Impact of Bif idobacterium longum NSP001 on DSS-induced colitis in conventional and humanised mice
2023-01-03MenglinChenHongYaoHuiziTanWenqiHuangQuanyongWuShaopingNie
Menglin Chen, Hong Yao, Huizi Tan, Wenqi Huang, Quanyong Wu, Shaoping Nie*
State Key Laboratory of Food Science and Technology, China-Canada Joint Laboratory of Food Science and Technology (Nanchang),Nanchang University, Nanchang 330047, China
Keywords:Bif idobacterium longum Ulcerative colitis Humanised mice Inf lammation Metabolism pathways
A B S T R A C T Most scientific investigations regarding inflammatory bowel disease (IBD) pathogenesis or therapeutic strategies use dextran sulfate sodium (DSS)-induced models performed on mice. However, differences between human and animal microbiota may confound the data reproducibility from rodent experiments to clinical trials. In this study, the intervention effects of Bif idobacterium longum NSP001 on DSS-induced colitis were investigated using mice colonized with either native or humanised microbiota. Disorders in disease activity index (DAI), morphology and histology of colon tissue, intestinal permeability, and secretion of MPO,TNF-α and IL-6 were ameliorated by daily intake of live B. longum NSP001 cells in both conventional and humanised colitis mice. But the abnormal thymus index, and colonic production of ZO-1 and iNOS were improved only in colitis mice treated with B. longum NSP001 and humanised microbiome. The accumulation of acetic acid and propionic acid in colon microbiome, and the optimization of primary bile acid biosynthesis and glycerophospholipid metabolism pathways in cecum commensals were likely to explain the benef icial effects of B. longum NSP001. These data revealed that intestinal microbiome baseline would possibly affect the manifestation features of interventions by probiotics or dietary components and highlighted the necessity to include humanised microbiome while investigating potential therapeutic strategies based on rodent models.
1. Introduction
Inflammatory bowel disease (IBD) is chronic and idiopathic,mainly including ulcerative colitis (UC) and Crohn’s disease (CD) [1].UC is often conf ined to the mucosal layer, and Crohn’s disease usually involves deeper tissues that can spread throughout the gastrointestinal tract. IBD has become a global health issue during the past few decades, due to frequent intake of the processed food and overuse of antibiotics followed with the development of industrialization [2].The pathogenesis of IBD still remains unknown, which may relate to a variety of factors such as heredity, environment, living habit and intestinal microbiota [3]. Disturbed intestinal microbiota were diagnosed in IBD patients according to clinical data, with significantly decreased Firmicutes and increased Bacteroidetes [4]. Ideal treatments for IBD have yet been established. 5-Aminosalicylic acid precursor such as mesalazine and sulfasalazine has been widely applied in clinic [5],but long-term use of which can cause side effects, such as allergic reactions and liver damage.
Bifidobacterium longumis the predominant species ofBifidobacteriumgenus colonized in the human gut, and has been approved to be supplemented in daily diet by the United States Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) [6]. Considerable evidences showed thatBifidobacteriumcould bind closely with the mucosal epithelium and thereby form advantages in niche occupation to prevent the colonization and invasion of pathogenic bacteria [7,8], and further improve intestinal disorders [9], tumorigenesis [10], immune system [11], and aging [12]. Moreover, the relative abundance ofBifidobacteriumin the intestine of the IBD patients was significantly lower than that of the healthy population [13]. Thus,Bifidobacteriummay serve as promising treatment method or adjuvant therapy for ameliorating UC, potentially through reducing inflammatory response and maintaining intestinal epithelial integrity [14,15].
The intestinal microbiota interact with host and play important roles in host immune system and metabolism, disturbance of which may trigger and contribute to the development of diseases [16]. For instance, transplantation of the intestinal microbiota from obese individuals resulted in obesity and impaired glucose tolerance in the recipient germ-free mice [17,18]. However, it is noteworthy that differences between human and animal microbiota may confound the disease analyses and the effects of dietary intervention based on conventional animal experiments. In order to comprehensively understand the probiotic functions and the associated mechanisms ofB. longumNSP001, in this study, mice models with DSSinduced colitis colonized with either native or humanised microbiota were established, and the impact ofB. longumNSP001 on organ injuries, intestinal barrier damages, inflammatory factors production, oxidative stress, and metabolites of gut microbiota were fully investigated.
2. Materials and methods
2.1 Materials
Pectin LM-13CG was supplied by CP Kelco (Atlanta, GA, USA)and was purified before experiments according to previous study [19].Dextran sulfate sodium (DSS, molecular weight 36 000-50 000 Da)was acquired from MP Bio (California, USA). Fluorescein isothiocyanate-dextran (dextran-FITC) with a molecular weight of 4 000 Da was purchased from Sigma-Aldrich (St Louis, MO, USA).Metronidazole, vancomycin, neomycin sulfate, and ampicillin were supplied by Beijing Solarbio Co., Ltd. (Beijing, China). Mouse ELISA kits were provided by SenBeiJia Biological Technology Co.,Ltd. (Nanjing, China). Other reagents were purchased from Shanghai Chemical sand Reagents Company (Shanghai, China). Acetic acid,propionic acid,n-butyric acid, andn-valeric acid (purity > 99%) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. All the other reagents used were of analytical grade and were purchased from Shanghai Chemicals and Reagents Co. (Shanghai, China).
2.2 Bacteria cultivation and fecal slurry preparation
B. longumNSP001 was isolated from fecal samples of a healthy Chinese adult and preserved in-house at the State Key Laboratory of Food Science and Technology of Nanchang University (Nanchang,China). The strain was cultured anaerobically using DeMan-Rogosa-Sharpe medium (Hopebio, China) supplemented withL-cysteine hydrochloride (Sangon Biotech, China) at 37 °C till stationary phase.Cell pellets were prepared in phosphate-buffered saline (PBS, pH 7.2)supplemented with 20% glycerol after centrifuging at 6 000 r/min for 10 min and kept at -80 °C before animal experiments. The cell viabilities after freezing and thawing were confirmed using colonyforming unit (CFU) counting on MRS agar plates supplemented withL-cysteine hydrochloride. Fecal slurries (10%,m/V) were prepared under anaerobic conditions by thoroughly mixing fecal sample from a healthy Chinese adult with PBS supplemented with 20% glycerol.The fecal slurries were aliquoted, preserved at -80 °C and thawed before administration to mice.
2.3 Animals
Five-week-old male C57BL/6 mice (SPF, 16-20 g) were purchased from Vital River Laboratories (Beijing, China). The mice were raised in the SPF-grade laboratory under temperature of(22 ± 2) °C, humidity of (55 ± 10)%, and a 12-h light/dark cycle.One-week acclimatization was allowed before experiments with standard diet and distilled water providedad libitum. All procedures used in this study involving animals were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Experimental Animal Care and Use Committee of Nanchang University.
2.4 Experimental protocol
Mice were randomly assigned in to two categories in the first two weeks: mice with native intestinal microbiota (NIM, 32 mice in total) which were given sterile saline by gavage and mice with humanised intestinal microbiota (HIM, 24 mice in total) which were given antibiotics cocktail (ampicillin 100 mg/kg body weight (BW),vancomycin 50 mg/kg BW, neomycin 100 mg/kg BW, metronidazole 100 mg/kg BW) [20,21] by oral administration. The effects microbiota depletion were confirmed by quantitative PCR of fecal samples as described [20,21] before fecal microbiota transplantation (data not shown). Then, mice in the NIM category were further divided into four groups (eight mice per group): the normal control group (NC),the DSS-treated model group (M), theB. longumNSP001 group (BD),and the pectin group (P) which was considered as the positive control according to our previous data [19]; and those in the HIM category were distributed into 3 groups (8 mice per group): the humanised group supplemented withB. longumNSP001 (AFBN), the DSStreated humanised group (AFD) and the DSS-treated humanised group supplemented withB. longumNSP001 (AFBD). During the next 14 days, mice in AFBN, AFD and AFBD groups were given 7-day microbiota transplantation with 0.2 mL fecal slurry per day; And mice in the NC, M and AFD groups were gavaged daily with sterile saline; mice in the BD, AFBN and AFBD were provided with daily administration of 1 × 108CFU ofB. longumNSP001; while mice in the P group were given a daily dose of 300 mg/kg BW of pectin LM-13CG. All the mice, except for those in the NC and AFBN group,received drinking water containing 3% DSS for the last seven days(Fig. 1). At the end of the experiment, all the mice were sacrificed by cervical dislocation with sodium pentobarbital anesthesia. Serum samples, colon tissues, colon and cecum contents were collected and were kept at -80 °C for further analysis.
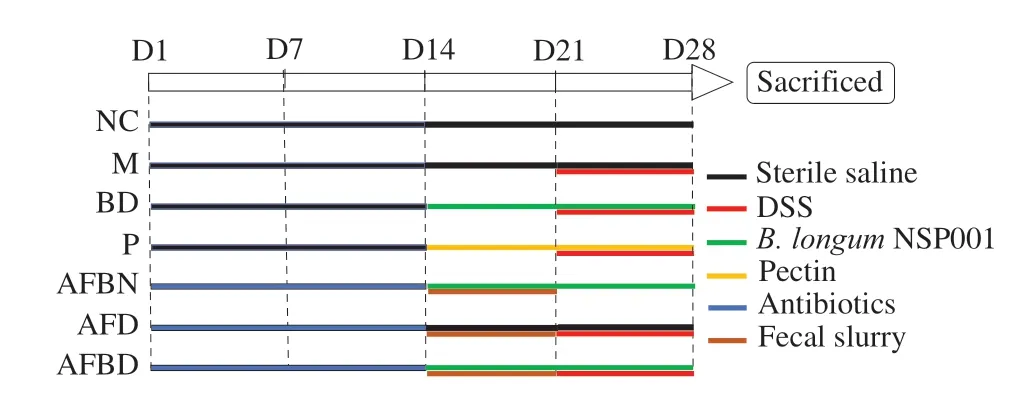
Fig. 1 Experiment design.
2.5 Pathological index evaluation
The length of colon tissue from the cecum to rectum and the weight of thymus were measured individually for calculating organ indexes. The disease activity index (DAI) score consists of three parts: body weight change rate, fecal traits and hematochezia degree of mice. The DAI calculating formulais as follows:
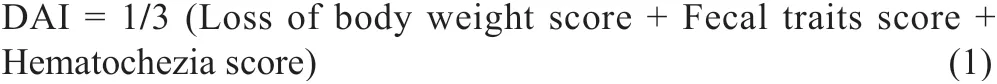
The specific scoring rules are shown in Table 1 [22,23].
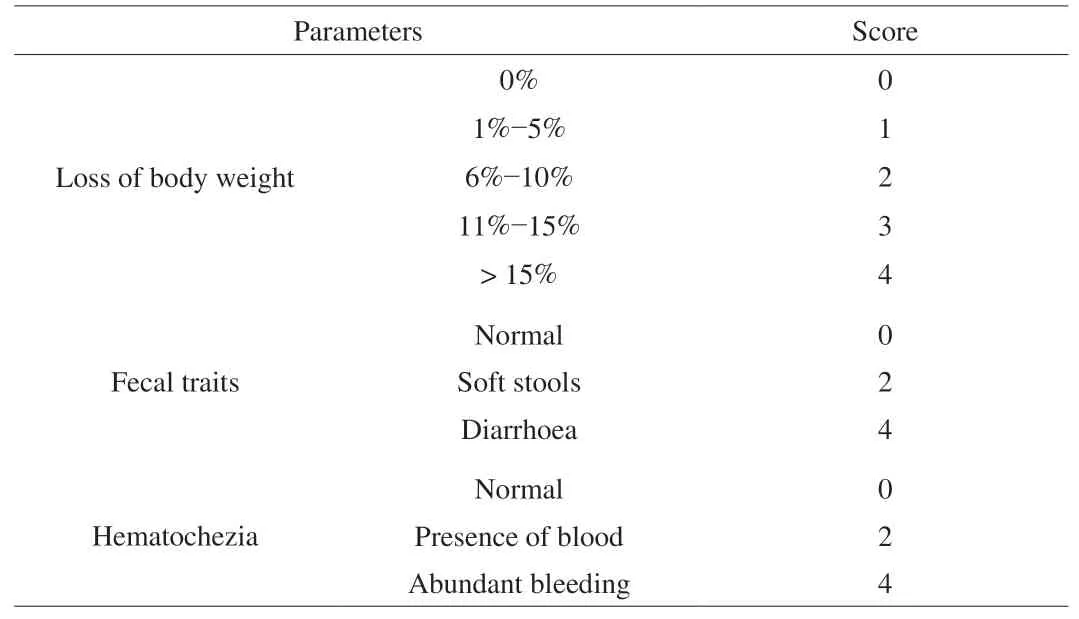
Table 1Scoring standards of DAI of the mice.
2.6 Histopathology analysis
Colon tissues of one centimeter were prepared and were made as Swiss rolls, fixed with 4% paraformaldehyde, and embedded in conventional paraffin. Hematoxylin-Eosin (HE) staining was carried out afterwards to assess the histopathological changes of colon tissue [24].
2.7 Intestinal permeability test
Intestinal permeability was evaluated by detecting serum levels of dextran-FITC and the expression of tight junction protein in colon. The mice were sacrificed 4 h after gavage with dextran-FITC (0.6 g/kg BW) to collect serum samples. Dextran-FITC was serially diluted by serum from untreated healthy mice to create a standard curve for calculation [25]. The concentration of dextran-FITC was determined by fluorescence spectrophotometer (excitation wavelength: 488 nm; emission wavelength: 520 nm) [26,27]. Colonic levels of tight junction proteins, including zonula occluden-1 (ZO-1)and occludin, were extracted by homogenization with PBS and were tested by corresponding ELISA kit according to the instruction of the manufacturer.
2.8 Measurement of inflammatory and oxidative stressrelated indicators
The colonic concentration of inflammatory factors including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and transforming growth factor (TGF)-β, and oxidative stress-related indicators such as myeloperoxidase (MPO) and inducible nitricoxide synthase(iNOS) were measured by corresponding ELISA kits according to the instructions of manufacturer.
2.9 Determination of short-chain fatty acids (SCFA)
Gradient mixed solution of standard substances including acetic
acid, propionic acid,n-butyric acid, andn-valeric acid were prepared to generate standard curve for calculation. 100 mg of colon contents were weighed and processed to determine the content of SCFA in the sample, as described [28]. Agilent 7890B gas chromatographic system with a flame ionization detector was used. Three independent tests were performed on each sample.
2.10 Metabolomics analysis of cecum contents
Forty milligram of cecum contents were freeze dried, and were homogenized and ultrasound treated, followed by mixing with methanol (1:8) so as to extract the total metabolites. Samples were subjected to the ultra-high performance liquid chromatography(UPLC, Shimadzu Nexera X2) for chromatography separation after deproteinization and filtration. And data acquisition was carried out on the AB SCIEX TripleTOF 5600 mass analyzer.
2.11 Statistical analysis
Results are expressed as means ± standard deviation (SD).Statistical analysis was performed by one-way analysis of variance(ANOVA) by the GraphPad Prism 6.01 (GraphPad, San Diego, CA,USA). Differences withPvalues less than 0.05 were considered to be statistically significant.
3. Results
3.1 Establishment of mice models colonized with native or humanised microbiota
Treatments of antibiotic cocktails significantly reduced intestinal bacterial counts by about 3 orders of magnitude according to RT-qPCR based on 16S V3 area, and by more than 4 orders of magnitude based on 16S V6 section (Fig 2A), which was in accordance with the published [21]. And the SCFA produced by colitis mice with native intestinal microbiota were significantly distinguished from those with humanised gut microbiota(Fig. 2B). Collectively, the establishment of humanised mice could be considered as successful.
In order to evaluate the feasibilities of applying humanised mice in studying the impact ofB. longumNSP001 on colitis, physiological indicators were carefully compared between the M and AFD groups,and between the NC and AFBN groups. As the results shown in Fig. 2C, there were no significant differences between colitis mice colonized with native and humanised microbiota in aspects of the body weight and colonic levels of the tight junction protein (Occludin),the inflammatory cytokine (TGF-β) and the oxidative stress-associated enzyme (MPO), which indicated that the fecal inocula from healthy human donor exhibited no protective effects on the DSS-induced colitis. Furthermore, healthy humanised mice supplemented withB. longumNSP001 did not perform any abnormity according to the body weight, intestinal barrier, inflammation- and oxidative stressrelated indicators (Fig. 2D), which revealed that the fecal slurry andB. longumNSP001 had no side effects on mice. Therefore, the mice models colonized with humanised microbiota can be used for further analysis of the probiotic functions and associated mechanisms ofB. longumNSP001.
3.2 Impact of B. longum NSP001 on body weight of mice
The alterations of body weight were monitored and recorded daily throughout the entire experiment. Before the DSS treatment started,7-day administration ofB. longumNSP001 displayed no significant influences on the body weight of mice regardless of the intestinal microbiota type (Fig. 3A). Pectin remarkably reduced the body weight of healthy mice (P< 0.05) in accordance with our previous data [19].Then, mice received drinking water containing 3% DSS performed obvious body weight loss (P< 0.001) compared to the NC group (Fig. 3B),which could be alleviated byB. longumNSP001 under both native and humanised intestinal micro-ecology, yet not significantly.

Fig. 2 Establishment of mice models colonized with native or humanised microbiota. (A) Intestinal bacteria elimination by antibiotic cocktails. (B) Microbial metabolites alterations after fecal microbiota transplantation in DSS-treated mice. (C) Impact of the human fecal slurry on DSS-treated mice, and (D) the potential side effects of human fecal slurry supplemented with B. longum NSP001 on healthy mice, in aspects of body weight alterations and the colonic concentration of ZO-1, TGF-β and MPO.

Fig. 3 The body weight alterations of mice during (A) pre-intervention period (Day 15-21) of B. longum NSP001 and pectin, and (B) DSS-treatment period (Day 22-28). Data are expressed as the mean ± SD. #P < 0.05,###P < 0.001 compared with the NC group.
3.3 Impact of B. longum NSP001 on the pathological indicators of DSS-induced colitis
DAI is widely used to appraise the pathological degree of colitis.During the DSS-treatment period, the DAI scores of mice in all groups except the NC group showed a gradually increasing trend,and the gradient of which the M group was the highest (Fig. 4A).The enhanced DAI were significantly reduced byB. longumNSP001(P< 0.05) and pectin (P< 0.001) intervention under native intestinal micro-ecology, and byB. longumNSP001 in DSS-challenged mice with humanised microbiota (P< 0.05) (Fig. 4A).
Thymus is considered an important immune organ in human body, decrease of the thymus index was positively correlated with the severity of inflammation. As shown in Fig. 4B, the thymus index of mice in the M group decreased significantly (P< 0.05) compared with the NC group. Thymus atrophy induced by DSS could be improved by interventions with pectin andB. longumNSP001, especially under humanised intestinal micro-ecology (P< 0.001).
DSS treatment induced obvious shortening of colon length(Fig. 4C), damaged epithelial mucosa, ulcerative crypt, and reduced goblet cells (Fig. 4D), which were typical symptoms of UC.The shortened colon of DSS-treated mice were improved by the intervention ofB. longumNSP001 in both native and humanised intestinal micro-ecology. The colonic surface of mice in the BD and AFBD groups were more smooth compared to those in the M group,with less bleeding spots and normal morphology of stool pellets inside were observed (Fig. 4C). And the histological scanning showed that administration ofB. longumNSP001 was capable of ameliorating the inflammatory cells infiltration, maintaining the integrity of the mucosa and crypt and stimulating sufficient goblet cells in the DSS-challenged mice regardless of the intestinal microbiota circumstance (Fig. 4D). Pectin improved the appearance and histology of colon in accordance with our previous data [19].
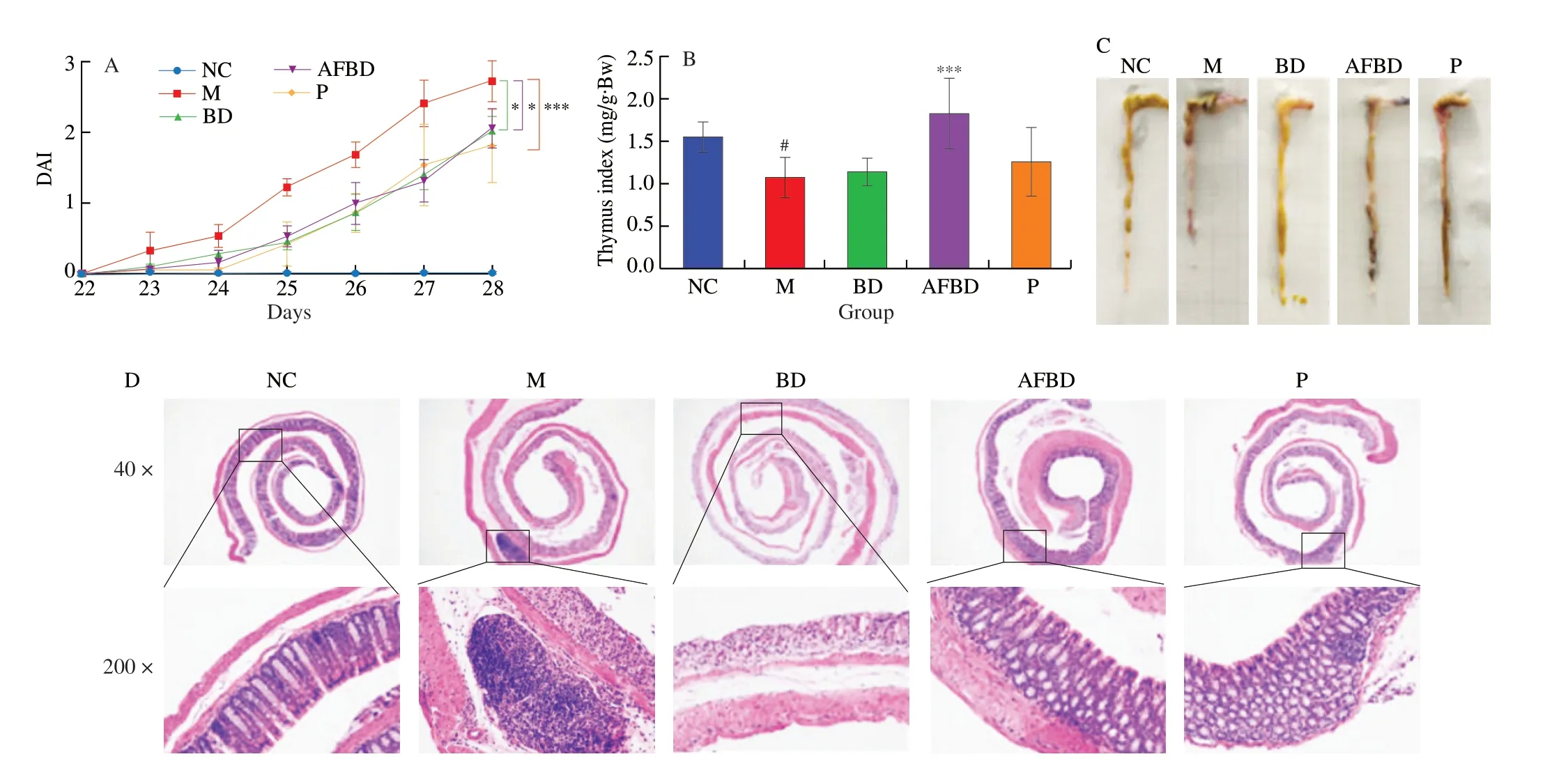
Fig. 4 Impact of the B. longum NSP001 on the pathological indicators of DSS-induced mice with native or humanised intestinal microbiota. (A) The DAI alterations during the 7-day DSS treatment; (B) thymus index; (C) pictures of colon; (D) the histological analysis of colon tissue at original magnification of 40 fold and 200 fold. Data are expressed as the mean ± SD. #P < 0.05 compared with the NC group; *P < 0.05, ***P < 0.001 compared with the M group.
3.4 Impact of B. longum NSP001 on the intestinal barrier function
We further determined the concentration of dextran-FITC in the blood samples of all mice and the concentration of ZO-1 and Occludin in the colon to assess the effects ofB. longumNSP001 on the intestinal barrier function in the DSS-treated mice (Fig. 5).Compared with those in the NC group, the serum levels of dextran-FITC in mice of the M group increased significantly (P< 0.001),indicating that the intestinal permeability was severely disturbed.However, the excessive penetration of dextran-FITC were eliminated in the mice of BD (P< 0.001), AFBD (P< 0.001) and P groups(P< 0.01) (Fig. 5A). Tight junction proteins ZO-1 and Occludin play an important role in regulating cell polarity and tight junction barrier function. Mice with DSS-induced colitis produced less ZO-1(P< 0.001) (Fig. 5B) and Occludin (P< 0.05) (Fig. 5C) in comparison with the normal mice. While intervention withB. longumNSP001 increased the concentration of tight junction proteins, especially the secretion of ZO-1 in the assistance of human intestinal microbiota(P< 0.05) (Fig. 5B).

Fig. 5 Impact of the B. longum NSP001 on the intestinal barrier of DSSinduced mice with native or humanised intestinal microbiota. (A) Serum concentration of dextran-FITC; (B) colonic concentration of ZO-1; (C) colonic level of Occludin. Data are expressed as the mean ± SD. #P < 0.05,###P < 0.001 compared with the NC group; *P < 0.05, **P < 0.01,***P < 0.001 compared with the M group.
3.5 Effects of B. longum NSP001 on the oxidative stressassociated indicators
As the result shown in Fig. 6, the activities of both MPO and iNOS in colon were significantly enhanced in the DSS-treated mice compared to those of the healthy mice (P< 0.001).B. longumNSP001 effectively inhibited the colonic MPO levels in mice of both BD (P< 0.01) and AFBD group (P< 0.05) (Fig. 6A). Nevertheless,the production of iNOS was remarkably reduced byB. longumNSP001 in the humanised mice (P< 0.05) (Fig. 6B). The content of colonic MPO (P< 0.001) and iNOS (P< 0.01) in mice of the P group was greatly decreased compared to those in the M group.
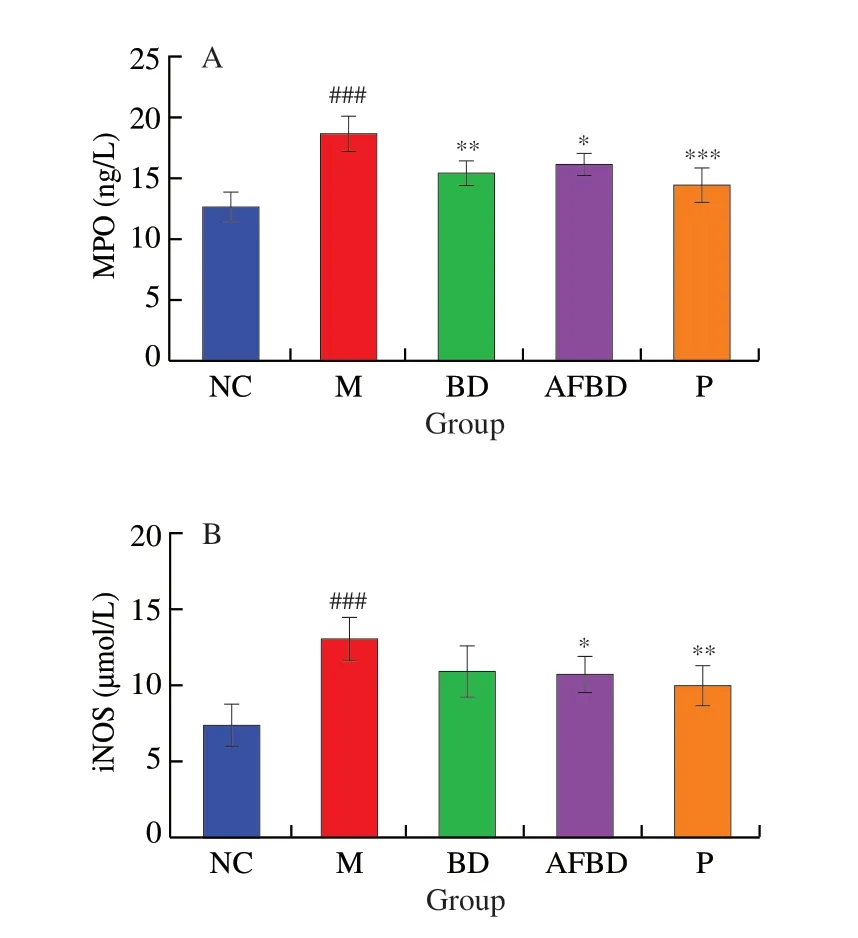
Fig. 6 Impact of the B. longum NSP001 on the oxidative stress-associated indicators in the colon tissues of DSS-induced mice with native or humanised intestinal microbiota. (A) MPO; (B) iNOS. Data are expressed as the mean ±SD. ###P < 0.001 compared with the NC group; *P < 0.05, **P < 0.01,***P < 0.001 compared with the M group.
3.6 Effects of B. longum NSP001 on the inflammatory cytokines
Measurements of pro-inflammatory cytokines like TNF-α and IL-6, and anti-inflammtory factors like TGF-β can directly reveal the degree of colon lesions in mice. After DSS intake, the content of TNF-α (P< 0.05) and IL-6 (P< 0.001) in the colon of mice in the M group increased significantly in comparison with those of the NC group (Figs. 7A and B). The treatments ofB. longumNSP001 decreased the secretion of pro-inflammatory TNF-α and IL-6 in mice with native intestinal microbiota (P< 0.01) and humanised intestinal microbiota (P< 0.001). Administration of pectin also relieved the over-expression of these pro-inflammatory molecules(P< 0.001). The abilities of producing anti-inflammatory TGF-β were severely impaired in mice of the M group (P< 0.001), which was improved in the BD, AFBD and P groups, but in a non-significant way (Fig. 7C).
3.7 Effects of B. longum NSP001 on the SCFA production in colon contents
As shown in Fig. 8, the concentrations of acetic acid, propionic acid andn-valeric acid in colon contents of mice in the M group decreased significantly after DSS treatment compared with the NC group (P< 0.001). Acetic acid was obviously increased in the BD(P< 0.05) and AFBD groups (P< 0.05), as well as propionic acid in the BD (P< 0.01) and AFBD groups (P< 0.001).B. longumNSP001 helped accumulatingn-butyric andn-valeric acid in the mice with DSS-induced colitis, yet not notably. The treatment of pectin significantly enhanced the abilities of intestinal microbiota to produce
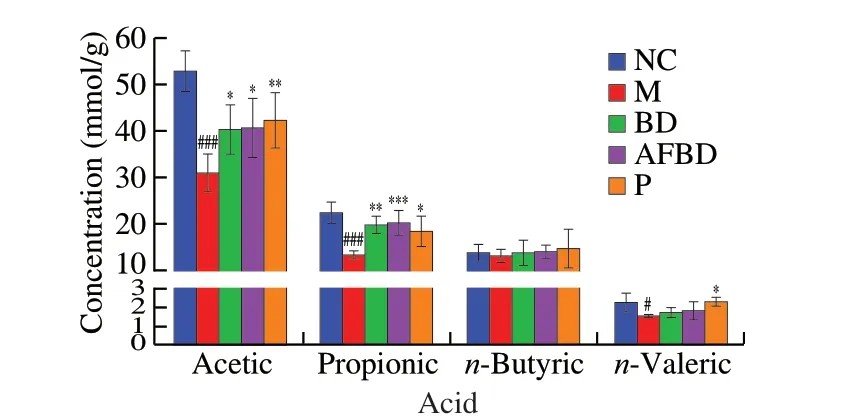
Fig. 8 Impact of the B. longum NSP001 on SCFA levels in the colon content of DSS-induced mice with native or humanised intestinal microbiota. Data are expressed as the mean ± SD. #P < 0.05, ###P < 0.001 compared with the NC group; *P < 0.05, **P < 0.01, ***P < 0.001 compared with the M group.
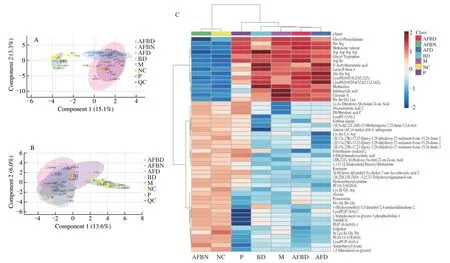
Fig. 9 Impact of B. longum NSP001 on the metabolites in the cecum contents of DSS-induced mice with native or humanised intestinal microbiota. (A, B)sPLSDA score plots; (C, D) the hierarchical clustering heatmap; (E, F) summary plot for pathway analysis of positive and negative models.

Fig. 9 (Continued)
acetic acid (P< 0.01), propionic acid (P< 0.05) andn-valeric acid(P< 0.05).
3.8 Effects of B. longum NSP001 on the metabolites in cecum contents
The reproducibility of the metabolomics investigation method and the statistical quality was evaluated by sPLSDA analysis (Figs.9A and B), and the overlap of data from the M and AFD groups, the NC and AFBN groups based on both positive and negative models further revealed that fecal slurryper seand supplemented withB. longumNSP001 did not impact the metabolomics of DSS-treated and normal mice respectively. According to the sPLSDA score plot(Figs. 9A and B) and the hierarchical clustering heatmap (Figs. 9C and D), the metabolites of cecum contents from mice in the NC and M group were obviously variant. The reduced levels of PE (16:0/0:0)and lysoPC (18:0) by DSS-challenged native mice were improved by administration ofB. longumNSP001 (Figs. 9C and 9D). While,the production of small peptides such as Arg-Gln-Leu-Thr and Pro-Ala-His-Gly in mice of the AFBD group were more than those of the AFD group (Figs. 9C and D). And the differentially expressed metabolites contributed to the significant differences in activation of pathways between the model and the BD groups (P< 0.05), including glycine, serine and threonine metabolism, cysteine and methionine metabolism, taurine and hypotaurine metabolism, primary bile acid biosynthesis, arginine and proline metabolism, aminoacyl-tRNA biosnthesis, glycerolipidmetabolism, glycerophospholipid metabolism and arginine biosynthesis (Figs. 9E and F).
4. Discussion
Most scientific investigations regarding IBD pathogenesis or therapeutic strategies use DSS-induced models performed on mice [25,29]. However, a recent study which revealed the significant differentiation in the gut microbiome among conventional and humanised mouse or rat with colitis [30], raised concerns about the data reproducibility from rodent experiments to clinical trials.Hence, we proposed that both native and humanised intestinal microecologies should be involved in primary investigations of dietary interventions using rodent models.
In this study, the 7-day DSS treatment in both conventional and humanised mice resulted in obviously reduced body weight, colon length and thymus index, hyperemic and erosive colon, and abnormal levels of colonic tight junction proteins, cytokines and oxidative stress associated indicators, most of which were ameliorated by pectin as reported in our previous study [19]. These data indicated that the UC had been successfully established. Meanwhile, the transplanted gut microbiome showed neither harmful nor preventive effects on healthy and colitis mice, which eliminated concerns about whether fecal transplantation would confound the functions ofB. longumNSP001.
Body weight loss is one the of typical and visible symptoms of UC [31],which has happened to the DSS-treated mice. However, DSStreated mice under supplementation ofB. longumNSP001 displayed less body weight loss than those without probiotics intervention,which directly reflected the potentials ofB. longumNSP001 in alleviating colitis. Furthermore, the disruption of intestinal barrier allows penetrations of microbial molecules into circulation, triggers or exacerbates intestinal and systematic inflammation.B. longumNSP001 inhibited the intrusion of dextran-FITC through epithelial cells in cooperation with either native or humanised microbiome and sustained the integrity of intestinal mucosal structures, which oriented the hypothesis thatB. longumNSP001 could interact with intestinal epithelial cells to reduce water secretion, enhance the production of mucin, and restore impaired colon barrier function.The tight junctions located at the apical and lateral region of the epithelial cells and are important parts of the maintenance of mucosal permeability [32]. However, tight junction protein of ZO-1 could only be recovered in DSS-treated humanised mice supplemented withB. longumNSP001. Further interaction analysis between gut microbiota and the potentially associated signaling pathways such as AMPK phosphorylation [33] should be considered.
Over-expression of oxidative stress and pro-inflammatory cytokines play important roles in UC. The activity of phagocytosis of colonic leukocytes is significantly increased in UC patients, which gives rise to the accumulation of free radical molecules [34], such as nitric oxide generated by iNOS, followed by serious damages in DNA, proteins and fats.B. longumNSP001 exhibited downregulatory effects on the vitality of neutrophil infiltration by decreasing colonic MPO in both DSS-treated conventional and humanised mice [33].However, it is noteworthy that the excessive iNOS induced by DSS was only ameliorated byB. longumNSP001 in colon accommodated humanised microbiome. Therefore, generation of iNOS is predicted to be closely dependent on the host-gut microbiota interactions. The impaired antioxidant capacity in colon of UC patients may be caused by excessive activation of inflammation [35]. Thymus is one the most important immune and endocrine organs, which releases most of the lymphocytes. The thymic atrophy is closely related with impaired immunity. Accordingly, notable decrease of the thymus index was observed in mice with DSS-induced colitis.B. longumNSP001 restored the thymic disorder in mice with humanised microbiota which implied potential connection between gut microbiota and systematic immunity. NF-κB signaling is one of the critical pathways in regulating innate immunity, activation of which results in secretion of cytokines including TNF-α and IL-6 [36]. In the meantime, IL-6 is capable of shifting Treg/Th-17 profiles, which is crucial for the adaptive immune homeostasis and is regarded as the therapeutic target of IBD,through suppressing the Treg cells and the expression of transcription factor Foxp3 and inducing the differentiation of Th-17 cells [27]. Thus,in lines with the relevant studies, the decreased levels of colonic TNF-α and IL-6 mediated byB. longumNSP001 were possibly to be mediated by NF-κB pathway or Treg/Th-17 cells [37,38].
SCFAs are saturated fatty acid consisting of less than six carbon atoms, and are generally produced by gut commensals during fermentation of undigested fiber. SCFAs can be absorbed by epithelial walls, provide energy and actively exert multiple beneficial characteristics [39]. Acetic acid is the dominant SCFA metabolites,and may protect the intestinal mucosa from the Shiga toxin produced by pathogenicEscherichia coliO157:H7 [40]. Propionic acid is capable of apoptosis of the colonic carcinoma cells in human [41,42].Andin vitroexperiments revealed that both acetic acid and propionic acid could stimulate the upregulation of IL-22 expression in CD4+T and ILC cells, which displayed negative correlations with intestinal inflammation [43]. Therefore, the recovery of inadequately produced acetic acid and propionic acid mediated byB. longumNSP001 may explain its mitigative effects on the DSS-induced colitis.Although butyric acid can inhibit carcinogenesis through suppressing NF-κB signaling [44,45], the levels ofn-butyric acid did not show a remarkable change in all DSS-treated mice, which were in accordance with the clinical data that there were no significant differences in fecal butyrate levels of UC patients compared to those of healthy individuals [46].
Moreover, the underlying mechanism of the anti-inflammatory effects ofB. longumNSP001 may be closely related with the microbial metabolic pathways. Metabolome of cecum microbiota were significantly disturbed by DSS treatment, regardless of the microbiome types. Excessive represetatives involved in glycerophospholipid metabolism in epithelial cells, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were regarded as colitis-associated carcinogenesis [47]. Our data suggested that the administration of live cells ofB. longumNSP001 remarkably regulated the glycerophospholipid metabolism pathway, and promoted the release of these threatening biomarkers from host and elimination from system with feces. Nevertheless, the upregulated pathway of primary bile acid biosynthesis was inhibited byB. longumNSP001.The elevation of primary bile acid in colon has been found positively correlated with the development of metabolic syndrome and tissue inflammation [48,49]. Recent scientific evidences explained that bile acid deconjugation by probiotics includingB. longumwas potentially related with the synthesis of bile salt hydrolases and the activation of farnesoid X receptor (FXR) while interacting with the intestinal mucosa [50,51].
5. Conclusions
Daily intake ofB. longumNSP001 could ameliorate acute colitis caused by DSS in both conventional and humanised mice,in aspects of reducing DAI index, maintaining morphology and histology of colon tissue, and intestinal permeability, downregulating the excessive secretion of MPO, TNF-α and IL-6, and improving the fecal production of acetic acid and propionic acid, and the metabolism pathways of cecum microbiome. While the abnormal thymus index, colonic production of ZO-1 and iNOS were improved only in colitis mice treated withB. longumNSP001 and humanised microbiome. These data revealed that intestinal microbiome baseline would possibly affect the manifestation features of interventions by probiotics or dietary components, highlighted the necessity to include humanised microbiome while investigating potential therapeutic strategies based on rodent models, and may contribute to the further mechanism investigations ofB. longum, expansion of the strain diversity, and industrial development.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The financial supports from the National Natural Science Foundation of China for Distinguished Young Scholars (31825020),the Special and General Fund of China Postdoctoral Science Foundation (2019TQ0138 and 2019M662281), and the Postdoctoral Financial Support from Human Resources and Social Security Department of Jiangxi Province (2019RC13 and 2020KY23) are gratefully acknowledged.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
