含异恶唑二铁配合物的合成、表征及电催化产氢性能和抗菌活性
2023-12-21刘旭锋李玉龙刘幸海
刘旭锋 李玉龙 刘幸海
(1宁波工程学院材料与化学工程学院,宁波 315211)
(2天津大学浙江研究院,宁波 315201)
(3四川轻化工大学化学与环境工程学院,绿色催化四川省高校重点实验室,自贡 643000)
(4浙江工业大学化工学院,杭州 310014)
[FeFe]-hydrogenases, which are found in natural bacteria, can efficiently catalyze the reduction of protons to evolve dihydrogen as a renewable and clean energy[1]. The structural elucidation of the active site of[FeFe]-hydrogenases was established in 1998[2]and 1999[3], featuring a butterfly di-iron cluster coordinated by carbonyls, cyanides, a bridged three-atom dithiolate, along with a [Fe4S4]-bearing cysteine. Previous studies focused on the biomimics for the bridged threeatom dithiolate and a lot of SCH2CH2CH2S or SCH2N(R)CH2S bearing complexes were prepared[4-9]. Encouraged by the ligands found in the di-iron center of the active site of [FeFe]-hydrogenases, some ligands like cyanides[10-11], phosphines[12-13],N- heterocyclic carbenes(NHC)[14-15]and thioethers[16-17]have been introduced into the di-iron center. Moreover, the electrocatalytic dihydrogen production by some biomimics was also investigated[18-20]. However, research on the SCH2CH2S bearing complexes is more limited and only a few examples of SCH2CH2S bearing complexes have been studied[21-23].
The five-membered heterocycle isoxazole derivatives have attracted great concerns as a result of their wide application in biological activities such as antifungal[24], cytotoxic[25], herbicidal[26], insecticidal[27], and anti-inflammatory[28]. We have previously reported that 1,2,3-thiadiazole[29]or pyrazole[30]containing di-iron complexes displayed some antifungal activities.Inspired by these findings, we recently developed research on the linkage of an isoxazole moiety with the di-iron cluster. Consequently, we have successfully synthesized four derivatives by esterification or CO exchange.Herein, in this paper, we report the synthesis and structural characterization of four isoxazole- containing di-iron complexes, namely [Fe2(CO)5(L)(μ-SCH2CHCH2OOC(5-C3HNOCH3)S)], where L=P(4-C6H4CH3)3(3), P(4 -C6H4F)3(4), P(2-C6H4OCH3)3(5), as the biomimics for the[FeFe]-hydrogenases active site.Moreover,the electrocatalytic properties and the antifungal activity of these complexes are also presented.
1 Experimental
1.1 Materials and methods
Tri(p-tolyl)phosphine, tris(4-fluorophenyl)phosphine,tris(2-methoxyphenyl)phosphine,4-dimethylaminopyridine (DMAP),N,N′-dicyclohexylcarbodiimide(DCC) and Me3NO∙2H2O were available commercially and used as received. Complex [Fe2(CO)6(μ-SCH2CH(CH2OH)S)] (1)[31]and 5-methylisoxazole-4-carboxylic acid[32]were synthesized by the reported methods.FTIR spectra were measured on a Nicolet 6700 FT-IR spectrometer. NMR spectra were obtained by a Bruker Avance 500 MHz spectrometer.Elemental analyses were performed on an Elementar Vario EL cube analyzer.
1.2 Synthesis of complex [Fe2(CO)6( μ-SCH2CH CH2OOC(5-C3HNOCH3)S)](2)
To a mixture of complex 1 (0.201 g, 0.5 mmol),DMAP (0.024 g, 0.2 mmol) and 5-methylisoxazole-4-carboxylic acid (0.076 g, 0.6 mmol) in CH2Cl2(20 mL),DCC (0.124 g, 0.6 mmol) was added. The mixture was stirred overnight. The solvent was evaporated. The crude product was purified by thin layer chromatography (silica gel G) with a mixture of CH2Cl2/petroleum ether (2∶3,V/V) as the eluent to afford complex 2(0.228 g,yield:89%).IR(CH2Cl2,cm-1):νC≡O2 077(s),2 037 (vs), 2 004 (vs), 1 996 (vs);νC=O1 730 (m).1H NMR (500 MHz, CDCl3):δ8.49 (s, 1H, CH=N), 4.21(dd,J=9, 14.5 Hz, 1H, OCH2), 4.12 (dd, J=9.5, 14.2 Hz, 1H, OCH2), 3.03-2.96 (m, 1H, SCH), 2.73 (s, 3H,CH3),2.72 (dd,J=10,16 Hz,1H,SCH2),1.95 (dd,J=7,16.5 Hz, 1H, SCH2). Anal. Calcd. for C14H9Fe2NO9S2(%): C, 32.90; H, 1.78; N, 2.74. Found(%): C, 33.39;H,1.63;N,2.81.
1.3 Synthesis of complexes 3-5
To a mixture of complex 2 (0.053 g, 0.1 mmol)and the corresponding phosphine ligand (0.1 mmol) in CH2Cl2(5 mL), Me3NO·2H2O (0.011 g, 0.1 mmol) in MeCN(5 mL)was slowly added.The mixture was stirred at room temperature for 1 h. The solvent was evaporated. The crude product was purified by thin layer chromatography (silica gel G) with a mixture of CH2Cl2/petroleum ether (1∶1,V/V) as the eluent to afford complexes 3-5.
Complex 3: 0.061 g, yield: 77%. IR (CH2Cl2,cm-1):νC≡O2 046(vs),1 987(vs),1 937(m);νC=O1 730(m).1H NMR (500 MHz, CDCl3):δ8.41 (s, 1H, CH=N), 7.47-7.43 (m, 6H, PhH), 7.18 (d,J=7 Hz, 6H,PhH),3.97-3.89(m,2H,OCH2),2.68(s,3H,CH3),2.36(s, 9H, 3CH3), 1.88-1.85 (m, 1H, SCH), 1.30 (s, 2H,SCH2).31P{1H} NMR (200 MHz, CDCl3, 85% H3PO4):δ60.26 (s). Anal. Calcd. for C34H30Fe2NO8PS2(%): C,51.86; H, 3.84; N, 1.78. Found(%): C, 51.75; H, 3.83;N,1.71.
Complex 4: 0.053 g, yield: 66%. IR (CH2Cl2,cm-1):νC≡O2 050(vs),2 039(m),1 991(vs),1 942(m);νC=O1 732 (m).1H NMR (500 MHz, CDCl3):δ8.42 (s,1H, CH=N), 7.54-7.52 (m, 6H, PhH), 7.14-7.10 (m,6H, PhH), 3.99-3.89 (m, 2H, OCH2), 2.68 (s, 3H, CH3),1.80 (s, 1H, SCH), 1.46-1.44 (m, 2H, SCH2).31P{1H}NMR (200 MHz, CDCl3, 85% H3PO4):δ61.15 (s).Anal. Calcd. for C31H21F3Fe2NO8PS2(%): C, 46.58; H,2.65;N,1.75.Found(%):C,46.85;H,2.78;N,1.79.
Complex 5: 0.058 g, yield: 70%. IR (CH2Cl2,cm-1):νC≡O2 052 (vs), 1 996 (vs), 1 969 (sh), 1 950(sh);νC=O1 728 (m).1H NMR (500 MHz, CDCl3):δ8.39 (s, 1H, CH=N), 7.84 (s, 2H, PhH), 7.39 (s, 4H,PhH), 6.89-6.84 (m, 6H, PhH), 3.92-3.87 (m, 2H,OCH2), 3.74 (s, 2H, SCH2), 3.55 (s, 9H, 3OCH3), 2.66(s, 3H, CH3), 1.21-1.20 (m, 1H, SCH).31P{1H} NMR(200 MHz, CDCl3, 85% H3PO4):δ49.29 (s). Anal.Calcd. for C34H30Fe2NO11PS2(%): C, 48.88; H, 3.62; N,1.68.Found(%):C,49.29;H,3.65;N,1.65.
1.4 X-ray crystallography
The single crystals with suitable sizes were mounted on a Bruker D8 QUEST diffractometer. Data were collected at 296 K by using a graphite-monochromatic with MoKαradiation (λ=0.071 073 nm) in theω-φscan mode. Using OLEX2, the structure was solved by direct methods using the SHELXS program and refined by full-matrix least-squares techniques SHELXL onF2. Hydrogen atoms were located using the geometric method. Non-hydrogen atoms were refined with anisotropic thermal parameters. Note that the largest difference peaks of complexes 2(2 710 e·nm-3)and 5(2 520 e·nm-3) are relatively high because the isoxazole ring is disordered without treatment. Details of crystal data,data collections, and structure refinements are summarized in Table 1.
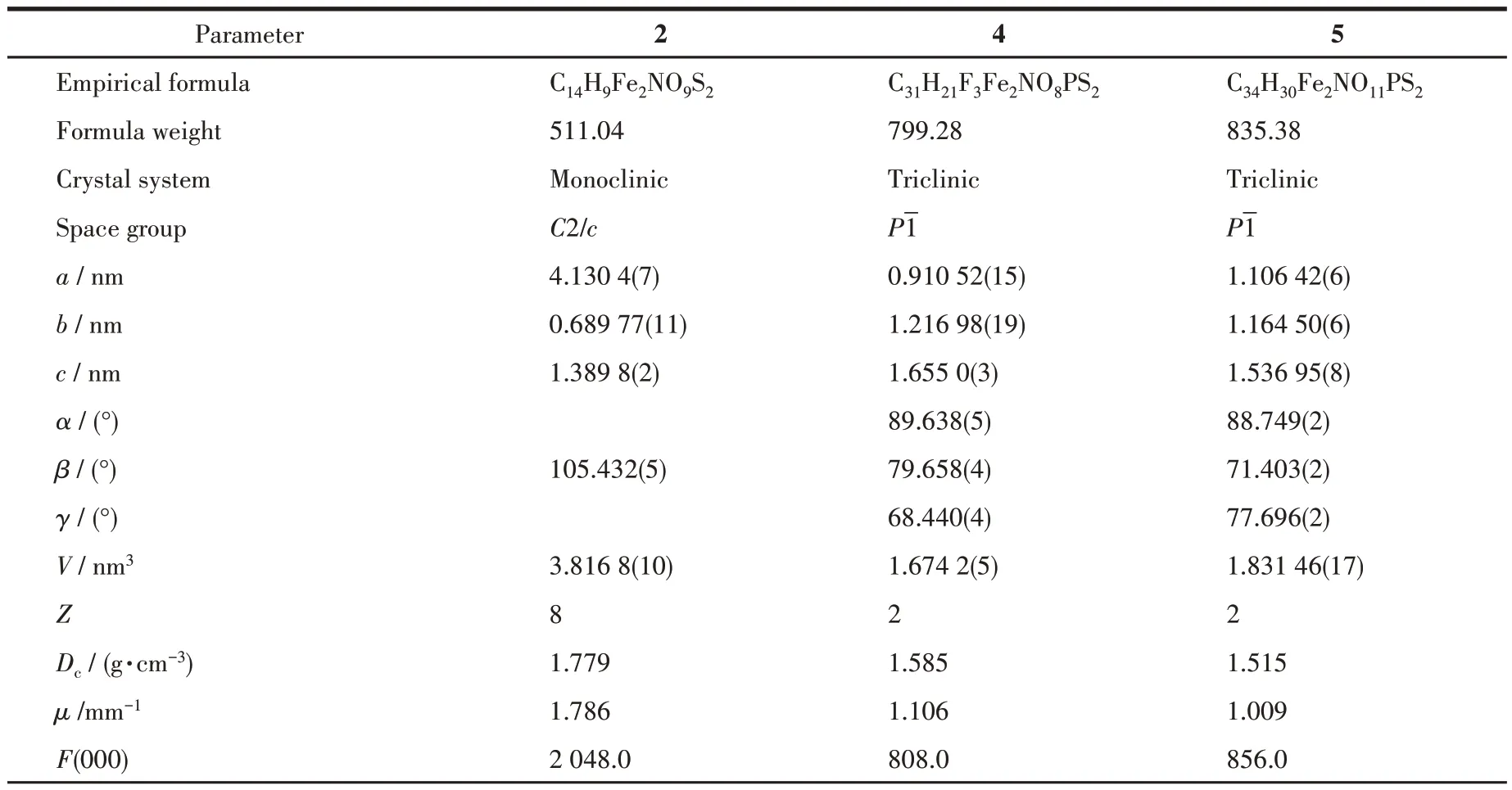
Table 1 Crystal data and structure refinement details for complexes 2,4,and 5
CCDC:2118524,2;2118526,4;2118527,5.

Continued Table 1
1.5 Electrochemical experiment
Electrochemical properties were studied by cyclic voltammetry (CV) in MeCN solution. Electrochemical measurements were carried out under nitrogen using a CHI 620 Electrochemical workstation. As the electrolyte,nBu4NPF6was recrystallized several times from a CH2Cl2solution by the addition of hexane. CV scans were obtained in a three-electrode cell with a glassy carbon electrode (3 mm diameter) as the working electrode, a platinum wire as the counter electrode, and a non-aqueous Ag/Ag+electrode as the reference electrode.The potential scale was calibrated against the Fc/Fc+couple and reported versus this reference system.
2 Results and discussion
2.1 Synthesis and characterization of complex 2
As shown in Scheme 1,the ester-product 2 can be made by the reaction of complex 1 with 5-methylisoxazole-4-carboxylic acid assisted by the reagents DCC and DMAP. Complex 2 was isolated as a red solid and structurally identified by elemental analysis and FTIR,1H NMR spectroscopies. Four bands ranging from 2 077 to 1 996 cm-1(Fig.1) were found in the FTIR spectrum, which can be designated as the stretching mode of terminal carbonyls, close to the corresponding values of the hexacarbonyl di-iron analogues[4,6,29]. The1H NMR spectrum exhibited two quartets atδ4.21 and 4.12 for the OCH2group.

Scheme 1 Synthesis of complex 2
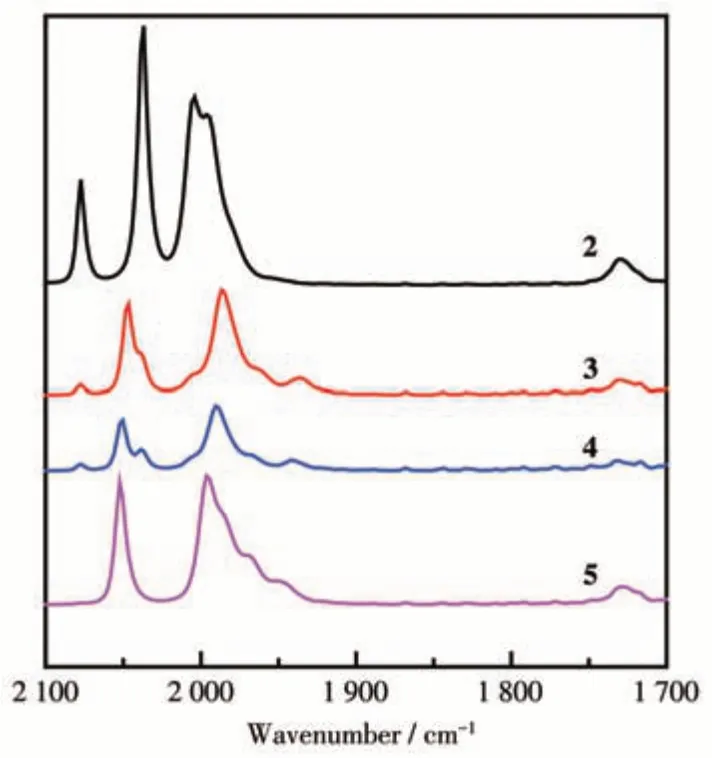
Fig.1 FTIR spectra of complexes 2-5
2.2 Synthesis and characterization of complexes 3-5
As displayed in Scheme 2, the phosphine-bearing analogues 3-5 can be got from the CO-interchange of complex 2 with a phosphine ligand tri(p-tolyl)phosphine,tris(4-fluorophenyl)phosphine,or tris(2-methoxyphenyl)phosphine assisted by the reagent Me3NO∙2H2O.Complexes 3-5 were isolated as red solids and structurally identified by elemental analysis and FTIR,1H NMR,31P{1H}NMR spectroscopies.Three to four bands in the region of 2 052-1 937 cm-1(Fig.1)were observed in the FTIR spectra of complexes 3-5, assigning to the stretching mode of terminal carbonyls C≡O, migrating to lower energies with respect to those of complex 2 because of the phosphine ligands having better donation than carbonyl[33-35]. In the1H NMR spectra of complexes 3-5, a multiplet at approximatelyδ3.90 was found for the OCH2group, shifting to the higher field with respect to complex 2 probably due to the shielding effect of the phosphine ligand. In the31P{1H} NMR spectra of complexes 3-5, the singlets atδ49-61 were observed for the ligated phosphine ligand, very close to those of phosphine-containing di-iron analogues[36-38].

Scheme 2 Synthesis of complexes 3-5
2.3 X-ray crystallography
Single crystal X - ray diffraction analysis was applied to characterize the molecular structures of the new complexes. The molecular structures presented as thermal ellipsoids are depicted in Fig.2 and Table 2 lists the selected bond lengths and angles. Complex 2 crystallizes in monoclinic space groupC2/cwith four molecules in the unit cell whilst complexes 4 and 5 crystallize in triclinic space groupP1 with two molecules in the unit cell. Fig.2 shows that all the complexes consist of a di-iron sub-cluster with a bridged SCH2CHS bearing an isoxazole moiety, six terminal CO or five terminal CO, and a phosphine ligand. Notably,all the P-donor ligands in complexes 4 and 5 possess an apical position of the pseudo-octahedral coordination arrangement of the Fe atom, in accord with monosubstituted di-iron analogues[29-30,39-40]. The Fe1—Fe2 bond length of complex 2 (0.249 6(4) nm) is very similar to that of complex [Fe2(CO)6(μ-SCH2CH(CH2OO CCH3)S)] (0.249 67(11) nm)[41]as well as 1,2,3-thiadiazole-bearing analogue[Fe2(CO)6(μ-SCH2CHCH2OOC(4-C2N2SCH3)S)] (0.249 2(3) nm)[29]indicating that the effect of isoxazole is consistent with methyl or 1,2,3-thiadiazole. The Fe1—Fe2 bond lengths of complexes 4 (0.250 29(9) nm) and 5 (0.252 00(12) nm) are mildly longer than complex 2 due to the coordination of Pdonor ligand. Moreover, the Fe1—Fe2 bond lengths of complexes 4 and 5 are shorter than those in natural[FeFe]-hydrogenases[2-3]along with those of P-donor ligand chelated analogues[33-34].

Fig.2 Molecular structures of complexes 2(a),4(b),and 5(c)as thermal ellipsoids at a 30% probability level

Table 2 Selected bond lengths(nm)and angles(°)for complexes 2,4,and 5
2.4 Electrochemical property
CV technique was applied to study the electrochemical and electrocatalytic properties of the new complexes. The CV curves of complexes 2-5 are overlapped in Fig.3 and the corresponding CV data are demonstrated in Table 3. The CV curve of complex 2 only had an irreversible reduction wave at -1.67 V,assigning to the one-electron reduction of FeⅠFeⅠto Fe0FeⅠaccording to the reported work[42], and the potential was analogous to complexes [Fe2(CO)6(μ-SCH2CHCH2OOC(4 - C2N2SCH3)S)] (-1.64 V)[29]and[Fe2(CO)6(μ-SCH2CHCH2OOC(CF3C3N2CH3)S)] (-1.64 V)[30]revealing that the electronic effect of isoxazole is close to 1,2,3-thiadiazole and pyrazole. Nevertheless,the CV curves of complexes 3-5 have an irreversible reduction wave at -1.87, -1.81, or -1.83 V together with an irreversible oxidation wave at 0.51, 0.60, or 0.58 V assigning to the one-electron oxidation of FeⅠFeⅠto FeⅡFeⅠ[42]. The potentials for the reduction and oxidation of complexes 3-5 were comparable to other phosphine-bearing analogues[29,30].It is noteworthy that the reduction potentials for complexes 3-5 moved cathodically by approximately 0.2 V with respect to that of complex 2, which is consistent with the fact that the phosphine ligand can increase the electron density on the Fe center.

Fig.3 CV curves of complexes 2,3,4,and 5 in 0.1 mol·L-1 nBu4NPF6/MeCN at a scan rate of 0.1 V·s-1

Table 3 CV data for complexes 2-5
Moreover, the electrocatalytic properties of these complexes have been studied by adding HOAc as a proton source into the MeCN solution, and the results are displayed in Fig.S1-S4 (Supporting information). It can be seen that upon an increment of HOAc concentration (2-10 mmol·L-1), the current heights of the reduction waves for complexes 2-5 were slightly grown up. However, new waves at -2.14 V (2), -2.26 V (3),-2.38 V (4), and -2.32 V (5) emerged and the current heights were significantly grown up linearly (Fig. 4)with successive increment of HOAc concentration. The catalytic potentials moved positively with respect to blank (HOAc without the di-iron complex)[43]. Further,the catalytic current heights for complexes 3 and 4 were higher than HOAc blank[43]. These electrochemical observations indicate that proton reduction catalyzed by complexes 2-5 occurs[42,44-46].
To estimate the catalytic capabilities of these complexes, overpotential and turnover frequency (TOF)were calculated by the known method[47].As revealed in Table 3, the overpotential for complex 2 (0.63 V) was lower than other complexes reflecting that the phosphine coordination will increase the overpotential, in agreement with recently reported work[48]. Also notable in Fig.4, the gradients of the dependence of catalytic current on the HOAc concentration of complexes 3 and 4 were steeper than other complexes suggesting that complexes 3 and 4 are more sensitive to HOAc[42]. As observed in Fig.5, the TOF values of complexes 3-5 were higher than complex 2 indicative of the phosphine coordination will increase the catalytic capability possibly due to the more basicity on the di-iron core for easily binding protons. Further, the TOF of complex 4 was higher than those of complexes 3 and 5 revealing that the phosphine ligand with electron-withdrawing group is more favorable for H2production than the corresponding ligand with electron-donating group. According to the results described above, an EECC (E=electrochemical, C=chemical) mechanism can be speculated for the electrocatalysis of H2production catalyzed by the isoxazole-bearing di-iron analogues.

Fig.4 Dependence of catalytic current on the HOAc concentration for complexes 2-5

Fig.5 Plots of TOF versus the HOAc concentration for complexes 2-5
2.5 Antifungal activity
The antifungal activity againstP.infestans(PI),G.zeae(GZ),P.oryae(PO),P.capsici(PC),C.fragariae(CF),B.cinerea(BC),R.solani(RS),F.oxysporum(FO),C.arachidicola(CA)andP.piricola(PP)was tested at a mass fraction of 0.005% by a reported method[49]and Table 4 lists the results.It revealed that complex 2 displayed moderate (40%-70%) activity against BC and RS and weak (10%-40%) activity against PI, GZ,PO,CF,and PP.Complexes 3-5 displayed weak activity against PI,PO,PC,BC,RS,and PP.

Table 4 Antifungal activity of complexes 2-5 at a mass fraction of 0.005%
3 Conclusions
In summary, we have presented the synthesis and spectroscopy of four isoxazole-bearing di-iron complexes. The X-ray crystallographic studies have shown that they consist of a di-iron sub-cluster with a bridged SCH2CHS containing an isoxazole moiety, six terminal CO or five terminal CO, and an apically-ligated Pdonor ligand. CV studies have revealed that these complexes can electrocatalyze the reduction of protons to dihydrogen by adding an acetic acid as a proton source into the solution. The antifungal activity studies have displayed that some complexes show moderate or weak activity.
Supporting information is available at http://www.wjhxxb.cn
