The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
2023-01-03HuiTengHongtingDengChngZhngHuiCoQunHungLeiChen
Hui Teng, Hongting Deng, Chng Zhng, Hui Co,b,*, Qun Hung*, Lei Chen,*
a College of Food Science and Technology, Guangdong Ocean University, Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety,Guangdong Province Engineering Laboratory for Marine Biological Products, Guangdong Provincial Engineering Technology Research Center of Seafood,Key Laboratory of Advanced Processing of Aquatic Product of Guangdong Higher Education Institution, Zhanjiang 524088, China
b Faculty of Food Science and Technology, University of Vigo, Vigo 36310, Spain
c Meat Processing Key Laboratory of Sichuan Province, School of Food and Biological Engineering, Chengdu University, Chengdu 610106, China
Keywords:Heterocyclic aromatic amines Flavonoids Hazardous control Precursors
A B S T R A C T Meat products are an important part in our daily diet, providing valuable nutrients for the human body.However, heating processes cause the meat to become more appetizing with changes in texture, appearance,flavor, and chemical properties by the altering of protein structure and other ingredients. As one kind of cooking-induced contaminants, heterocyclic aromatic amines (HAAs) are widely present in protein aceous food products with strong carcinogenic and mutagenic properties. In order to promote the safety of traditional meat products, this review focused on the formation, metabolism, biological monitoring and inhibitory mechanism of HAA. An overview of the formation pathways, hazards, and control methods of HAAs during food processing in recent years was studied, aiming to provide some valuable information for exploring effective methods to inhibit the production of associated hazards during food processing. Systematic selection of different types of f lavonoids to explore their effects on the formation of HAAs in an actual barbecue system can provide theoretical reference for effectively controlling the formation of HAAs and reducing their harm to human health.
1. Introduction
Meat products are important food materials in our daily diet,providing us with essential nutrients, including fat, protein, vitamins,essential amino acids and some trace elements, such as iron,magnesium, selenium, copper and zinc [1]. However, excessive heating or improper cooking method for the meat may generate toxic and harmful substances, such as nitrosamines, polycyclic aromatic hydrocarbons, and heterocyclic aromatic amines (HAAs) [2,3]. Most of the substances have been approved for their potential mutagenic or carcinogenic effects on the human body [4]. As early as 1939,Widmark found the substances extracted from roasted horse meat could induce breast cancer by repeatedly smearing on the back of mice [5]. And Lijinsky and Shubik [6] also found benzo(a)pyrene,a mutagenic compound of carcinogen, generated in broiled steak.But not enough attention was attracted until 1977, when HAAs were f irstly extracted from charred f ish and meat by Nagao et al. [7]. Up until now, more than 30 kinds of carcinogenic and mutagenic HAAs were isolated and identif ied from over cooked meat products [8-10].Even trace amounts of HAAs in cooked food was found to cause serious illness in human organs, f inally leading to the onset of various cancers in long term. And excepting for meat products, different kinds and concentrations of HAAs were detected in other food commodities as well, such as coffee [11] and alcoholic beverages like whisky,wine beer, brandy, and Japanese sake, which are summarized in Fig. 1 [12-14]. Since the ubiquity of HAA in food matrices and also for the scrupulosity of their potential hazards on human health, it becomes a mandatory requirement to well understand the generation mechanism and find a possible way for hindering the reaction process [15].This review provided comprehensive information about HAAs according to relative published studies. Various sample pre-treatment and clean-up methods in different food samples have been explained and different analytical techniques for the isolation and determination of HAAs have been gathered and discussed. This paper reviews the classification, potential harm, formation process, and analysis of HAAs, to increase the understanding of HAAs for consumers and relative food processors.

Fig. 1 Formation of HAA.
2. Classification and formation of heterocyclic HAAs
The generation of mutagens in proteinaceous foods such as fish and meat is highly dependent on cooking temperature. In accordance with the formation process, they could be classified into 2 categories,including pyrolytic amino-carboline (AC, 2-amino-pyridinemutagens) generated above 300 °C and 2-amino-imidazo-azaarenes(AIAs, 2-amino-imidazole-type mutagens) formed under moderate temperature between 100-300 °C (Fig. 1). The pyrolytic AC could be divided into 4 subgroups ofα-carboline (AαC/MeAαC),β-carboline(Harman/Norharmane),γ-carboline (Trp-P-1/Trp-P-) andδ-carboline(Glu-P-1/Glu-P-2). And quinoline (imidazoquinoline (IQ), etc.),quinoxaline (IQx, etc.) and pyridine (PhIP, etc.) are well studied subgroups of AIAs. International Agency for Research on Cancer (IARC 1993) had assessed IQ as a probable human carcinogen as Group 2A (high carcinogenic) and classified AaC, MeAaC, MeIQ, PhIP,Trp-P-1, TrpP-2, Glu-P-1, and Glu-P-2 as possible human carcinogens as Group 2B (low carcinogenic). However, Harman nor Norharmane,which belong toβ-carbolines, are not reported for carcinogenic effects as they lack an exocyclic amine group. Since the exocyclic amine group of HAAs can undergo metabolic activation byN-hydroxylation,producing an intermediate (arylnitrenium ion), which has been implicated in general toxicity and DNA damage [16].
And as a matter of fact, the mechanism for HAAs formation in well cooked food matrice is much more complex, involving multiple compounds such as free amino acids, creatine, creatinine,monosaccharides, disaccharides, or dipeptides, which also act as precursors of HAAs [17-19]. In general, there are two possible generation ways for HAAs: Maillard reaction or free radical reaction pathways [20]. On one hand, the heterocyclic pyridine and pyrazine might be formed via the Maillard process that reacts between hexose and amino acids, after which these intermediate products further transformed into imidazo quinoxaline by free radical reaction with Strecker aldehyde and creatinine (Fig. 2). And in fact, the heating temperature is a critical variable for the occurrence of radical reaction, when it exceeds 300 °C, many reactive fragments are produced and further condensed to form a heterocyclic structure [21].Therefore, the radical reaction might be the cause for the emergence of pyrolytic mutagen [22]. Zöchling and Murkovic [23] speculated that the pyridine and pyrazine, as the products of Maillard reaction,were produced by Strecker degradation and lately formed HAAs via aldol condensation. Lee and co-authors [24] added Maillard reaction products of tetrahydrothiophene and 2,3-dimethylpyrazine into the boiled pork juice, through which he observed enhanced mutagenicity,indicating the 2 Maillard products might be involved in the formation of IQ mutagens. However, the addition of 2-acetylpyrrole and imidazole greatly inhibited the mutagenicity of pork juice, so the Maillard reaction product could also inhibit the formation of mutagens [25]. On the other hand, Pearson and other workers [26] considered that the reaction of dialkyl pyrazin radicals and creatinine could produce methylimidazoquinoline (MeIQx) and 2-amino-3,4,8-trimethyl-3H-imidazole[4,5-f]quinoxaline (4,8-DiMeIQx). The above results further confirmed that a single theory or reaction mechanism is not able to clearly explain the generation process of HAAs.

Fig. 2 Circulation of digestion, absorption and metabolic process for HAAs.
In addition, the food matrix is a relatively complex system, and the formation of HAAs might involve a more intricate interplay between multiple components that is still obscure. As the most abundant HAAs in food products, the formation mechanism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) has been wildly reported [27,28]. It is clear that PhIP could be formed through the reaction between phenylalanine with creatine/creatinine,producing colon and mammary gland tumors in rodents [29].Zochling and Murkovic [23] also reported detailed reaction steps for PhIP formation as follows: firstly, phenylalanine was degraded into phenylacetaldehyde by Strecker degradation, while at the same time, creatine was cyclized into creatinine under high temperature heating conditions. Afterward, the intermediate product was formed via the condensation of phenylacetaldehyde and creatinine, and PhIP was finally formed by a series of condensation cyclization. It can be seen from the reaction process that the formation of PhIP does not require the participation of glucose, which further indicates that Maillard reaction is not the only way to generate HAAs. Furthermore,β-carboline HAAs are mainly formed by the pyrolysis of amino acids or other small molecular peptides, and among them, Norharmane and Harman are the most abundantly found in cooked meat products [30].Compared with other types of HAAs, Norharmane and Harman reactions can be generated at lower temperatures [31,32]. As shown in Fig. 1, the tryptophan in the form of furanose amadori rearrangement product (ARP) initially undergoes dehydration, and is then conjugated with oxonium ions as assisted by the lone pair electron. Afterward,the reactive intermediate further dehydrates to stabilize and forms an extended conjugated system or undergoes C-C bond to produce neutral furan derivatives and imine cations. Finally, an intramolecular nucleophilic substitution reaction occurs, leading to the generation ofβ-carboline [33].
3. HAAs formation dynamics
As we knew, the type of HAAs and their corresponding contents generated during meat cooking depend on heating temperature and time [34]. For examples, the pyrolysis HAAs cannot be detected in meat products when the heating temperature is below 200 °C [35], but when the heating temperature is higher than 250 °C, the thermal HAAs in the simulated chemical system significantly decrease [36]. On the other hand, creatine, creatinine, free amino acids, hexose and small molecular peptides are revealed as the precursor substances to form HAAs in muscle tissue [37]. Thus, the thermal mechanical behavior of HAAs can be explored by mixing these compounds to simulate the external environment of meat products [38]. Novak et al. [39]studied the formation kinetics of polar HAAs using creatinine,creatinine, amino acids and glucose, which proposed the formation of HAAs follows the first order kinetics (equation 1). Further data reported by Göker and co-workers [40] confirmed that the effect of temperature on rate constant during HAAs formation fitted the Eyring equation (equation 2) rather than typical Arrhenius equation(equation 3). Because the Eyring equation gives activation entropyδS*, which may reflect the mechanism of chemical reactions.Skog et al. [18] used beef juice as a model system to test the formation kinetics of HAAs, in which he verified the formation of 2-amino-3-methyl-3H-imidazo[4,5-f]quinoxaline (IQx) followed the first order kinetic model, and the temperature dependence fitted the Eyring equation. Another kinetic study as accomplished by Ahn and Grün [41] using a real meat system also revealed heating time,temperature and precursor substances were significant factors for the generations of either polar or nonpolar HAAs and kinetic patterns followed the first order reaction. It is worth noting that the kinetic behavior of HAAs formation is quite different when different models are used. And the real muscle system is more miscellaneous since the variations in heat and mass transfer, and tissue structure, and also the evaporation of surface water should be considered [42]. In such a case, further researches are still needed to figure out the relationship between precursors and HAAs formation.
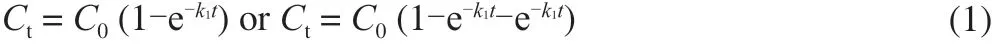
whereCtrepresents concentration HAAs as a function of time(HAA (mmol/L)/creatinine (mmol));C0represents concentration of HAAs compounds (HAA (mmol)/creatinine (mmol));trepresents heating time (min);k1represents degradationC0rate constant. At the same time, it is the rate constant that forms theCt(min-1);ktrepresents HAAs degradation rate constant.

whereκrepresents rate constant;kbrepresents Boltzmannh constant;hrepresents planck constant;Trepresents temperature(K); R represents gas constant (8.314 J/(mol·K));ΔGrepresents free energy for activation (kJ/mol).

wherekrepresents rate constant; -Earepresents the minimum energy required for the reaction (J/mol); R represents gas constant(8.314 J/(mol·K));Trepresents temperature (K);Arepresents pre-index factor.
4. Metabolism of HAAs and intestinal absorption
As shown in Fig. 2, once people intake cooked foods with HAAs generated, the HAAs would enter the circulation of digestion,absorption and metabolism. A great quantity of studies was available on the biotransformation of HAAs by mammalian phase I and phase II enzymes in the liver [43-45]. But recent studies revealed many times that the intestinal microflora might be a key role in the conversion of HAAs into genotoxic metabolites [46-48]. Since the extent of DNA damage in conventional rats was found 4 times higher than that in the same strain lacking of intestinal bacteria [49]. Thus, it might be inferred that intestinal microflora plays at least an equally or even more important role in the HAAs conversion. However, only limited studies could be found dealing with the metabolic effects of the intestinal microflora and lactobacilli from dairy products on HAAs.The most important mechanism for HAAs activation by intestinal bacteria is probably because they can cleave glucuronides-conjugates of HAAs [50], but more evidence is still needed. Meanwhile, if the glucuronidase activity of the intestinal microflora was involved for HAAs conversion, its activity could be modulated via the alteration of diet constitution. Our earlier published work revealed an increase of glucuronidase activity in rats through diet supplementation with other exogenetic compounds [51]. In another feeding study of rats,it was found that the activity of intestinal enzymes increased when different diets were provided and ranked in the following orders: high fats > high fibers > beef diet [52]. A similar experiment fed rats with either a “low risk” diet consisting of polysaccharides mixture, or a“high risk” diet to induce colon cancer, which found that intestinalβ-glucuronidase activity in the low risk diet group was substantially reduced [53]. Intestinal bacteria possess several other enzymes,such as sulfatase and nitro-reductase, which may influence the genotoxicity of the HAAs as well [54], but no experimental data on such interactions are available so far.
5. Detection and Biological monitoring of HAAs exposure in human studies
Urine samples are easy to collect and have no trauma to the human body. Therefore, most studies take the amount of HAAs in urine as the basis of biological monitoring [55,56]. MeIQx and PhIP are considered as the commonly used markers when analyzing the original HAAs in urine [57-59]. The detection of HAAs in urine samples can be separated by reversed-phase chromatographic column,and determined by a series of liquid-liquid extraction, blue cotton absorption and gas chromatography-mass spectrometry (GC-MS)methods or high performance liquid chromatography (HPLC) [58,60].The urine sample can be directly injected and accurate results can be obtained only by dilution, for example, 8 kinds of HAAs are detected:IQ, MeIQ, MeIQx, 4,8-DIMeIQx, 7,8-DiMeIQx, and PhIP [7,60]. In the detection of the same sensitivity, a rapid and simple biological monitoring method for HAAs in urine was proposed, which was separated by a series solid phase extraction (SPE) chromatography and analyzed quantitatively by ESI-MS, and the results showed that four HAAs were detected, the detection limit was 1-8 pg/mL [55].This method can be used to evaluate the daily exposure level of various HAAs [61]. In recent years, with the development of detection technology, the detection ability of HAAs in urine has been further improved, and the detection limit has been reduced [61,62].
Publication of intra- and inter-group differences in MeIQx and PhIP exposure of the human body showed that there was a good correlation between the amount of urinary MeIQx and PhIP prototypes and the intake of HAAs [63]. Human bioavailability of main HAAs in cooked beef with a known amount of MeIQx and found there was 1.8%-4.9% MeIQx prototypes in urine collected within 12 h after the meal, but no MeIQx prototypes were detected after 12-24 h [64]. As mentioned above, there are only 2 HAAs:MeIQx and PhIP metabolites have been reported in the monitoring of HAAs metabolites in urine. As markers, MeIQx metabolites includeN2-glucosidate conjugate ofN-OH-MeIQx, IQX-8-COOH, etc, and the metabolites of PhIP include 5-OH-PhIP etc. [65,66].N-OHMeIQx is an important metabolite marker of MeIQx in urine, and the monitoring of its content in urine is an indirect method to determine the metabolic capacity of MeIQx [59]. Additionally, cytochrome CYP1A2 andN-acetyltransferase iso-enzymes are the biological activation mediators of HAAs, and the metabolic reactions of MeIQx and PhIP are mainly mediated by CYP1A2 [67]. Turesky and others analyzed the metabolites in urine by online spectroscopy and liquidmass spectrometry.14C-labeled MeIQx, and 4 main metabolites were detected by immune-affinity purification: sulfonamide,N2-glucoside conjugate of MeIQx,N2-glucoside conjugate of N-OH-MeIQx, and C8-OH-MeIQx [68,69]. The first two metabolites are unstable acidic two-stage metabolites of MeIQx, while the latter two metabolites are the oxidation derivatives of CYP1A2, and their total amount of 7.6%-8.0% was after the intake of MeIQx, indicating the importance of CYP1A2 mediatedN-oxidation of MeIQx in the human body [69].
Meanwhile, the content ofN2-glucosidic acid conjugate inN-OHMeIQx can reflect the level ofN-oxidized MeIQXin the human body.Stillwell et al. [66] evaluated the level ofN-oxidation MelQxin vivoby a quantitative determination ofN2-glucosidate conjugate in urine.Methods including SPE, immune-affinity purification and hydrolysis ofN-OH-MeIQxN2-glucoside conjugate were used to form MelQx.AndN2-glucosidic acid conjugate ofN-OH-MeIQx was used as an internal standard. Another metabolite marker of MeIQx is IQx-8-COOH, which is the main oxidation product of MeIQx in urine and a pre-carcinogen [66,68]. Capillary gas chromatography-anion mass spectrometry can also be used for the determination of IQx-8-COOH. On the other hand, published data have shown that CYP1A2 participates not only in the metabolic activation of MeIQx, but also in the detoxification of IQx-8-COOH [43]. In addition, another study showed that the quantity of IQ in the urine of the volunteers after eating roast beef was much higher than that of MeIQx and PhIP, while IQ was also detected in the urine of vegetarians, which suggested that IQ may be produced in the bladder or other biological changes [70]. 5-OH-PhIP is a biomarker of PhIP metabolism in urine. As a by-product of PhIP-DNA adduct, 5-OH-PhIP is the final metabolite of PhIP, which is of great significance in the study of longterm exposure of PhIP [57].
In conclusion, the investigation of human biological monitoring of HAAs has made great progress in the detection of urine as a biological material. However, there are still some problems that existed at present, such as the single selection of biomaterials and limited types of HAAs in urine that could be detected. In addition,it is necessary to further study the role of supplemented exogenousmaterials in the detoxification of HAAs. Biological monitoring of urinary excretion levels is a good short-term exposure assessment, but long-term exposure to HAAs intake and tumor risk assessment should also be considered.
6. Inhibition of HAAs and their mechanism
As shown in Table 1, cooked meat products are made from raw meat by over-heating processing, which not only contains the precursors needed for the formation of HAAs, but also has the generation conditions of HAAs, so it is necessary to control the formation of HAAs in cooked meat products. Firstly, it is well known that different heating times, heating temperatures and heating methods have a strong influence on the type and amount of HAAs [34].It was found that higher heating temperature and longer heating time,producing more HAAs. However, when the heating time was too long in the humid heat condition, the HAAs content decreased [22].Salmon and co-authors found that the production of 2-amino-3,4-8-trimethyl-imidazo[4,5-f]quinoxaline (4,8-DiMeIQx) eventually stabilized with the prolonged heating time, while the production of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (8-MeIQx) and 2-amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline (7,8-DiMeIQx)showed a different trend [71]. It follows a fact that the degradationof HAAs may also occur along with their formation under different prolonged heating conditions. Jägerstad et al. [72] found that PhIP was most susceptible to degradation, followed by 7,8-DiMe, IQx,8-MeIQx, 4,8-DiMeIQx and IQx. In addition, mild heating methods such as steaming and boiling as well as microwave heating produced fewer HAAs, but dry air, such as pan frying, produced more HAAs [73]. Accordingly, the inhibition of HAAs formation is based on the differences in heating time, temperature and heating method,thus, these factors need to be taken into account when consideration of hazard control of meat processing [74].

Table 1Inhibitors of exogenous substances, mechanism and reduction amount of HAAs for different food materials.
As mentioned earlier, sugars, amino acids and creatine (creatinine)are important precursors for the formation of HAAs, and they could be reduced by controlling the type and level of precursors. Sugars have been reported to show a significant effect on the formation of HAAs [75]. It could inhibit the formation of HAAs when its content exceeds the content of creatine, which is probably due to excessive sugars preferentially producing 5-hydroxymethyl-2-furaldehyde in the Maillard reaction, inhibiting the formation of HAAs as reacted with creatinine [73]. Meanwhile, the type of sugar differs in its effect on the HAAs production, and Hasyimah et al. [76] found that honey,rich in glucose and fructose, was a good inhibitor of HAAs and could replace sucrose in sweetened marinades. Free amino acids such as phenylalanine and tryptophan are also considered to be important precursors for the formation of PhIP, Harman and Norharmane,and reducing in the amount of free amino acids in the raw material could inhibit the formation of these HAAs [77]. It has been found that the sugars naturally present in meat account for approximately half the amount of creatine and amino acid substances, and they will greatly increase the probability of the formation of HAAs. However,the amount of HAAs formation will be reduced if the sugar content exceeds this proportion [78]. Metal cations on the other side could affect the reaction between phenylethylaldehyde and creatinine during the formation of PhIP [79,80]. Yu and co-workers [80] found that Fe2+and Fe3+could inhibit the formation of PhIP, possibly because Fe2+and Fe3+react with the amino group in creatinine to form a new compound or Strecker aldehyde, thus blocking the reaction of phenyl-ethyl aldehyde with creatinine. It follows that the reduction of creatinine content inhibits the formation of HAAs.
7. Natural inhibitors of HAAs
7.1 Phenolics act as inhibitors of HAAs
There are three commonly recognized mechanisms of HAAs inhibition: the first is that phenolics react with free radicals to form a relatively stable semi-quinone free group, blocking the chain reaction; The second is that they inhibit the generation of free radicals by binding metal ions with antioxidant compounds: the third is that phenolics scavenge free radicals by supplying hydrogen oxidation by themselves [26]. Structurally, the reactive oxygen group on the benzene ring of phenolic substances acts as an antioxidant, scavenging the free radicals generated during the reaction and inhibiting the formation of HAAs [37,81]. Plant tissues and fruits contain many phenolic compounds, grape seeds have a high content of grape polyphenols, and tea contains 20%-35% of tea polyphenols [82].Adding these spices and plant extracts to cooked meat products can inhibit the formation of HAAs, and at the same time, they play a role in flavoring and preserving [83,84]. Sepahpour et al. [85] found that the addition of turmeric, curry leaves, torch ginger and lemon grass,either singly or in combination, reduced the total formation of HAAs in marinated roast beef. Shao and coworkers [86] found that alkoxy radicals are important in the formation of MeIQx and 4,8-Di MeIQx,and nine flavonoids can inhibit the formation of HAAs by scavenging alkoxy radicals, but the specific inhibition pathway is more complex than scavenging alkyl radicals alone. Numerous studies have shown that the inhibitory effects of grape seed extract, red pepper extract [87],apple peel extract [88], rose tea extract [89], olive extract [90], sugar cane molasses extract [91], chrysanthemum extract [92] and tea extract on HAAs are related to their free radical scavenging ability.
Although spices and plant extracts could inhibit HAAs, they do not show single inhibition effects for different HAAs. For example,herb spices significantly inhibited the formation of Harman and Norharmane in soya beef, but promoted the formation of MeIQ [85];ginger inhibited the formation of Harman and Norharmane [93], while quercetin and kaempferol significantly promoted the formation of Harman and Norharmane [94]. In addition, ascorbic acid can scavenge pyrazine cation radicals, thus inhibiting the production of HAAs [95].Sulphur-contained amino acids such as cysteine and acetylcysteine may inhibit the production of HAAs by scavenging pyrazine cation radicals during the Maillard reaction, but the exact mechanism still needs further verification [96]. Beer marination reduced the amount of HAAs in charcoal-grilled pork and artichoke extract reduced the amount of HAAs in beef and chicken breast, which was related to the antioxidant capacity of beer and artichoke extract [97].
7.2 Intermediate modulators act as inhibitors of HAAs
Phenylacetaldehyde is an important intermediate in the formation of PhIP (Fig. 3). Flavonoids can form adducts with phenylacetaldehyde and thus inhibit the formation of PhIP [98].They compared the inhibition of PhIP formation in roasted meat by 8 flavonoids with the correlation between the radical scavenging ability and the scavenging ability of phenylethylaldehyde, and found that the inhibition of HAAs was positively correlated with their capacities on inhibition of phenylethylaldehyde in the roasted meat [98]. Fan et al. [99] found that quercetin-phenylacetaldehyde adducts were detected after the addition of quercetin to beef under a microwave field, and their content increased with increasing in quercetin content, leading to an increased inhibition of PhIP formation. Besides, dihydromyricetin could trap phenylethylaldehyde [100]; naringenin has a scavenging effect on phenylethylaldehyde, formaldehyde and acetaldehyde [101]; certain amino acids can bind to the carbonyl groups of phenylethylaldehyde and act as inhibitors of HAAs [102].Furthermore, amides can also scavenge intermediates and inhibit the production of HAAs [103].For an instance, pepper amides, capsaicin has been verified to have a significant inhibitory effect on the intermediates of PhIP and phenylethylaldehyde [104].

Fig. 3 Postulated pathways for the inhibitory activity of flavonoids against PhIP generation.

Fig. 4 Chemical structure of flavonoid affects its inhibitory effect on HAAs.
7.3 Maillard reaction inhibitors act as inhibitors of HAAs
Many studies have shown that HAAs are formed through complex Maillard reactions. Sulphur-contained compounds can inhibit the Maillard reaction, thereby reducing the amount of HAAs. Previously,6 organosulphur compounds, such as diallyl disulfide, dipropyl disulfide, diallyl sulfide, allyl sulfide, allyl methyl sulfide, and allyl mercaptan, have been reported as effective inhibitors in suppressing the HAAs formation [105]. Diallyl disulfide, dipropyl disulfide,diallyl sulfide, allyl methyl sulfide, allyl mercaptan and cysteine were added to pork juice and all of them were found enable to reduce the productions of IQ, MeIQ and MeIQx [106]. The author suggested that diallyl disulfide, dipropyl disulfide and amino acids all reacted with glucose, thus inducing a competitive reaction which could inhibit the Maillard reaction, and might be responsible for the reduced production of HAAs. In addition, sulphur-contained amino acids can effectively inhibit the non-enzymatic browning reaction under the pyrolytic amino acid glucose system [107]. It has been shown that sulphur-contained amino acids such as glutathione,L-cysteine,L-cystine and deoxyallicine can significantly reduce the levels of HAAs in model systems and fried beef patties [108].
Controlling other ingredients in the food can also be effective in reducing the formation of HAAs in cooked meat products. The presence of moisture can have an inhibitory effect on the formation of HAAs [109]. Water-holding substances will reduce the formation of HAAs. For example, microcrystalline cellulose and carboxymethyl cellulose, which have good water-holding and water-retaining properties, are used in roast beef patties so as to inhibit the formation of HAAs [110,111]; sodium chloride and tripolyphosphates could be added to cooked meat products to significantly reduce the PhIP amount [112]. The formation of HAAs is promoted by the oxidation of fats to produce free radicals, whereas polyunsaturated fatty acids have a certain antioxidant capacity that counteracts the promotion of HAAs formation by the oxidation of free radicals, therefore virgin olive oil rich in polyunsaturated fatty acids has a certain inhibitory effect on the formation of HAAs [113].
On the other hand, it seems that free radicals may be involved in Maillard reaction and affect the generation of HAAs. The antioxidants, such as flavonoids showed that they could scavenge free radicals and reduce HAAs formation [114]. For example, the main tomato antioxidant, quercetin, gave an inhibition of MeIQx formation between 9% and 57% with a maximum effect of 67% at 10 mg/kg [4]. The levels of different HAAs, such as MeIQx or PhIP,can be lowered by heating model systems with flavonoids, such as quercetin, rutin, catechin, catechin gallate, andn-propyl gallate [115].The results indicated that these flavonoids having 2 hydroxy groups at meta positions of the aromatic ring were the most efficient inhibitors.The presence of alkyl or carboxylic groupsas additional substituentsin the aromatic ring reduced the inhibitory effects lightly [115]. On the other hand, the introduction of additional hydroxyl and amino groups mostly cancelled the inhibitory effect, which was also mostly absent in ortho and para dihydroxy derivatives; in complex flavonoids, the presence of several rings with opposite effects produced a reduced inhibitory effect [17].
8. Flavonoids inhibits the formation of HAAs
Flavonoids are natural antioxidants, which can be divided into flavonols, isoflavones, flavanols and dihydroflavones according to their structures. Studies have shown that some flavonoids such as epigallocatechin gallate (EGCG) [4], luteolin, and quercetin [105]could effectively inhibit the formation of HAAs such as MeIQx, PhIP and IQ. According to the traditional view as discussed in the section 6, free radical reaction is involved in the formation of HAAs, and antioxidants or plant extracts can eliminate pyridine, pyrazine and other free radicals as produced in Maillard reaction, thus inhibiting the formation of HAAs [116]. When studying the effect of 12 flavonoids on the content of PhIP in beef, Cheng et al. [101] found that naringenin, quercetin and other flavonoids can combine with phenylacetaldehyde to form specific compounds and therefore to quench the active carbonyl group, thus blocking the further reaction between phenylacetaldehyde and creatinine, and finally inhibit the formation of PhIP.
As shown in Fig. 4, through the investigation of 15 flavonoids on inhibition of HAAs, Zhao and co-authors [94] revealed that:1) daidzein and puerarin in isoflavones had a relatively poor inhibitory effect on HAAs. It was speculated that 5-hydroxy and 7-hydroxy in flavonoid mother nucleus might effectively inhibit the formation of HAAs; 2) the inhibitory effect of quercetin, kaempferol, catechin and epicatechin on the formation of HAAs, among them, dihydroflavones,naringenin has a better inhibitory effect on the formation of HAAs than hesperidin, which may be related to the 4’-hydroxyl group in the B ring of flavone [94]. Lu et al. [9] showed that the presence of 4’-hydroxyl group of B ring in flavonoids can prolong the conjugation system of xanthone body, making the electron cloud of the whole molecule out of the range, and help flavonoids to form relatively stable free radical intermediates.
At the same time, the intra-molecular hydrogen bond was formed between 4’-hydroxy and 3’-oxygen, which further improved the stability of 3’,4’-O-dihydroxyflavone radical [117]. It is concluded that the inhibition of apigenin and luteolin on the formation of HAAs may be related to their antioxidant activities [118]. It is speculated that the simultaneous presence of 5- and 7-hydroxyl groups in ring A and 4’-hydroxyl group in ring B play an important role in the effective inhibition of flavonoids on the formation of HAAs in broiler breast, while the presence of 3-hydroxyl group in ring C reduces the inhibition of flavonoids on the formation of HAAs [119]. In addition,the strong inhibitory effect of EGCG on HAAs may be related to its 6 ortho phenolic hydroxyl groups.
At present, the simulation system is mainly used to study the effect of flavonoids on the formation of HAAs. Whether the simulation system theory is applicable in the real system remains to be verified, and the selection of flavonoids is also lack of systematicness.The role of active sites and important functional groups of flavonoids in inhibiting the formation of HAAs remains to be further studied [6].Systematic selection of different types of flavonoids to explore their effects on the formation of HAAs in an actual barbecue system can provide theoretical reference for effectively controlling the formation of HAAs and reducing their harm to human health.
Declarations of interest
The authors declare no competing financial interest.
Acknowledgements
The present work was supported by National Postdoctoral Program (2020M682073), the National Natural Science Foundation of China (NSFC, 32061160477), The National Natural Science Foundation of China (32272315, 32072209), and the Natural Science Foundation of Guangdong Province (2022A1515010694).
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
- Flowers: precious food and medicine resources
