杂化纳米材料光热治疗肿瘤的研究进展
2023-09-15黄小秀李虎杨松
黄小秀 李虎 杨松

摘 要:光热治疗(photothermal therapy,PTT)具有高效快速、侵入性小、无创等优点。通常在光热治疗中采用单一的纳米材料作为光热剂,然而使用单一纳米材料通常会面临生物相溶性差、稳定性差、毒性大、靶向性能差、缺少成像性等问题。本文主要综述了最近几年开发的杂化纳米材料,通过多种光热材料的掺杂、包覆、表面修饰等方法开发了碳基杂化、无机杂化、有机-无机杂化等具有优良光热性能的光热材料,解决了使用单一光热材料面临的问题。此外,通过融合光热材料、靶向材料、成像材料、化疗材料和放射性材料等达到联合协同治疗的效果。杂化材料促进高效治疗癌症技术的发展,为光热协同治疗提供材料支持。这些方法为进一步开发新型杂化光热材料提供了思路,有望基于当前报道的各类具有光热潜力的材料,开发出高光热效率、高安全性的杂化光热材料,使光热杂化纳米材料在肿瘤的治疗临床应用具有广阔的前景。
关键词:光热治疗;光热材料;靶向特异性;生物相容性;癌细胞
中图分类号:TB383.1;R730.5
文献标志码:A
癌症一直是威胁人类生命健康的主要疾病之一。目前,癌症的治疗方法主要依赖手术、化疗、放疗等传统技术[1]。但這些原始的治疗方案往往会对正常人体组织产生不可避免的损害,而且具有疗效低、靶向特异性差、耐药性等缺点[2]。近年来,光热疗法(photothermal therapy,PTT)已成为治疗癌症的一种有效且重要的方法。其主要原因是治疗时间短(约几分钟),侵入性小,疗效显著,副作用少(大多数光敏剂在低浓度下相对无害)等[3]。PTT是一种典型的光子触发治疗方式,它通过光热剂(photothermal agents,PTAs)在可见光或近红外光(near-infrared,NIR)(600~900 nm)的激发下产生的局部高温杀死肿瘤细胞[4]。癌细胞对高热的敏感性高于正常细胞,高温会对癌细胞膜造成不可逆的损伤,促进蛋白质变性[5]。近红外光因其具有较高的组织穿透能力,对皮肤的吸光度较低,并且能够聚焦于特定的组织部位,被广泛用作光热治疗的外部激光源[2]。因此,PTT是一种对传统癌症治疗的补充,具有创伤性小、靶向性高、恢复速度快等特点,被认为是一种极具应用前景的癌症治疗策略。PTAs是PTT应用中的一个重要的组成部分,它能通过非辐射机制将光能直接转变成热能,高温消融病变组织或细胞。光热转换效率(photothermal conversion efficiency,PCE)是PTAs的一个关键因素,它直接决定光热治疗过程中所需要的激发光强度。已经报道了许多不同类型的光热剂,包括无机纳米材料(如贵金属纳米粒子、金属硫化物纳米粒子、碳基材料、过渡金属纳米粒子以及新兴的二维纳米材料等[1-2,6]),有机化合物纳米材料(如吲哚菁绿[7]、聚苯胺[8]、卟啉环[9])。早期对PTAs的研究主要集中在贵金属纳米材料,这些贵金属纳米材料具有良好的光吸收能力,同时具有较高的光热转换效率[4]。但无机材料存在生物相容性低、不可降解等特点,而有机材料具有良好的生物相容性。因此,杂化纳米材料是一种由有机或无机物质组成的混合物,它提供了每一组分的最佳特性,使杂化纳米材料具有独特的物理和化学性能[10],成为生物医学研究的热点领域之一。
随着基因疗法(gene therapy,GT)、免疫疗法、光动力疗法(photodynamic therapy,PDT)等新兴疗法的相继发展,利用光热治疗与其他治疗方案联合应用,设计开发出多功能的杂化纳米复合材料,实现多种具有协同效应治疗策略的集成,从而提高治疗效果和优化肿瘤治疗方案[11]。在本综述中,总结了不同杂化纳米材料在光热治疗癌症中的研究进展,并详细讨论了光热治疗的机理和3种典型的杂化材料的制备策略,如多种光热材料掺杂、包覆、表面修饰等方法,解决了使用单一材料面临生物相溶性差、稳定性差、毒性大、靶向性能差、缺少成像性等问题。此外,杂化纳米材料通过融合光热材料、靶向材料、成像材料、化疗材料和放射性材料等从而达到联合协同治疗的效果。光热治疗与化疗、放疗、光动力治疗的协同运用为其在临床的应用提供可能。
1 碳基杂化纳米材料
近年来,碳纳米材料由于具有优异的热学和光学性质被广泛应用于生物医学领域。碳基纳米材料晶格中的杂化形式有利于分子中的电子在近红外激光照射下从低轨道态激发到高轨道态。随后,通过非辐射弛豫的方式将吸收的激光能量从被激发的电子传递到整个晶格的振动中,进而产生热能[12]。因此,在近红外波段具有较高的光热转换效率。其次,碳纳米材料具有较低的细胞毒性,可在肿瘤环境中被过氧化物酶降解[13]。再者,碳纳米材料具有较大的比表面积和孔体积[14],可作为药物载体,用于化疗和热疗协同治疗肿瘤。
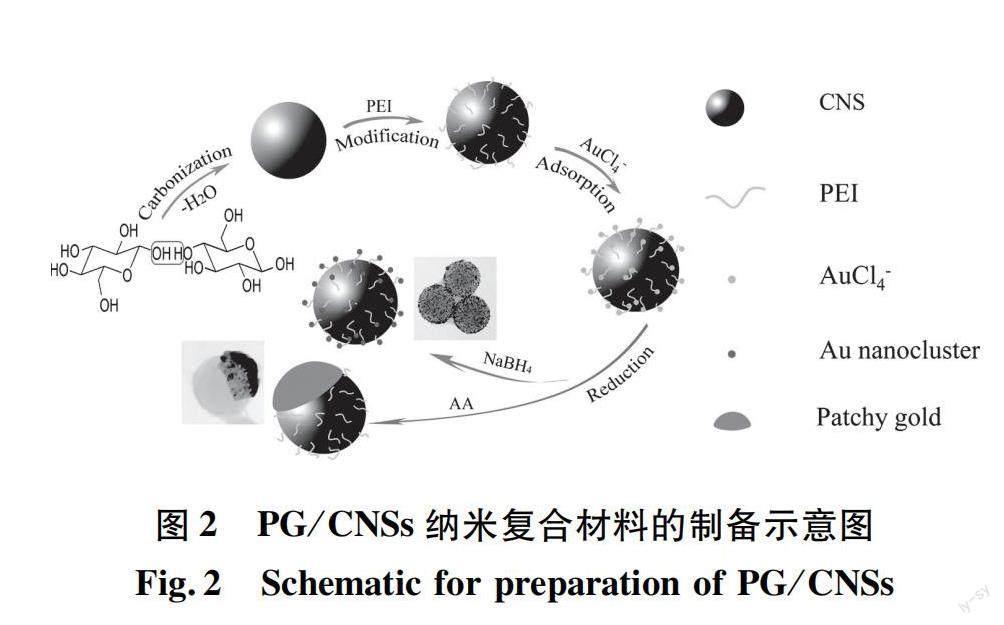
目前,癌症发病率不断上升,但传统的给药系统受到低生物利用度的限制,导致肿瘤部位药物积累很少,循环时间短[15],缺乏选择性[16]。此外,由于光散射的原因,单一的PTT技术难以治愈肿瘤。传统药物疗效显著,但副作用和耐药性较大。与单独PTT或化疗相比,两者联合应用可协同治疗肿瘤,达到药物可控释放,增强疗效[17-19]。据此,Liu等[20]设计并合成了一种多孔核壳结构、且具有良好生物相容性的多功能纳米载体Fe3O4@C/ZnO-DOX-FA。碳和ZnO均由ZIF-8于适当的温度下,在Ar或空气氛中煅烧获得(图1)。通过体外和体内实验证实,复合材料中的介孔碳不仅可以作为光热剂,还可与ZnO一起作为有效的药物载体。Fe3O4和ZnO同时存在,可以实现药物靶向细胞给药和pH控制释放,并且叶酸分子实现了癌细胞的特异性靶向[20]。Wang等[16]通过吸附-还原方法制备了一种金/碳纳米球(PG/CNSs)材料(制备过程如图2所示),其斑片状金的形成主要是一个热力学驱动的自发过程。通过葡萄糖水热碳化法合成碳纳米球,然后使用聚乙烯亚胺(polyethyleneimine,PEI)接枝到CNSs表面,将改性后的碳纳米球移入HAuCl4溶液中,AuCl-4离子吸附在主粒子表面,最后使用抗坏血酸(ascorbic acid,AA)弱还原剂将AuCl-4离子还原为Au,获得斑片状金/碳纳米球(PG/CNSs)。向体内注射一定量的PG/CNSs颗粒(0.2 mg),在激光照射下,肿瘤局部温度迅速达到64.4 ℃,足以消融恶性细胞。结果表明,PG/CNSs纳米材料以Janus结构的形式存在时,暴露在表面的斑片状金和碳的组合吸收NIR,从而增加NIR吸收系数。与单独的金纳米棒相比,由于对碳的保护,这种薄金贴片的热稳定性有望更高,所得的纳米复合材料不仅具有更高的光热转换效率,而且具有更高的热稳定性。
碳纳米管(carbon nanotube,CNT)包括单壁碳纳米管 (single-walled carbon nanotube,SWCNT)和多壁碳纳米管(multi-walled carbon nanotube,MWCNT),在NIR区域有强光吸收和高光热转换效率[21-22]。此外,碳纳米管具有高比表面积和体积,是核酸、蛋白质和药物分子等细胞内转运的潜在载体[23]。利用碳纳米管可有效地将化疗和光热治疗结合起来,Dong等[24]开发了一种新型的基于TAT-壳聚糖功能化多壁纳米管(MWCNTs/TC)的阿霉素(DOX)给药体系,并初步研究了抗肿瘤作用,探讨了MWCNTs/DOX/TC在化学光热联合治疗中的应用潜力,并评估药物体外释放、光热效应、细胞摄取和细胞毒性。结果表明,该新型给药体系不仅实现了DOX的显著缓释,而且保留了MWCNTs的光学特性,在近红外照射下具有较高的光热效应;同时,通过化疗和光热消融的协同作用,表现出显著增强的抗肿瘤疗效。
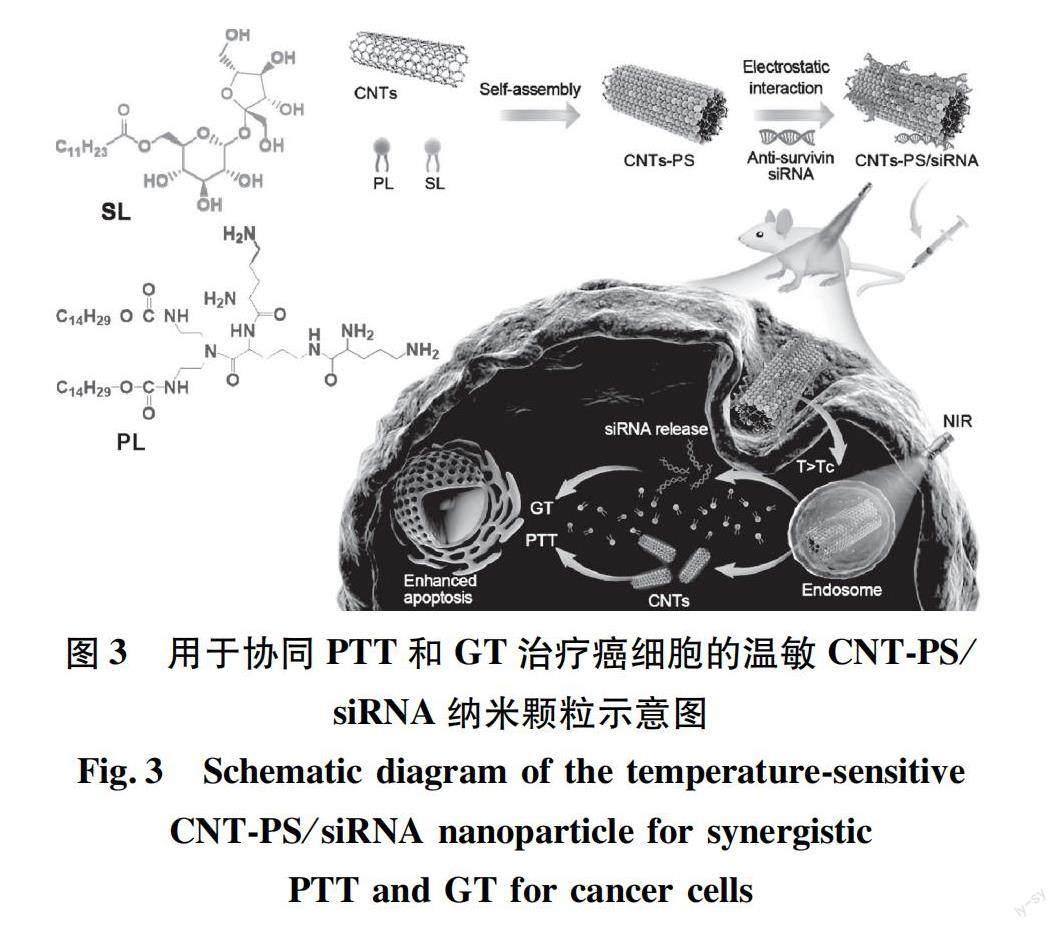
PTT与GT的联合应用在协同抗肿瘤方面同样具有很大潜力。光热转换材料作为基因传递载体,可以有效结合带负电荷的基因[25]。目前,碳纳米管被用作药物载体和生物成像探针[26]。Zhao等[27]采用肽脂质和月桂酸蔗糖包裹单壁碳纳米管(SWCNTs)和多壁碳纳米管(MWCNTs)(图3),形成了具有温度敏感性和光热性能的双功能传递体系(分别为SWCNT-PS和MWCNT-PS),并将siRNA加载到碳纳米管上,形成siRNA可控释放的载体CNT-PS/siRNA。结果表明,CNT/siRNA能抑制肿瘤的生长,同时在NIR光照下表现出光热效应。相较于MWCNT-PS/siRNA,SWCNT-PS/siRNA表现出非常高的抗肿瘤活性,可完全抑制部分肿瘤生长。
氮掺杂石墨烯量子点(N-GQDs)具有强电子供体基团[28]、较佳的生物相容性和光热稳定性,是良好的光热转换剂(photothermal conversion agents,PTCAs)。Zhang等[29]通过在核壳氮掺杂石墨烯量子点(N-GQD)@中空介孔二氧化硅纳米球(HMSN)上包覆介孔氮化碳(C3N4)层,并用P-PEG-RGD聚合物装饰,构建了一种智能纳米调节器R-NCNP(图4)。N-GQDs使得R-NCNP纳米调控器对高温具有光热效应,表现出较强的光热效应和红外热成像(IRT)。
2 无机杂化纳米材料
贵金属纳米材料具有较强表面等离子体共振效应(surface plasmon resonance effect,SPR)和可调谐性[30],特别是金颗粒(Au NPs),由于具有粒径小、光热转换率高、增强渗透性和保留效果(enhanced permeability and retention,EPR)等优点,成为PTT的主要介质[31]。氧化石墨烯(graphene oxide,GO)具有良好的水相分散性、易于表面改性[32]、具有良好的生物相容性、无毒性和稳定性,以及大型离域π-电子系统有助于氧化石墨烯在可见光和近红外区域获得良好的吸附能力。球形金纳米壳和石墨烯基纳米材料是两种主要的NIR热疗剂,已被报道用于癌症的光热治疗。基于两种材料的特征,He等[33]通过在氧化石墨烯涂上一层介孔二氧化硅,利用种子生长法构建了一层金纳米片,形成GO@SiO2@AuNS材料(图5)。氧化石墨烯上的二维结构和介孔二氧化硅层不仅为锚定金种子和金壳生长提供了模板,而且提高了氧化石墨烯的生物相容性和稳定性。GO@SiO2@AuNS杂化材料在近红外区域具有较强的吸收能力和光热转换效率,在低激光剂量(0.3 W/cm2)下表现出良好的光热稳定性,对癌细胞具有显著的光消融作用。与GO@SiO2相比,GO@SiO2@AuNS杂化物在808 nm处的吸光度提高了近40倍。GO@SiO2@AuNS杂化物的光热转换效率约为30%,高于金纳米棒(21.0%)。结果表明,GO@SiO2@AuNS杂化纳米材料具有突出的癌症光热治疗前景。
Liu等[34]通过DNA与稀土-金杂化纳米粒子(UCNP-Au NPS)的静电络合,制备了一种新型的近红外光响应的、可注射性的DNA杂化水凝胶DNA-UCNP-Au。采用溶剂热法制备了Yb3+和Er3+共掺杂的单分散NaYF4纳米粒子,并以其为核与NaYF4∶Nd形成杂化物。以聚醚酰亚胺(polyetherimide,PEI)为表面包覆剂将NaYF4∶Yb和Er@NaYF4∶Nd(UCNP)转化为亲水性的UCNP-PEI。接着,PEI与HAuCl4发生氧化还原反应生成UCNP-Au NPS(图6)。与原始无机纳米材料相比,DNA-UCNP-Au水凝胶具有较低的细胞毒性;同时,在相同的近红外光辐照下,表现出较快的加热速率,PTT可有效抑制肿瘤复发。
在各種捕光剂中,二元Cu基半导体(即Cu2-xS、Cu2-xSe和Cu2-xTe)由于无毒、成本低和铜空位在NIR区域表现出局域表面等离子体共振(localized surface plasmon resonance,LSPR)而具有较高的光热转换效率,近年来受到了广泛关注[35]。为了提高光疗效果,将PDT和PTT整合到一个系统中被认为是一种有效的策略[36]。Chen等[37]提出了由Cu-In-S(CIS)异质结纳米棒(HS-rod)、氯素e6(Ce6)和透明质酸(HA)组成的Ce6-HA-CIS光热治疗纳米杂化体(图7),用于靶向PDT/PTT。杂化体中CIS-HS-rod作为PTT剂将光能转化为热能,Ce6作为PDT剂产生单线态氧(1O2)。HA包裹CIS-HS纳米棒表面,并实现CIS-HS纳米棒的水溶性。制备的Ce6-HA-CIS纳米杂化体表现出较高的光热转换效率、良好的光稳定性和光动力活性。且体外和体内实验表明,与单独的PTT或PDT相比,Ce6-HA-CIS具有较低的细胞毒性和良好的协同光动力和光热杀伤癌细胞的作用。
Yang等[38]设计并合成了空心结构的hCu2-xS@Au纳米壳复合材料。通过在HCu2-xS@Au纳米颗粒表面修饰二硫键桥接Au纳米壳和多羧基石墨烯量子点(MC-GODs),实现可控给药、监测和高光热转换效率的多模式治疗。结果表明,在808 nm近红外光照射下,hCu2-xS@Au@MC-GODs的光热转换效率为32%。
在临床医学应用中,由于血液和软组织的吸收和散射,PTT需要具有相当深穿透力的近红外光[39]。先前的大量研究主要集中在近红外第一窗口(NIR-I ,750~1 000 nm),相比之下,近红外第二窗口(NIR-II, 1 000~1 350 nm)具有更深的组织穿透、更低的光散射和更大的最大允许曝光量(maximum permissible exposure,MPE)[40]。窄带隙的p型纳米半导体和具有LSPR的纳米金属都有良好的NIR光响应特性[41-45],将等离子体金属和窄带隙半导体集成到异质结构单元中,可促进高效的PTT。金纳米粒子作为最常用的等离子体纳米材料,在光催化[46]、生物成像、光热治疗等领域得到了广泛的应用[47]。传统的研究主要集中在纳米结构的形状和尺寸上。当相同的物质在种子上生长(同金属生长)时,界面完美匹配,生长材料将形成一个保形层,类似于液体层的润湿性。由于其中有强键合,很少有方法可以调整同金属界面。基于此,Jia等[48]报道了一种Au-on-AuNR杂化纳米珊瑚结构材料,通过嵌入小的有机硫醇分子增加生长材料与底层种子之间的界面能,导致典型的润湿生长转变为岛状非润湿生长模式,成功获得了一系列Au-on-AuNR杂化结构。金纳米棒上Au的生长情况可经优化结合界面能和反应动力学得到调节。通过改变AuNR上出现的Au域,可以在可见NIR光谱范围内有效且连续地微调,结合对AuNRs的尺寸和长宽比的常规控制,进而有利于功能材料的结构精细化调控。通过体外和体内实验得出材料在NIR-II区域具有强烈吸收和出色的光热转换行为,表明纳米珊瑚结构具有较好光热疗法和光声显像剂。Li等[49]制备了超薄层状双氢氧化物(layered double hydroxides,LDHs)负载的Ag@Ag2O核壳纳米颗粒(Ag@Ag2O/LDHs-U),极大地提高了NIR-II光热性能,在1 064 nm激光下光热效率高达76.9%。研究表明,超细Ag@Ag2O核壳纳米颗粒(约3.8 nm)高度分散并固定在超薄LDHs纳米片内,而Ag2O外壳具有丰富的空位型缺陷。此外,体内外活性测试进一步证实了Ag@Ag2O/LDHs-U在NIR-II区域具有良好的生物相容性和突出的PTT治疗效果。
3 有机-无机杂化纳米材料
近年来,开发的近红外光热材料可分为有机材料和无机材料两大类。然而,现有的有机近红外光热材料往往存在光稳定性差、光热转换效率低、血液清除速率快等问题[50],而无机光热材料通常存在生物相容性差、不可降解性、毒性大等缺点[51]。近红外有机/无机纳米杂化材料不仅可以结合有机和无机部分的原始性能,而且通过单个成分的协同作用(如理想的光学、生物和药物特性等)[52-53],有望产生新的特性,在PTT领域引起了广泛关注。通过无机纳米活性结构与功能有机分子的偶联和/或组装,不仅可以提高NIR治疗癌症的疗效,还可以增强NIR光热治疗剂的生物相容性和稳定性。
LDHs作为一种二维阴离子插层功能纳米材料或前驱体,因其在物理化学性质和结构上的可变性而受到纳米材料领域的广泛关注[54]。Zhang等[55]报道了一种低带隙的电子给体-受体效应诱导的有机/无机纳米杂化物(ICG/Ag/LDHs)。将Ag纳米粒子原位沉积到CoAl-LDHs表面(Ag/LDHs),然后将ICG耦合到Ag/LDHs上。结果表明,在808 nm激光照射下,ICG/Ag/LDHs的光热转换效率(~45.5%)比ICG(~28.4%)提高了1.6倍。且体外和体内实验结果均证实了ICG/Ag/LDHs在NIR引发的癌症治疗中具有良好的生物相容性。
此外,Sun等[56]将金纳米棒(gold nanorods,GNRs)封装在聚吡咯(polypyrrole,PPy)外壳中,并通过修改SiO2硬模板来控制它们之间的空隙空间,形成具有可调谐的空隙空间(GNRs@Void@PPy)。双NIR吸收的组分提供了协同增强光热性能的作用,并通过体外和体内实验证实了该杂化物具有较高的抗肿瘤活性。
目前,在NIR-II生物窗中已經开发了一系列用于肿瘤PTT和成像的PTAs,如WO3-x纳米点(NDs)、Bi2S3-Ag2S-DATS@BSA-N3纳米系统、Au NDs和TeO2/(NH4)xWO3NCs[57-60]等。此外,据报道,Cu2Se比Cu2S或Cu2SSe具有更好的光热效应[61],可能是由Se2-最外层的电子产生的,比S2-更容易吸收光子并被激光激发。基于此,Hu等[62]报道了一种新型的NIR-II响应纳米平台(硒化镍@聚多巴胺纳米复合材料,NiSe@PDA NCs),用于双模型成像引导光热治疗,在NIR-II激光(1 064 nm)照射下,NiSe@PDA NCs的光热转换效率可达48.4%,光热转换效率高于单个NiSe NPs。
无机纳米材料二硫化钼(MoS2)[63 ]作为PTT纳米剂或放射增敏剂得到了广泛的研究。由于无机纳米级材料不能被生物降解,通常会在体内停留很长时间,造成潜在的长期毒性。聚苯胺(polyaniline,PANI)具有良好的光热转换效率、显著的生物相容性和良好的光稳定性等特征。鉴于此,Wang等[64]通过静电作用采用溶剂热法制备了二硫化钼量子点@聚苯胺(MoS2@PANI)杂化多功能纳米材料。聚苯胺作为可生物降解和无毒的有机材料,可有效降低无机材料的治疗浓度,进而降低MoS2在体内的保留量。通过与偶联剂形成稳定的酰胺键,得到可溶性良好和稳定高的MoS2@PANI杂化纳米材料,其具有较强的X射线衰减和较高的NIR吸收效率,可作为肿瘤X射线计算机断层扫描(X-ray computed tomography,X-CT)和光声(photoacoustic,PA)成像的造影剂。二硫化钼纳米量子点能产生强烈的荧光,有望作为体外和体内成像的探针或放射增敏剂。由于PTT诱导的适当水平的热疗可以增加肿瘤内血流,进而增强肿瘤微环境中的氧条件,导致细胞对放射治疗(radiation therapy,RT)更加敏感[65]。简言之,MoS2@PANI杂化纳米材料可实现同步CT/PA成像和协同PTT/RT联合治疗癌症。
霍氏小组报道了钨氧化物纳米颗粒(WO NPs)可以在NIR-II范围内吸收光,并且具有良好的光热转换效果和稳定性。一些光热剂,如PANI细胞毒性低,不仅具有优异的光热转化性能,而且可作为表面涂层材料,与其他光热剂[66-68]形成复合光热纳米载体。鉴于PANI和W18O49在光热治疗中的优势,Yang等[69]制备PANI@W18O49@Fe3O4(PWF)和PANI@W18O49(PW)的有机-无机杂化纳米颗粒。不同波长(808和1 064 nm)下的纳米颗粒的吸光度和光热测量结果表明,其光热性能是稳定的。PANI@W18O49在808和1 064 nm激光照射下的光热转换效率可分别达到50.43%和30.69%,PANI@W18O49@Fe3O4的光熱转换效率也可达到63.9%和32.55%。药物释放实验证明,混合纳米颗粒可以在双刺激反应(pH和温度)条件下控制DOX的释放,从而减少生理条件下药物不受控制释放引起的副作用。研究结果表明,制备的杂化纳米颗粒具有应用于光热和化疗联合治疗的潜力;同时,杂化纳米颗粒不仅不影响W18O49的光热性能,而且提高了其抗氧化性能。
4 总结与展望
光热治疗由于具有高效快速、侵入性小、无创等优点,被广泛开发用于治疗恶性肿瘤、消炎、抗菌等生物医学应用领域。本文总结了最近几年光热杂化材料的制备方法及其功能活性。随着无机、有机光热材料的发展,通过多种光热材料的掺杂、包覆、表面修饰等方法开发杂化纳米材料(包括碳基杂化材料、无机杂化材料、有机-无机杂化材料等)解决了使用单一材料面临的生物相溶性差、稳定性差、毒性大、靶向性能差、缺少成像性等问题。杂化纳米材料通过融合光热材料、靶向材料、成像材料、化疗材料和放射性材料等达到联合协同治疗的效果。同时,光热治疗与化疗、放疗、光动力治疗的协同运用为其在临床上的应用提供了可能。这些方法为进一步开发高光热效率、高安全性的新型杂化光热材料提供了思路,促进高效治疗癌症技术的发展,为光热协同治疗提供材料支持,使得光热杂化纳米材料在肿瘤的治疗临床应用具有广阔的前景。
参考文献:
[1] LV Z Q, HE S J, WANG Y F, et al. Noble metal nanomaterials for NIR-triggered photothermal therapy in cancer[J]. Advanced Healthcare Materials, 2021, 10(6): 2001806.
[2] BAO Z H, LIU X R, LIU Y D, et al. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy[J]. Asian Journal of Pharmaceutical Sciences, 2016, 11(3): 349-364.
[3] ZOU Y, LI M L, XIONG T, et al. A single molecule drug targeting photosensitizer for enhanced breast cancer photothermal therapy[J]. Small, 2020, 16(18): 1907677.
[4] ZHAO R X, ZHU Y L, ZHOU J L, et al. Dual glutathione depletion enhanced enzyme catalytic activity for hyperthermia assisted tumor therapy on semi-metallic VSe2/Mn-CS[J]. ACS Nano, 2022, 16(7): 10904-10917.
[5] BIAN W Q, WANG Y K, PAN Z X, et al. Review of functionalized nanomaterials for photothermal therapy of cancers[J]. ACS Applied Nano Materials, 2021, 4(11): 11353-11385.
[6] NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Two-dimensional gas of massless Dirac fermions in graphene[J]. Nature, 2005, 438(7065): 197-200.
[7] YU J, JAVIER D, YASEEN M A, et al. Self-assembly synthesis, tumor cell targeting, and photothermal capabilities of antibody-coated indocyanine green nanocapsules[J]. Journal of the American Chemical Society, 2010, 132(6): 1929-1938.
[8] YANG J, CHOI J, BANG D, et al. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells[J]. Angewandte Chemie-International Edition, 2011, 123(2): 461-464.
[9] LOVELL J F, JIN C S, HUYNH E, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents[J]. Nature Materials, 2011, 10(4): 324-332.
[10]ALAMDARI S G, AMINI M, JALILZADEH N, et al. Recent advances in nanoparticle-based photothermal therapy for breast cancer[J]. Journal of Controlled Release, 2022, 349: 269-303.
[11]WANG J, WU X, SHEN P, et al. Applications of inorganic nanomaterials in photothermal therapy based on combinational cancer treatment[J]. International Journal of Nanomedicine, 2020, 15: 1903.
[12]HUANG J S, WANG J Q, HUANG Z C, et al. Photothermal technique-enabled ambient production of microalgae biodiesel: mechanism and life cycle assessment[J]. Bioresource Technology, 2023,369: 128390.
[13]KOTCHEY G P, ALLEN B L, VEDALA H, et al. The enzymatic oxidation of graphene oxide[J]. ACS Nano, 2011, 5(3): 2098-2108.
[14]CHENG L, RUAN W M, ZOU B F, et al. Chemical template-assisted synthesis of monodisperse rattle-type Fe3O4@C hollow microspheres as drug carrier[J]. Acta Biomaterialia, 2017, 58: 432-441.
[15]ZHANG Y L, LIU G N, WEI J Y, et al. Platelet membrane-based and tumor-associated platelettargeted drug delivery systems for cancer therapy[J]. Frontiers of Medicine, 2018, 12(6): 667-677.
[16]WANG J H, LI D, FAN Y, et al. Core-shell tecto dendrimers formed via host-guest supramolecular assembly as pH-responsive intelligent carriers for enhanced anticancer drug delivery[J]. Nanoscale, 2019, 11(46): 22343-22350.
[17]ZHANG M Y, LIU X J, LUO Q, et al. Tumor environment responsive degradable CuS@mSiO2@MnO2/DOX for MRI guided synergistic chemo-photothermal therapy and chemodynamic therapy[J]. Chemical Engineering Journal, 2020, 389: 124450.
[18]WANG Y Y, LIU X J, DENG G Y, et al. Se@SiO2-FA-CuS nanocomposites for targeted delivery of DOX and nano selenium in synergistic combination of chemo-photothermal therapy[J]. Nanoscale, 2018, 10(6): 2866-2875.
[19]LEE J H, GIBSON K J, CHEN G, et al. Bipyramid-templated synthesis of monodisperse anisotropic gold nanocrystals[J]. Nature Communications, 2015, 6: 1-9.
[20]LIU X, WANG C, WANG X Y, et al. A dual-targeting Fe3O4@C/ZnO-DOX-FA nanoplatform with pH-responsive drug release and synergetic chemo-photothermal antitumor in vitro and in vivo[J]. Materials Science & Engineering C-Materials for Biological Applications, 2021, 118: 111455.
[21]BAO Z H, LIU X R, LIU Y D, et al. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy[J]. Asian Journal of Pharmaceutical Sciences, 2016, 11(3): 349-364.
[22]HUANG J S, JIAN Y M, LI H, et al.Lignin-derived layered 3D biochar with controllable acidity for enhanced catalytic upgrading of Jatropha oil to biodiesel[J]. Catalysis Today, 2022, 404: 35-48.
[23]QI X L, RUI Y, FAN Y C, et al. Galactosylated chitosan-grafted multiwall carbon nanotubes for pH-dependent sustained release and hepatic tumor-targeted delivery of doxorubicin in vivo[J]. Colloids and Surfaces B-Biointerfaces, 2015, 133: 314-322.
[24]DONG X, SUN Z T, WANG X X, et al. An innovative MWCNTs/DOX/TC nanosystem for chemo-photothermal combination therapy of cancer[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2017, 13(7): 2271-2280.
[25]KIM J H, KIM J H, JEONG C, et al. Synergistic nanomedicine by combined gene and photothermal therapy[J]. Advanced Drug Delivery Reviews, 2016, 98: 99-112.
[26]LIU Z, SUN X M, NAKAYAMA-RATCHFORD N, et al. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery[J]. ACS Nano, 2007, 1(1): 50-56.
[27]ZHAO Y A, ZHAO T Y, CAO Y N, et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy[J]. ACS Nano, 2021,15(4): 6517-6529.
[28]LIU Q, GUO B D, RAO Z Y, et al. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging[J]. Nano Letters, 2013, 13(6): 2436-2441.
[29]ZHANG X, ONGACHWA MACHUKI J, PAN W Z, et al Carbon nitride hollow theranostic nanoregulators executing laser-activatable water splitting for enhanced ultrasound/fluorescence imaging and cooperative phototherapy[J]. ACS Nano, 2020, 14(4): 4045-4060.
[30]LV Z Q, HE S J, WANG Y F, et al. Noble metal nanomaterials for NIR-triggered photothermal therapy in cancer[J]. Advanced Healthcare Materials, 2021, 10(6): 2001806.
[31]CHEN H J, SHAO L, MING T, et al. Understanding the photothermal conversion efficiency of gold nanocrystals[J]. Small, 2010, 6(20): 2272-2280.
[32]MACKEY M A, ALI M R K, AUSTIN L A, et al. The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments[J]. The Journal of Physical Chemistry B, 2014, 118: 131326.
[33]HE S Y, LI J Y, CHEN M J, et al. Graphene oxide-template gold nanosheets as highly efficient near-infrared hyperthermia agents for cancer therapy[J]. International Journal of Nanomedicine, 2020, 15: 8451.
[34]LIU B, SUN J, ZHU J J, et al. Injectable and NIR-responsive DNA-inorganic hybrid hydrogels with outstanding photothermal therapy[J]. Advanced Materials, 2020, 32(39): 2004460.
[35]ALONSO M I, WAKITA K, PASCUAL J, et al. Optical functions and electronic structure of CuInSe2, CuGaSe2, CuInS2, and CuGaS2[J]. Physical Review B, 2001, 63(7): 075203.
[36]LI Q, HONG L, LI H G, et al. Graphene oxide-fullerene C60(GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light[J]. Biosensors & Bioelectronics, 2017, 89: 477-482.
[37]CHEN S H, HUANG W W, DEHVARI K, et al. Photosensitizer-conjugated Cu-In-S heterostructured nanorods for cancer targeted photothermal/photodynamic synergistic therapy[J]. Materials Science & Engineering C-Materials for Biological Applications, 2019, 97: 793-802.
[38]YANG L, HU B, LIU A H, et al. A hollow-structured nanohybrid: intelligent and visible drug delivery and photothermal therapy for cancer[J]. Talanta, 2020, 215: 120893.
[39]LIU Y J, BHATTARAI P, DAI Z F, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer[J]. Chemical Society Reviews, 2019, 48(7): 2053-2108.
[40]LIN H, GAO S S, DAI C, et al. A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows[J]. Journal of the American Chemical Society, 2017, 139(45): 16235-16247.
[41]JI M W, XU M, ZHANG W, et al. Structurally well-defined Au@Cu2-xS core-shell nanocrystals for improved cancer treatment based on enhanced photothermal efficiency[J]. Advanced Materials, 2016,28(16), 3094-3101.
[42]LI H, LI Y, FANG Z, et al. Efficient catalytic transfer hydrogenation of biomass-based furfural to furfuryl alcohol with recycable Hf-phenylphosphonate nanohybrids[J]. Catalysis Today, 2019, 319: 84-92.
[43]HUANG J S, JIAN Y M, ZHU P, et al. Research progress on the photo-driven catalytic production of biodiesel[J]. Frontiers in Chemistry, 2022, 10: 904251.
[44]ZHOU Z, LI B, SHEN C, et al. Metallic 1T phase enabling MoS2nanodots as an efficient agent for photoacoustic imaging guided photothermal therapy in the near-infrared-ii window[J]. Small, 2020, 16(43): 2004173.
[45]LI H, ZHAO W F, RIISAGER A, et al. A Pd-catalyzed in situ domino process for mild and quantitative production of 2, 5-dimethylfuran directly from carbohydrates[J]. Green Chemistry, 2017,19(9): 2101-2106.
[46]JIA H L, DU A X, ZHANG H, et al. Site-selective growth of crystalline ceria with oxygen vacancies on gold nanocrystals for near-infrared nitrogen photofixation[J]. Journal of the American Chemical Society, 2019, 141: 5083-5086.
[47]LIU Y J, BHATTARAI P, DAI Z F, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer[J]. Chemical Society Reviews, 2019, 48: 2053-2108.
[48]JIA J, LIU G Y, XU W J, et al. Fine-tuning the homometallic interface of au-on-au nanorods and their photothermal therapy in the NIR-II window[J]. Angewandte Chemie, 2020, 132: 14551-14556.
[49]LI K L, MA X T, HE S, et al. Ultrathin nanosheet-supported Ag@Ag2O core-shell nanoparticles with vastly enhanced photothermal conversion efficiency for NIR-II-triggered photothermal therapy[J]. ACS Biomaterials Science & Engineering, 2022, 8(2): 540-550.
[50]FORGACS E, CSERHTI T, OROS G. Removal of synthetic dyes from wastewaters: a review[J]. Environment International, 2004, 30(7): 953-971.
[51]LIU Z, FAN A C, RAKHRA K, et al. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy[J]. Angewandte Chemie-International Edition, 2009, 48(41): 7668-7672.
[52]ZHAO N N, YAN L M, ZHAO X Y, et al. Versatile types of organic/inorganic nanohybrids: from strategic design to biomedical applications[J]. Chemical Reviews, 2018, 119(3): 1666-1762.
[53]LI H, ZHAO W F, FANG Z. Hydrophobic Pd nanocatalysts for one-pot and high-yield production of liquid furanic biofuels at low temperatures[J]. Applied Catalysis B: Environmental, 2017, 215: 18-27.
[54]GUAN S Y, WENG Y Z, LI M N, et al. An NIR-sensitive layered supramolecular nanovehicle for combined dual-modal imaging and synergistic therapy[J]. Nanoscale, 2017, 9(29): 10367-10374.
[55]ZHANG G J, LI K, HE S, et al. Electron donor-acceptor effect-induced organic/inorganic nanohybrids with low energy gap for highly efficient photothermal therapy[J]. ACS Applied Materials & Interfaces, 2021, 13(15): 17920-17930.
[56]SUN X H, WANG J, WANG Z Y, et al. Gold nanorod@void@ polypyrrole yolk@shell nanostructures: synchronous regulation of photothermal and drug delivery performance for synergistic cancer therapy[J]. Journal of Colloid and Interface Science, 2022, 610: 89-97.
[57]ZHENG Z L, CHEN Q, DAI R, et al. A continuous stimuli-responsive system for NIR-II fluorescence/photoacoustic imaging guided photothermal/gas synergistic therapy[J]. Nanoscale, 2020, 12(21): 11562-11572.
[58]CHENG Y R, YANG F, XIANG G L, et al. Ultrathin tellurium oxide/ammonium tungsten bronze nanoribbon for multimodality imaging and second near-infrared region photothermal therapy[J]. Nano Letters, 2019, 19(2): 1179-1189.
[59]YIN C, LI X Z, WANG Y, et al. Organic semiconducting macromolecular dyes for NIR-II photoacoustic imaging and photothermal therapy[J]. Advanced Functional Materials, 2021, 31(37): 2104650.
[60]LI J, JIANG R C, WANG Q, et al. Semiconducting polymer nanotheranostics for NIR-II/photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy[J]. Biomaterials, 2019, 217: 119304.
[61]WANG X W, ZHONG X Y, LEI H L, et al. xHollow Cu2Se nanozymes for tumor photothermal-catalytic therapy[J]. Chemistry of Materials, 2019, 31(16): 6174-6186.
[62]HU W X, ZHEN W Y, ZHANG M C, et al. Development of nickel selenide@polydopamine nanocomposites for magnetic resonance imaging guided NIR-II photothermal therapy[J]. Advanced Healthcare Materials, 2021, 10(23): 2101542.
[63]YIN W Y, YAN L, YU J, et al. High-throughput synthesis of single-layer MoS2nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy[J]. ACS Nano, 2014, 8: 6922-6933.
[64]WANG J P, TAN X X, PANG X J, et al. MoS2quantum dot@polyaniline inorganic-organic nanohybrids for in vivo dual-modal imaging guided synergistic photothermal/radiation therapy[J]. ACS Applied Materials & Interfaces, 2016, 8: 24331-24338.
[65]YONG Y, CHENG X J, BAO T, et al. Tungsten sulfide quantum dots as multifunctional nanotheranostics for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy[J]. ACS Nano, 2015, 9: 12451-12463.
[66]LI H, GUO H X, SU Y Q, et al. N-formyl-stabilizing quasi-catalytic species afford rapid and selective solvent-free amination of biomass-derived feedstocks[J]. Nature Communications, 2019,10(1): 699.
[67]YU C C, XU L J, ZHANG Y Y, et al. Polymer-based nanomaterials for noninvasive cancer photothermal therapy[J]. ACS Applied Polymer Materials, 2020, 2: 4289-4305.
[68]LI H, ZHANG Q S, BHADURY P S, et al. Furan-type compounds from carbohydrates via heterogeneous catalysis[J]. Current Organic Chemistry, 2014 18(5): 547-597.
[69]YANG S S, YANG P F, XIE Y L, et al. Organic-inorganic hybrid photothermal nanomaterials for combined photothermal and chemotherapy therapy of tumors under the dual biological window[J]. Journal of Materials Science, 2021, 56: 18219-18232.
(責任编辑:曾 晶)
Advances in Photothermal Therapy of Tumors
Using Hybrid Nanomaterials
HUANG Xiaoxiu1,2, LI Hu*2, YANG Song2
(1.Panzhou Peoples Hospital, Liupanshui 553500, China; 2.Center for Research & Development of Fine Chemicals, Guizhou University, Guiyang 550025, China)
Abstract: Photothermal therapy (PTT) is an efficient, rapid, less invasive or even non-invasive technique. A single nanomaterial is usually used as a photothermal agent in the photothermal therapy, which, however, is affected by problems such as poor biocompatibility, poor stability, high toxicity, poor targeting performance, and lack of imaging. Therefore, the author mainly reviews the hybrid nanomaterials developed in recent years. Through a variety of photothermal materials doping, coating, surface modification and other methods, carbon-based hybrid, inorganic hybrid, organic-inorganic hybrid and other photothermal materials with excellent photothermal properties have been developed to solve the problems faced by using single photothermal materials.In addition, the fusion of photothermal materials, targeting materials, imaging materials, chemotherapy materials and radioactive materials are used to achieve the effect of joint synergistic therapy. Hybrid materials promote the development of efficient cancer treatment technology and supply material support for photothermal synergistic therapy. These methods provide ideas for the further development of new hybrid photothermal materials. It is expected to develop hybrid photothermal materials with high photothermal efficiency and high safety based on the currently reported materials with photothermal potential, which makes photothermal hybrid nanomaterials have broad prospects in the clinical photothermal therapy of tumors.
Key words: photothermal therapy; photothermal materials; targeting specificity; biocompatibility; cancer cell
