Plasma-activated hydrogel: fabrication,functionalization,and effective biological model
2023-10-08JiachengLI李嘉诚CuntaoLAN兰存涛LanlanNIE聂兰兰DaweiLIU刘大伟andXinpeiLU卢新培
Jiacheng LI(李嘉诚),Cuntao LAN(兰存涛),Lanlan NIE(聂兰兰),Dawei LIU(刘大伟),2,∗and Xinpei LU(卢新培),∗
1 State Key Laboratory of Advanced Electromagnetic Engineering and Technology,School of Electrical and Electronic Engineering,Huazhong University of Science and Technology,Wuhan 430074,People’s Republic of China
2 Wuhan National High Magnetic Field Center,Wuhan 430074,People’s Republic of China
Abstract Hydrogels are biomaterials with 3D networks of hydrophilic polymers.The generation of hydrogels is turning to the development of hydrogels with the help of enabling technologies.Plasma can tailor the hydrogels’properties through simultaneous physical and chemical actions,resulting in an emerging technology of plasma-activated hydrogels(PAH).PAH can be divided into functional PAH and biological tissue model PAH.This review systematically introduces the plasma sources,plasma etching polymer surface,and plasma cross-linking involved in the fabrication of PAH.The ‘diffusion-drift-reaction model’ is used to study the microscopic physicochemical interaction between plasma and biological tissue PAH models.Finally,the main achievements of PAH,including wound treatment,sterilization,3D tumor model,etc,and their development trends are discussed.
Keywords: plasma-activated hydrogel,plasma crosslinking,biological model
1.Introduction
A hydrogel is an extremely hydrophilic 3D network structure gel(figure 1) that can hold large amounts of water without dissolving [1].Hydrogel provides a regenerative framework for biological tissues [2],and controls the diffusion of molecules and cells simultaneously [3].The hydrogel’s flexible and controllable water content provides a suitable environment for cell seeding and encapsulation [2,4].Its porosity facilitates the transport of gases,nutrients,proteins,cells,and wastes [5].Hydrogel is one of the most promising polymer materials,avoiding the traditional biomaterials’limitations.
Due to their excellent structure and flexibility,hydrogels are ideal for medical applications.However,its low mechanical strength is an obstacle to the potential application of hydrogels in life medicine [6],especially when it is in a swollen state.In addition,the non-adherent quality [6],degradation behavior[7],and crosslink-dependent dissolution[8] of hydrosols also limit their application promotion.Therefore,the scientific community constantly seeks novel hydrogel preparation strategies and systems to improve these properties [9].
Plasma is an ionized gas that can generate high-density charged particles,reactive species,ultraviolet radiation,and electric fields.It enables many different applications,ranging from surface modification,and clean energy production to biomedicine,through the simultaneous action of physics andchemistry [10-15].For example,the highly reactive species generated by plasma not only modify surfaces by etching or attaching functional groups at or near the polymer surface,but also trigger crosslinking of polymers in the liquid phase[16],thus opening the application of plasma in the synthesis of hydrogel.On the other hand,hydrogels are used widely in plasma-medicine to localize and quantify plasma effects[17].

Figure 2.Plasma-activated hydrogel(PAH) can be divided into functional PAH and biological tissue model PAH depending on usage scenarios.
This review focuses on introducing plasma-activated hydrogel(PAH),a novel way of synthesizing gels,discusses the evolution of hydrogels and their fabrication methods,and points out that plasma can initialize and crosslink functional hydrogel synthesis through the simultaneous action of physical and chemical processes.Depending on usage scenarios,PAH can be divided into functional PAH and biological tissue model PAH(figure 2).Based on systematically introducing the PAH fabrication methods,the ‘diffusion-drift-reaction model’ is used to study the microscopic physicochemical process of the interaction between plasma and biological tissue models.Finally,the main achievements of PAH and their development trends are discussed.
2.Hydrogel materials
2.1.The development of hydrogel and its application in the plasma biomedicine
Related research on hydrogels began at the end of the 19th century,and colloidal gel products were first produced using inorganic salts [18].These polymers were physically or chemically linked,and the scientific community began to use‘hydrogels’ to describe the 3D networks of these hydrophilic natural/synthetic polymers [19].
Hydrogels have been continuously improved to meet the demands of biomedical applications.So far,three generations of hydrosols have been developed [20].The first generation was chemically cross-linked hydrogels,which were synthesized mainly through the polymerization of hydrosol monomers and the crosslinking of existing synthetic polymers,with good mechanical strength and high swelling properties [21].Second-generation hydrogels include hydrogels that can respond to specific stimuli,such as pH,ion concentration,or temperature in solution [22].The third generation of hydrogels includes stimuli-responsive development of the secondgeneration hydrogels with tunable mechanical and chemicalphysical properties,namely ‘smart hydrogels’ [5,22].
Hydrogels are the most commonly used models for plasma biomedicine,which are easily shaped into models with the same structure as organs [23],while being tuned to tissue-like physicochemical properties.Therefore,hydrogels are employed to study the transportation of plasma in biological tissues and to manufacture sustained-release modules of plasma active ingredients for tumor treatment [17,24].
2.2.Crosslinking strategy of hydrogels
The hydrogels’ integrity depends on the crosslinking in their structure,so hydrogels can be distinguished based on the crosslink network.Hydrogels can be synthesized through chemical or physical crosslinking.Figure 3 compares physical and chemical crosslinking in production mechanisms,network properties,and desired characteristics,and lists the main technical approaches.

Figure 3.Differences between physical and chemical crosslinking.

Figure 4.The usage scenarios of the biological model PAH.(a) Gel matrix,(b) barrier + gel matrix,and(c) gel matrix + liquid.
Chemically crosslinking hydrogel has more tunable physicochemical properties than physically crosslinking hydrogel,and also have higher crosslinking density and mechanical strength,which may be more beneficial for biomedical applications [25].However,too high crosslinking density will reduce the swelling ability and pore size ofhydrogels,and inhibit the diffusion and transport of biomolecules and cells in hydrogels.Therefore,we also need new technical means to modify crosslinking to improve hydrogels’biological interactions [26].
Physical crosslinking in hydrogels mainly includes hydrogen bonding,coinduced processes,heating or cooling of polymer solutions,and crystalline crosslinking.Hydrogen bond crosslinking is possible for polymers containing polar functional groups such as hydroxyl,amido,and carboxyl groups [27].The common term coexistence refers to the phase separation process in colloidal chemistry,which is caused by a change in the environment of the medium under controlled conditions.The simple co-factoring process refers to the copolymerization of a single polymer after adding salts or co-factors,while the complex co-factoring process involves at least two oppositely charged polyelectrolytes[28].
Hydrogels can also be generated by cooling or heating.The heating or cooling of polymer solutions is mainly applicable to polymers with helical formation and junction regions in their structures [29,30].Crystalline crosslinking relies on crystallites in the polymer chain that act as physical crosslinking sites in the network,and the crosslinking method can be performed at temperatures in the freezing point range [31].
Chemical crosslinking in hydrogels includes free radical polymerization,photopolymerization,enzymatic reactioninduced crosslinking,crosslinking and grafting by click chemistry.Photopolymerization is a unique method to form gels rapidly and controllably by interacting with photosensitive compounds via visible/ultraviolet light and converting liquid monomers or macromolecules into hydrogels via FRP [32].Enzymatic crosslinking allows rapid gelation and strong covalent bond formation under physiological conditions,and this method is an effective method for in situ hydrogel formation,similar to photopolymerization [31].Click chemistry represents a set of fast,versatile,purifiable,and high-yield reaction processes that mimic the formation of aldol condensations of natural materials.Natural products mainly linked through C-C bonds in their backbones [33],and their synthesis requires high thermodynamic driving forces;the click reaction solves this problem by combining C atoms with the abundant heteroatom X in polysaccharides.Instead of C-C bonds,click reactions ultimately form C-X-C bridges to connect proteins,nucleic acids and/or polysaccharides[34,35].Graft polymerization is a multifunctional technique for adding desired functional groups to the polymer backbone to generate hydrogels with tailored functionalities.
Physical and chemical methods for crosslinking hydrogels each have advantages and disadvantages.Researchers have been seeking an alternative efficient method for the synthesis of novel hydrogels that should effectively reduce the complex crosslinking process [36,37],namely the use of chemicals and high-energy manipulation;and achieve tunable functionality that is economical and safe without decreasing the structural biological integrity.Plasma is a new matter for physics(electric field,high-energy electrons/ions) and chemistry(UV,RONS) to work together,and its application in hydrogel synthesis will be discussed in depth in subsequent sections.
2.3.Hydrogel models for plasma-biomedicine
Hydrogel is a widely used model in plasma biomedicine to study the transportations of plasma active components.Hydrogels used in plasma biomedicine include agarose,gelatin,and polyvinyl alcohol(PVA).
Agarose is an organic substance with the chemical formula C24H38O19.Agarose is heated to 90°C to dissolve in water; afterward,a good semisolid gel is obtained when the temperature decreases to less than 40°C.Agarose has almost no charged groups.It rarely causes denaturation and adsorption to sensitive biological macromolecules.
Gelatin is a macromolecular hydrocolloid that is the product of partial hydrolysis of collagen.The collagen molecule is a helical structure of three polypeptide chains wrapped around each other.Therefore,gelatin is a polydisperse system with a certain molecular weight distribution.The raw materials for the production of gelatin are mainly animal skins,bones and tannery wastes.Gelatin is widely used in the field of medicine because of its biodegradability,good biocompatibility,and film-forming properties.
PVA is a synthetic polymer with the formula [C2H4O]n.Medical PVA aqueous gel has many applications in ophthalmology,wound dressings,and artificial joints.Since it is not yet generally applied to plasma biomedicine,this review will not discuss it in depth.Agarose and gelatin are the main reference materials for hydrogels in plasma biomedicine,and both gels have shown excellent results in practical applications as models of tissues.
The usage scenarios of hydrogel models in plasma biomedicine are mainly divided into three types(figure 4): gel matrix,barrier + gel matrix,and gel matrix + liquid.A gel matrix is the most commonly used configuration,consisting of a gel and a specific chemical reporter,simulating a certain volume of living biological tissue.It is widely used to study the transportation of plasma RONS in the biological tissue,which is usually determined by the plasma(type of working gas,driving voltage,and products,etc) and working environment(gel properties,distance between electrode and gel,processing angle and time,etc).The gel matrix used in the barrier + gel matrix case is covered by a layer of gel orliquid,so the transportation of RONS on the gel matrix through the barrier can be measured for plasma-treated skin tissue with intact epidermal structure or exudate-covered wounds application scenarios.The gel matrix works as a barrier in the gel matrix + liquid case.The transfer effect of RONS through the barrier and the buffering effect of the barrier tissue are studied by analyzing its delivery effect in liquid.
The goals of these model studies include(1) RONS distribution and penetration depth;(2) RONS transport effect through obstacles;(3) regulation of plasma parameters:working gas flow rate and mixing ratio,distance from the electrode to target,tilt angle,and plasma driving voltage;(4)model validity evaluation;(5) adding bacteria or cells to the model to simulate actual tissue.
3.Typical PAH fabrication sources
There are two types of PAH,the first is functional PAH,and the second is biological model PAH.Functional PAHs are mainly produced by dissolving granules hydrogel powder in plasma-activated water(PAW),discharging in hydrogel solutions,or plasma-treating hydrogel.
The system shown in figure 5(a)is to dissolve acrylamide directly,polymerize it in PAW,and then add a crosslinking agent,initiator,and polymerization inhibitor,and finally obtain PAH with antibacterial properties [38].The PAW is generated with an air plasma jet based on a hollow electrode dielectric barrier discharge.The whole system mainly consisted of copper electrodes and quartz dielectric,which are set at the end of a quartz tube with an inlet diameter of 1.5 mm.A uniform plasma is generated in a 0.5 mm discharge gap and a plasma jet is ejected through a 7.0 mm end outlet.The plasma jet can generate air plasma with an electron density of up to 1015cm-3,and the penetration depth of plasma below the water surface is 2 cm.The optimal time for plasma jet treatment is 15 min(PAH-15).
After the plasma jet treatment,the monomer powder of acrylamide(AAm,AR) is dissolved in PAW at a concentration of 2.1 M; then the crosslinking agent N,N’-methylenebisacrylamide(MBAA,CP) is added.After stirring for one minute,the gel solution is poured into the mold and kept at room temperature for 2 h to complete the preparation of PAH.Hydroxyl radicals and NO-3generated by plasma are the main antifungal active components in PAH.The comparison indicates that the antifungal ability of PAH-15 min was better than that of the fluconazole group.This indicates that PAHs have better inhibitory efficiency than traditional antifungal gel.The antifungal life of PAH is longer than that of PAW.
Figure 5(b) shows a bio-adhesive catechol-modified chitosan wound healing hydrogel system prepared by discharging in gallic acid(GA)and chitosan solutions(CS)[39].First,CS and GA are added to a flask,stirred,and bubbled for 22 h.The positive pin electrode of the glow discharge is placed in the mixed solution,and the copper ground electrode is pasted on the flask’s outer wall.After treating the solution with liquid phase glow discharge plasma(LP-GDP) for 20 min,the mixed CS-GA solution is heated to 85°C and kept for 3 h to obtain purified CS-GA after lyophilization.The powder is stirred in ultrapure water for 1 h,and after being placed in air for 3 h,it gradually turns into a non-flowable CSGA hydrogel.LP-GDP generates a large amount of highly active RONS,such as OH,H2O2,O-2,HONOO,and aqueous electrons through electrochemical reactions.The chitosan is oxidized and fragmented by RONS generated by plasma,resulting in a decrease in viscosity.Plasma can also promote the electrostatic interaction between chitosan NH+3and GA negative ions.Therefore,plasma stoichiometrically graft GA onto chitosan chains to prepare gallic acid-modified chitosan(CS-GA) hydrogel.The plasma-fabricated CS-GA hydrogels exhibit unusual adhesion and toughness properties while exhibiting high biocompatibility and hemocompatibility.

Figure 5.PAH fabrication sources:(a) dissolving granules hydrogel powder in plasma-activated water(PAW),(b) discharging in hydrogel solutions,(c) plasma-treating hydrogel.
Figure 5(c) shows the crosslinking of gelatin films containing econazole nitrate(ECN) by DBD [40].The plasma source consists of two parallel aluminum plate electrodes,the upper plate is covered by a 2.4 mm thick polyoxymethylene(POM-C) plate,the lower electrode is grounded,and the air gap spacing of the discharge is 1 mm.The plasma is driven by a pulsed high voltage of 15 kV,500 Hz,and a rising edge of 1μs.Plasma treatment is performed by placing the ECNcontaining gelatin film between electrodes,ensuring that both sides of the gelatin film are treated by plasma.Plasma induces rapid gelatin crosslinking and changes the chemical properties of the film surface.Plasma enhances mucoadhesive properties.It achieves more than two times the mucoadhesive strength value while retaining the antibacterial properties of the gelatin film.
The biological model PAH is mainly used to study the reaction and permeation process of plasma active components in biological tissues and semisolid food materials.Figure 6(a)shows a system for studying plasma penetration and microbial inactivation in semisolid materials by treating agar gels with large-area air DBD[41].The discharge device consists of two aluminum electrodes with a diameter of 15.24 cm,each covered with a polypropylene layer(0.22 cm in thickness) as a dielectric barrier,treating agar gel with a thickness of 3 cm(25 ml).The driving voltage of plasma is 90 kVpp(frequency at 120 Hz).A pH indicator is added to the gel to track the pH depth change corresponding to the penetration depth of the plasma active components.The maximum penetration depth is 1.5 cm; at the same time,the relationship between plasma penetration and killing salmonella and Escherichia coli in agar gel is also studied.Plasma treatment for 2 min reduces the bacterial content in applesauce by 6 orders of magnitude.

Figure 6.The biological model PAH.(a) The agar gel treated by large area air DBD,(b)3D collagen matrix encapsulated with cancer cells treated by plasma jet.

Figure 7.The interaction mechanism between plasma and water.
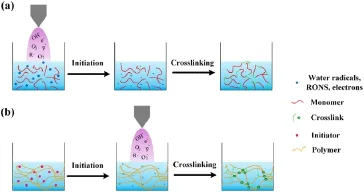
Figure 8.(a) Plasma work as an initiator during PAH synthesis.(b)Plasma crosslinking during PAH synthesis.
Currently,the research on the effect of plasma on cancer cells mainly uses traditional 2D monolayer cell culture.In contrast,the 2D model cannot fully simulate the 3D tumor environment,and the animal model is expensive and time-consuming.Employing 3D collagen matrix geometrically similar to cancer tissue can provide more physiologically relevant results than 2D models.Figure 6(b) shows that an air DBD is used to treat 3D collagen matrix encapsulated with A549 lung adenocarcinoma cells [42].The DBD system consists of a copper electrode inserted into a hollow cylindrical glass tube,which is packaged together with the larger test tube.The plasma is driven by a pulsed high voltage of 12 kV with 1 kHz frequency and 4μs pulse width.The plasma source generates a filamentary plasma with a length of 1 mm and a diameter of 0.6 mm between the test tube and the collagen sample.As the plasma treatment time increases,the RONS generated by the plasma penetrate vertically and laterally in the 3D collagen matrix,so the viability of the cancer cells in the collagen matrix decreases significantly.The diffusion area of RONS in the lateral direction in the collagen matrix is 100 times larger than the plasma’s cross-section,and the vertical penetration depth of the plasma is 1 mm.
4.Fabrication mechanism of PAH
4.1.Fabrication mechanism of functional PAH
The preparation of functional PAH systems shown in figures 5(a) and(b) first involves the interaction between plasma and water.As shown in figure 7,the interaction between plasma and liquid involves gaseous plasma,gaseous plasma-liquid interface,and liquid phase [43].The gaseous plasma mainly contains plasma interacting with ambient air to produce high-density RONS.When these RONS approach the interface,they react with the evaporated H2O to generate more reactive species; these highly reactive species penetrate into the liquid phase through collision,diffusion,and dissolution absorption,and further chemical reactions occur.At the same time,the plasma’s high-energy electrons,ions,and UV can also react directly with the liquid to promote the generation of active free radicals.
The free radicals generated by plasma in water can trigger the polymerization process as shown in figure 8(a);according to the synthesis conditions(plasma power,treatment time,mass ratio,and crosslinking agent),the hydrogel can be adjusted as reversible swelling-desolvation and stimulus-responsive behavior.The plasma-synthesized cellulose-based ionic hydrogels exhibit responsive behaviors to changes in solution pH,ionic species and density,and free radicals.The acidic-neutral solution after plasma treatment affects the swelling-desolubilization behavior,and the change of ion density can lead to volume shrinkage and a decrease of the swelling rate of the gel [44,45].
Plasma-generated free radicals in water are also able to promote crosslinking during hydrogel synthesis(figure 8(b)).DBD-based gelation of chitosan shows that the hydrogel viscosity depends on plasma treatment and chitosan concentration.At lower concentrations,chitosan is oxidized and fragmented by plasma,resulting in a decreasing viscosity; at higher concentrations,the viscosity increases due to the formation of higher molecular weight chitosan conjugates.Chitosan-acrylic acid blend hydrogel is prepared by plasma spraying method.Electrostatic interactions of chitosanand acrylic acid COO-control the polymer network.The treatment by plasma jet also establishes another connection via C-O bridges,for example,when a high concentration of chitosan(2.5 wt%)is treated by plasma,the changes of C=O and C-O bonds are more pronounced than those at lowchitosan concentrations,thereby establishing partial covalent crosslinks and the aggregation of micelles in the hydrogel network,leading to an increase in molar mass and viscosity.Ultimately,plasma treatment doubled the elasticity of the hydrogel [46,47].
4.2.The interaction mechanism between plasma and biological model hydrogel
The interaction between plasma and biological model hydrogel includes three regions,the gaseous plasma region,the plasma-gel interface region,and the gel region.The interaction time scale ranges from microseconds(short-lived reactive species) to hours(diffusion of long-lived reactive species in the gel).First,the highly reactive species generated by plasma interact with the gel when they reach the gel surface(shown in the figure 9).These plasma species continuously bombard the surface of gelatin,causing changes in the structure or morphology of the gel surface,as well as functionalization,crosslinking,and etching[48,49].Next,the permeation of plasma reactive species in the semisolid material of the gel depends on the molecular motions.The concentration gradient-diffusion effect and the electrostatic motion-drift effect affect the molecular motions,which can further react with the gel itself and the microorganisms in the gel [41].
The diffusion-drift-reaction model(equation(1)) is used to describe the permeation process of plasma reactive species in the gel(figure 10)
where i and j denote the species and gel,respectively,Ci,jis the concentration of plasma reactive species in the gel,Di,jis the diffusion coefficient of the reactive specie in the gel,ziis the charge number,μi,jis the drift mobility of a charged specie,E is the electric field,Cxis the concentration of reactant x reacting with plasma reactive species in the gel.Γi,diffis the diffusion flux of element i,Γi,driftis the drift flux of charged element i,and Γi,reacis the reaction between plasma reactive species and the gel phase [50].The gas composition and flow rate influence the concentration of gaseous plasma species at the plasma-gel interface.The electrode geometry influences the electric field
The model shows that the electric field,plasma working gas,and processing time all affect the transport effect of plasma reactive species in the gel.The increase of plasma power can increase the density of reactive species,thereby promoting the penetration of reactive species,while the increase of plasma electric field facilitates the transportation of ions into the gel [51].The electric field of the plasma can also promote the transfer of short-lived species(chargedparticles,metastable particles and highly reactive radicals,etc) to the gas-gel interface [52].
The penetration ability of plasma reactive species in the gel is also related to the material properties of the gel,such as density/concentration,porosity,chemical composition,etc.It is obvious that the lower density gel has higherDi,jandμi,jvalues,Therefore,the penetration rate of RONS in the low density gel is faster.Temperature affects Γi,diffand Γi,reacby affectingDi,jand ki,for example,plasma treatment for a longer time(1.5 h) increases the temperature of gel samples by ~(5-10) °C [41].Electric fields can also affect the diffusion and electrophoresis of reactive species in gels by inducing temperature gradients [53],because the diffusion coefficients and reaction rates in both gels and liquids are temperature-dependent.Various reactions occur during the penetration of RONS of plasma,such as 105 reactions between 33 kinds of reactive species under aqueous conditions [54],and the molecular reactions between bacteria and reactive species [55].
5.The role of PAH biomaterials
After more than ten years of development,plasma has become an effective technology to improve biological material’s properties.
Table 1 lists representative works on the generation of functional biomaterials by plasma synthesis or modification of hydrogels,and the PAH has become an emerging way to improve or introduce hydrogel properties.

Table 1.Representative works of functional PAH.
PAH better solves the problem that the reactive species in PAW are difficult to preserve for a long time.The long-lived reactive species,such as H2O2,,O3,generated by plasma and water can store in the hydrogel directly,and the short-lived high-active species,O,1O2,OH and etc,can react in the hydrogel,so the hydrogel becomes a comprehensive carrier for a variety of plasma generated RONS,which further expanding the application of plasma in various fields such as wound healing,antibacterial and tumor treatment.
Plasma can also synthesize thermosensitive hydrogels containing magnetic nanoparticles(Fe3O4)[68].The chemical composition and physical properties of the hydrogels are not affected by plasma,while the magnetic strength of magnetic hydrogels is significantly enhanced.Plasma can also be used as a neutral energy source for crosslinking preparation of gelatin-graphene oxide(gel-GO) nanocomposite hydrogel scaffolds[67].Plasma-treated gel-GO hydrogels have tunable viscoelasticity,gel strength,and mechanical properties,and have better water-holding capacity for cell growth and proliferation.
As a biological model,PAH is a simple,low-cost but powerful tool in plasma biomedicine.It is mainly used to study the generation of plasma,the transport of plasma reactive species in human tissue,and the mechanism of plasma action(with or without skin barrier,with or without exudate coverage,different organs,etc),so as to more effectively adjust the plasma power,working gas mixing,electrode configuration,treatment time and other parameters to improve the plasma treatment effect.
The main contributions of the biological model PAH include: the density and distribution of RONS generated by plasma are affected by the distance from the electrode to the model,gas mixing and flow velocity,model thickness and etc;the plasma induced apoptosis of cancer cells at a depth of 1 mm in the 3D matrix [42],indicating that plasma can be used in real tissue; although plasma can be switched on and off in time,the penetration and transport of plasma reactive species in hydrogel last from minutes to hours; the electric field enhances the penetration range of plasmonic reactive species.
6.Future direction of PAH
As the carrier of charged particles,electric field,RONS,and UV,plasma acts simultaneously through physical and chemical methods to facilitate the synthesis of hydrogels in the initial step and during the crosslinking process.The resulting PAHs have the crosslinking network through physical andchemical methods,and a RONS-rich environment.Combined with the development trend of smart hydrogels,plasma will be able to participate in the programmed process of hydrogels to respond to multiple stimuli(physical,chemical,and biological signals),so as to enable PAHs in biosensors,regenerative medicine,and targeting wider applications in areas such as drug delivery.
To enhance our comprehension and optimization of plasma’s therapeutic efficacy,and address the obstacles encountered by plasma biomedicine,subsequent advancements in the biological model of PAH encompass:(1) indepth exploration of plasma RONS penetration parameters,which will facilitate the improvement of plasma treatments for both deep-seated lesions(tumors) and superficial tissues(wounds);(2) the creation of larger and more precise biological models(such as gut and airway models) that will establish a foundation for plasma endoscopic therapeutic applications;(3) discerning the role of RONS diffusion in tissues and the intercellular communication initiated by RONS,which will aid in understanding the fundamental mechanisms of plasma biomedicine;and(4)investigating the impact of a tissue’s electrical properties and the target’s chemical composition on plasma treatment outcomes.
7.Conclusion
This review provides a comprehensive view of the use of PAH in plasma biomedicine.We first review the development of hydrogels and their applications in biomedicine,discuss the crosslinking strategies of hydrogels,and point out that plasma,as integrated carriers of charged particles,electric fields,and high-density RONS,can promote the crosslinking of hydrogels through simultaneous physical and chemical actions.
In order to expand the application scope of PAH,the typical PAH fabrication ways are listed in detail,namely dissolving granules hydrogel powder in PAW,discharging in hydrogel solutions,plasma-treating hydrogel,air DBD treating bacteria hydrogel model,and plasma jet treating 3D cancer cell tissue model.The structure of the plasma sources,the driving power source,the hydrogel type,and the PAH function are also discussed.
On the PAH fabrication mechanism of plasma-activated hydrogels(PAH),we review the PAW generation mechanism involved in the fabrication process,revealing that plasma works as initiator and plasma crosslinking during PAH synthesis.For the application process of the PAH biological model,we reveal the interaction process of various active components of plasma with the hydrogel surface,and analyze the penetration mechanism of plasma in the PAH model by using the diffusion-drift-reaction model.
Finally,we summarize the application of PAH in wound treatment,drug delivery,cancer treatment,wound model,tumor model,etc,and point out that plasma-activated smart hydrogels and more in-depth research on the mechanism of PAH biological model are the development direction of PAH.
Acknowledgments
This work was supported by National Natural Science Foundation of China(No.52277149) and the Interdisciplinary Program of Wuhan National High Magnetic Field Center(No.WHMFC202144),Huazhong University of Science and Technology.
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Propagation of surface magnetoplasmon polaritons in a symmetric waveguide with two-dimensional electron gas
- Investigation of electron cyclotron wave absorption and current drive in CFETR hybrid scenario plasmas
- Inversion techniques to obtain local rotation velocity and ion temperature profiles for the x-ray crystal spectrometer on EAST
- Impact of resonant magnetic perturbation on blob motion and structure using a gas puff imaging diagnostic on the HL-2A tokamak
- Comparison of methods for turbulence Doppler frequency shift calculation in Doppler reflectometer
- Invariant manifold growth formula in cylindrical coordinates and its application for magnetically confined fusion
