantiomers of toussaintine C,namely(+)-toussaintine C (412) and (-)-toussaintine C (413),displayed similar effects at 5 μM.
4.7 Immunomodulatory
As evidenced by multiple studies [2,139],SGE was found to enhance immunity by mediating immune response and balancing the proportion of lymphocytes in restraint mice models.Comparable immunoprotective effects were also observed inS.glabra-derived polysaccharides,wherein increased expression of cell surface molecules and immune factors (IL-1β,IL-10,and iNOS) was observed in RAW 264.7 cells [195].An acidic polysaccharide purified fromS.glabra(p-SGP) was found to stimulate anti-tumour immune responses,making it an ideal candidate as a tumour vaccine adjuvant.The mechanism involves the upregulation of delta-like ligand 4 (DLL4)gene,which in turn activates the differentiation and maturation of T-helper cells and dendritic cells [196].
4.8 Hepatoprotective
A group of sesquiterpene glycosides,namely sarcaglabosides A-E (2,3,165,166and148),together with chloranoside A (35) exhibited notable inhibitory activities in vitro at a concentration of 10-4M [14].Among them,sarcaglaboside B (3) exhibited the most pronounced hepatoprotective effect,displaying an inhibition rate of 65.9%.Furthermore,Li et al.[197] reported that SGE exerted hepatoprotective effects on mice models by inhibiting the activities of alanine aminotransferase level(ALT) and leukotriene B.The inhibitory rates were 78.5%,70.3%,and 55.1% at concentrations of 500,250,and 125 mg/kg,respectively.
4.9 Gastroprotective
Based on a histopathological examination and conjoint metabolomics and network analysis,SGE was also found to associate with multiple signalling pathways related to gastric cell inflammation,metabolism,apoptosis,and differentiation [198].The extract exerted profound protective effects on the gastric mucosa of rat models by alleviating oxidative stress,promoting antioxidant activity,and inhibiting the expression of inflammatory factors.
4.10 Hypoglycaemic and hypolipidemic
It was reported that SGP-2 and SERP 1,two proteoglycans isolated fromS.glabra,demonstrated remarkable α-glucosidase inhibitory activities,with IC50values of 87.06 ± 11.76 μg/mL and 49.01 μg/mL,respectively [199,200].These polysaccharides mitigated insulin resistance,improved lipid metabolism,and attenuated oxidative stress under hyperglycaemic conditions through α-glucosidase inhibition.Similarly,CMSERP,a carboxymethylated polysaccharide fromS.glabra,displayed substantial hypoglycaemic effects,achieving a maximum inhibition of 83.38% ± 2.30% at 1000 μg/mL concentration [201].
In addition to flavonoids [202],sesquiterpene dimers fromS.glabrawere discovered to possess notable hypolipidemic effects.Sarcaglarol A (117) reduced lipid droplets in L02 cells as indicated by oil red O staining,while sarglaromatics A and B (123and124) effectively mitigated lipid accumulation in L02 cells exposed to free fatty acids [39,41].The inhibitory potential of the dimers was postulated to be amplified by the free hydroxyl groups present in their structures,suggesting potential application in treating non-alcoholic steatohepatitis.
4.11 Other biological activities
Apart from the mentioned biological activities,S.glabraand its chemical constituents were evaluated for their anti-multidrug resistance,neuroprotective,and autophagy-inducing activities.Chi et al.[33] described the anti-multidrug resistance against MCG-7/DOX cells displayed by several sesquiterpenoids isolated from the leaves ofS.glabra,whose reversal fold values ranged from 11.8 to 129.2.
Du et al.[83] recently disclosed the acetylcholinesterase (AchE) inhibiting activity of coumarins fromS.glabra.Notably,5-methoxy-6,7-methylenedioxycoumarin (252) exhibited significant AchE inhibitory effi-cacy (IC50=1.982 ± 0.003 μM),surpassing donepezil(IC50=3.118 ± 0.006 μM).Molecular docking results showed that the main interaction involved hydrogen bonding with two target amino acids,Phe-288 and Arg-289.Additionally,Liu et al.[98] introduced the first report on the autophagy-inducing effects ofS.glabracompounds,including glabratin A (279),glabratins D-F(282-284),glabratins I-N (287-289and337-339),and adunctin E (293).The compounds demonstrated enhanced conversion of the protein light chain LC3-II to its non-lipidated form (LC3-I) in vitro.
5 Conclusions
This paper presents a comprehensive review on the compound isolation,biosynthesis,and pharmacological attributes ofS.glabra.In essence,S.glabrais a highly prolific producer of secondary metabolites,including terpenoids,coumarins,lignans,flavonoids,sterols,anthraquinones,organic acids,and organic esters,several of which hold substantial research value due to their unique chemical structures and extensive range of biological effects.The taxonomical markers of this plant include lindenane-type sesquiterpenoids,characterised by a distinct linear 3/5/6 polycyclic ring and typically formed through a [4+2] Diels-Alder cycloaddition.
Through an analysis of existing literature,several gaps in research have been discerned.In the domains of phytochemistry and pharmacology,certain subspecies ofS.glabraare underexplored.Continued research on these lesser-known variants holds the potential to unveil therapeutic properties or reveal hitherto unprecedented compounds.Despite the wide distribution ofS.glabraacross Asia,the majority of investigations have focused on specimens collected exclusively from China.Exploring specimens from diverse geographical origins would be of interest,facilitating a comprehensive understanding of common and distinct secondary metabolites among these plants.
From a biological activity viewpoint,S.glabraholds promise for further exploration of its pharmacological potential.Future studies are expected to analyse the mechanism of action of the active principles and evaluate the possible synergistic action within SGE.Besides,the establishment of precise analytical methods is crucial to standardise the secondary metabolites present in medicinal preparations likeZhongJieFeng,an herbal extract ofS.glabrawith excellent antitumour and anti-inflammatory activities.Such scientific validation is imperative to ensure the effectiveness and safety of this traditional Chinese medicine,thereby optimising its medicinal utility.
Abbreviations
DCM Dichloromethane
PE Petroleum ether
Acknowledgements
We thank REM Corporation Sdn.Bhd.(Dr Donald Chen) for inspiration and financial support.
Author contributions
JNC wrote and prepared the manuscript.KHL and PK reviewed the manuscript.All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no conflict of interest.
Author details
1School of Pharmacy,University of Nottingham Malaysia,Jalan Broga,43500 Semenyih,Selangor,Malaysia.2Foundation in Science,University of Nottingham Malaysia,Jalan Broga,43500 Semenyih,Selangor,Malaysia.
Received:23 October 2023
Accepted:13 November 2023

猜你喜欢
杂志排行
Natural Products and Bioprospecting的其它文章
- A recent update on development,synthesis methods,properties and application of natural products derived carbon dots
- l-Palmitoylcarnitine potentiates plasmin and tPA to inhibit thrombosis
- Ginsenoside compound-K attenuates OVX-induced osteoporosis via the suppression of RANKL-induced osteoclastogenesis and oxidative stress
- The alkynyl-containing compounds from mushrooms and their biological activities
- Natural product rhynchophylline prevents stress-induced hair graying by preserving melanocyte stem cells via the β2 adrenergic pathway suppression
- Kaemtakols A-D,highly oxidized pimarane diterpenoids with potent anti-inflammatory activity from Kaempferia takensis
