Effect of oxygen on regulation of properties of moderately boron-doped diamond films
2022-12-28DongYangLiu刘东阳LiCaiHao郝礼才WeiKangZhao赵伟康ZiAngChen陈子昂KunTang汤琨ShunMingZhu朱顺明JianDongYe叶建东RongZhang张荣YouDouZheng郑有炓andShuLinGu顾书林
Dong-Yang Liu(刘东阳) Li-Cai Hao(郝礼才) Wei-Kang Zhao(赵伟康) Zi-Ang Chen(陈子昂)Kun Tang(汤琨) Shun-Ming Zhu(朱顺明) Jian-Dong Ye(叶建东)Rong Zhang(张荣) You-Dou Zheng(郑有炓) and Shu-Lin Gu(顾书林)
1School of Electronic Science and Engineering,Nanjing University,Nanjing 210093,China
2The Shanghai Huahong Grace Semiconductor Manufacturing Corporation,Shanghai 201203,China
3Collaborative Innovation Center of Solid-State Lighting and Energy-Saving Electronics,Nanjing University,Nanjing 210093,China
Keywords: moderately boron doped diamond,crystal quality,suppression effect,boron and oxygen complex structures
1. Introduction
It is well known that boron is an acceptor in diamond and replacing a carbon atom with a boron atom can create an acceptor level near the top of valence band with an activation energy of 0.37 eV.[1,2]Therefore, boron-doped diamond is a promising material for p-type ultrawide bandgap semiconductors due to high carrier mobility,high thermal conduction,and extreme elastic properties.[3–5]Many efforts have been devoted to synthesizing boron-doped diamond with high crystalline quality.[6–8]Microwave plasma chemical vapor deposition (MPCVD) technique has been widely employed to grow p-type diamond with high crystalline quality and doping concentration by changing the methane concentration,[6]improving the power of plasma,[7]and increasing the growth temperature.[8,9]
In recent decades, a promising technology of co-doping was widely utilized for tuning the crystalline quality, dopant population, and electrical properties.[10–12]Especially in diamond, many groups have adopted the co-doping strategy to grow high crystalline quality diamond by MPCVD. The codoping with oxygen is considered as an effective way to improve the quality of diamond due to the etching effect of the oxygen plasma. The co-doping of boron and oxygen has been widely investigated by many groups.[13–15]For example, Issaouiet al.[16]have studied the effect of oxygen on the heavily boron-doped diamond and shown that the obtained films exhibit high crystalline quality with adding a large amount of oxygen in gas phase. However, most of the prior literature focuses on the effect of oxygen on the properties (such as crystalline quality,[17,18]growth rate,[18,19]and electrical property[17,19]) in heavily boron-doped diamond. The effect of oxygen on properties of moderately boron-doped diamond is lack of systematic research, especially effect of oxygen on regulation of acceptor concentration and donor concentration,and compensation of oxygen is still unclear.
In this work,regulation of oxygen on properties of moderately boron-doped diamond is fully investigated. The asgrown diamond films were characterized by atomic force microscopy (AFM), Raman scattering spectra, photoluminescence spectra,optical emission spectroscopy(OES),and Halleffect measurement. Effects of oxygen on the crystalline quality,surface morphology,and electronic properties are systemically studied. Moreover,regulation of oxygen on donor concentration and acceptor concentration are systemically investigated.
2. Experimental details
Diamond films were grown on (100)-oriented type Ib CVD single-crystalline chemical vapor deposited diamond substrates (3.0 mm× 3.0 mm× 1.0 mm) by MPCVD technique. The flow rates of CH4and H2were kept at 20 and 500 sccm, respectively. To ensure that boron and oxygen were incorporated, diborane and oxygen were added in the gas phase during the growth. The ratio of boron to carbon in the gas phase was fixed at 2.5 ppm for all the samples. The chamber pressure and the microwave power for all deposition were fixed at 135 Torr and 3.8 kW, respectively. The substrate temperature was set to 800–850◦C. To investigate the effect of oxygen on the boron-doped diamond films,the oxygen concentration,evaluated by the O/C gas ratio,was kept at 0%, 2.5%, 5.0%, 10.0%, and 20.0%. The growth duration is 2 h and the thickness of diamond is 6 µm (O/C=0%), 4 µm(O/C=2.5%), 2 µm (O/C=10.%), and 1 µm (O/C=20.0%).After deposition, in order to remove organic and metallic contaminants from the substrate surface, the substrates were cleaned in aqua regia(HNO3/HCl 1:3)at 60◦C for 30 min.
The surface morphology of diamond was performed by an AFM(NTEGRA)in the tapping mode. Micro-Raman scattering spectra and photoluminescence spectra were recorded by using a 514 nm laser as an excitation with a confocal microscope (Horiba Jobin Yvon) at 100 magnifications (numerical aperture, NA, 0.9). OES has been employed to monitor the composition of the plasma in the reaction chamber. The Hall effect measurement has been carried out at both room temperature(300 K)and variable temperatures(300–900 K)to study the electrical properties of the dopants and impurities.
3. Results and discussion
Figure 1(a)shows the growth rate of diamond as a function of the O/C ratio. As can be seen,a monotonous decreasing trend is observed,which is related to the etching effect of oxygen plasma.[19]In order to further elucidate the effect of oxygen on the growth rate of diamond,OES was employed to analyze the composition of the gas mixture. Figure 1(c)shows the OES of all samples while Fig.1(b)zooms in the spectrum of the O/C=0%sample to tick the peaks. The CH4–H2–B2H6mixture contains several optical emission lines,among which the ones located at 431.1 nm and 486.2 nm are originated from the carbon-hydrogen[20]and atomic Hβradicals,[21]respectively. Furthermore, the line located at 516.4 nm is the result of existence of C2radicals.[21]Figure 1(d)compares the peak height of the C2lines,and we plot the data in Fig.1(a),being quite consistent with the growth rate. The weakened intensity of the C2lines implies that more oxygen addition would consume the C2radicals,and researchers have reported in literature that the growth rate of diamond is related with the C2radicals.[21]Hence,the growth rate of diamond is decreased.

Fig.1. (a)Growth rate of diamond and intensity of C2 radical as a function of ratio of O/C;(b)OES of boron-doped diamond films;(c)OES of boron-doped,boron and oxygen co-doped diamond films;(d)the enlarged view of OES from 515 nm to 517 nm.
Generally, slower growth may lead to better surface and crystalline quality. Consequently,the AFM and Raman spectroscopy have been employed to characterize the quality of the samples.Figure 2 shows the AFM images of a 10µm×10µm area for all the samples. For the sample without oxygen addition as shown in Fig. 2(a), many etching pits are observed,and the surface is quite rough with a root-mean-square(RMS)roughness of 2.31 nm. By adding a small amount of oxygen(O/C=2.5%), the surface becomes smoother with the RMS roughness continuously decreasing to a minimum value of 0.28 nm for the O/C=0.5% sample. However, with further increasing the O/C ratio to 10.0% and 20.0%, the surface of diamond becomes rougher again and a few etching pits could be observed. The observation from the AFM shows an optimized O/C ratio for improving the surface morphology, the optimal point is also evidenced by the Raman spectroscopy.Figure 3(a)shows the Raman spectra and figure 3(b)plots the extracted width of the 1333 cm−1diamond characteristic peak.Since the width can represent the crystalline quality of the diamond film to some extent, the trend in Fig. 3(b), perfectly matching the AFM RMS roughness,points to a same optimal O/C ratio for both surface and crystalline quality.Combing the persistent decrease of the growth rate,it can be concluded that slower growth does not necessarily lead to better surface and crystalline quality. Excessive addition of oxygen may result in severe etching effect that forms more defects on the diamond surface.
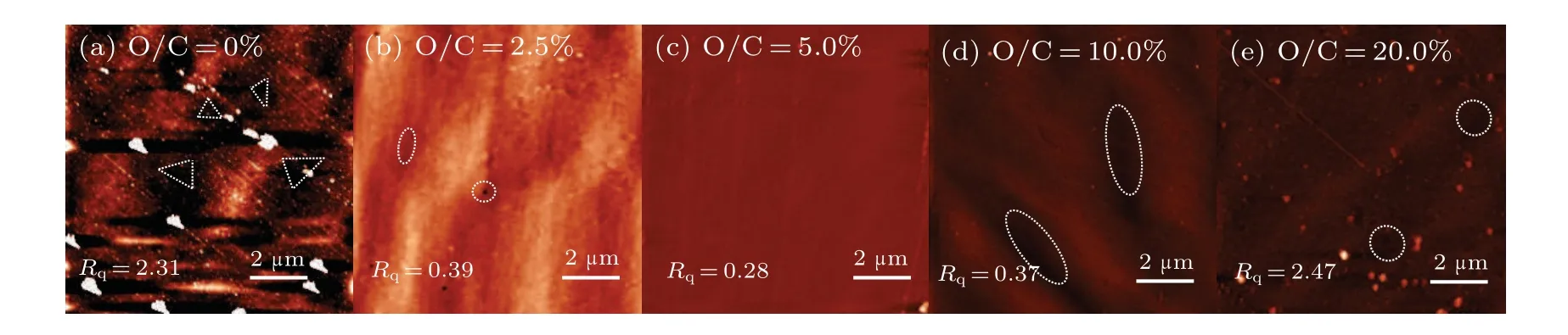
Fig. 2. AFM photographs of boron-doped, boron and oxygen co-doped diamond: (a) O/C=0%, (b) O/C=2.5%, (c) O/C=5.0%,(d)O/C=10.0%,(e)O/C=20.0%.

Fig.3. (a)Raman spectra of boron-doped,boron and oxygen co-doped diamond films;(b)the FWHM of Raman scattering peak of diamond as a function of ratio of O/C.
During the optical characterization of the samples, we have interestingly found the evidence of nitrogen incorporation since no intentional nitrogen doping is employed. Figure 4(a) shows the photoluminescence (PL) spectra of all the samples(the intensity of NV0and NV−centers have normalized by Raman intrinsic peak of diamond). The presence of the NV0(the peak located at 575 nm) and NV−centers (the peak located at 637 nm)with varied intensity have been found in the diamond films. The origin of nitrogen could be due to the residual nitrogen in the chamber walls or the impurities from the feeding gas precursors. However,what we are interested here is the tuning of the NV centers by different amount of oxygen. Figure 4(b)shows the NV intensity extracted from the PL. The trend is similar to Figs. 2 and 3, indicating that the surface and crystalline quality change due to the fact that the oxygen variation could be the reason for different levels of NV incorporation. For small amount of oxygen addition, the crystalline quality of diamond is improved due to the etching effect of oxygen plasma. In this situation,defects and vacancies are not easily formed on the surface of diamond.Also,the nitrogen precursors may be exhausted by oxygen addition via forming NO related gas product.Hence,the intensities of NV0and NV−centers are decreased with increasing ratio of O/C.However,for excessive oxygen addition,the severe etching effect would result in formation of more defects and vacancies on the surface of diamond. Vacancies would become mobile under high temperature(above 700◦C).[22]Then some of vacancies are trapped by substitutional nitrogen to form NV centers,leading to the enhanced NV-related PL for the O/C=20%sample.

Fig.4. (a)PL spectra of boron-doped,boron and oxygen co-doped diamond films with an excitation wavelength of 514 nm;(b)the intensity of NV0,NV−as a function of O/C ratio.
Since the oxygen in diamond is a donor-like defect and the boron and oxygen may form complex to affect the electrical properties,we have thus employed Hall-effect measurement to characterize the samples.Figure 5(a)shows the results of the hole concentration and the Hall mobility. Generally,the hole concentration increases firstly and then decreases with oxygen addition, implying that different amounts of oxygen addition can regulate the properties of the boron acceptors. In order to investigate more in depth,the OES is employed again.Figure 5(b)shows the OES from the CH4–H2–B2H6and CH4–H2–B2H6–O2mixtures in the MPCVD system. The optical emission lines located at 433.2 nm and 434.0 nm are originated from the BH radical[23]and the atomic Hγradical,[20]respectively. Moreover, we take the intensity ratio of the BH to C2radicals (IBH/IC2) to represent the active boron precursor in the plasma mixture and plot it in Fig. 5(c). The trend accords quite well with the one of the hole concentrations,indicating that the main reason for the change of the hole concentration with different amounts of oxygen addition is due to the different incorporation levels of boron acceptors.
The regulation could be understood by the following two mechanisms. On the one hand, the oxygen ions with larger radius could compensate and balance the stress caused by the incorporation of boron ion with smaller radius,[24]leading to more boron atoms incorporated into the diamond material.On the other hand, oxygen in gas phase would severely react with the boron precursors in the plasma and consume the active boron ions,[19]leading to a persistent decrease of the boron incorporation amount. These two mechanisms have opposite effects and would interpret the hole concentration trend in Fig. 5(a). Correspondingly, the mobility shows an inverse relation to the hole concentration in general, which could be easily understood by the scattering from ionized impurities.However, there is one mismatch for the O/C=5% and 10%samples, where both higher hole concentration and mobility have been achieved on the O/C=5%sample. It may be related to better surface and crystalline quality of the sample as shown by the AFM and Raman spectra.

Fig. 5. (a) Hole concentration and mobility of boron-doped, boron and oxygen co-doped diamond as a ratio of O/C; (b) OES of boron-doped, boron and oxygen co-doped diamond films with wavelength from 432 nm to 435 nm;(c)the intensity ratio of BH radical to C2 radical as a function of ratio of O/C.
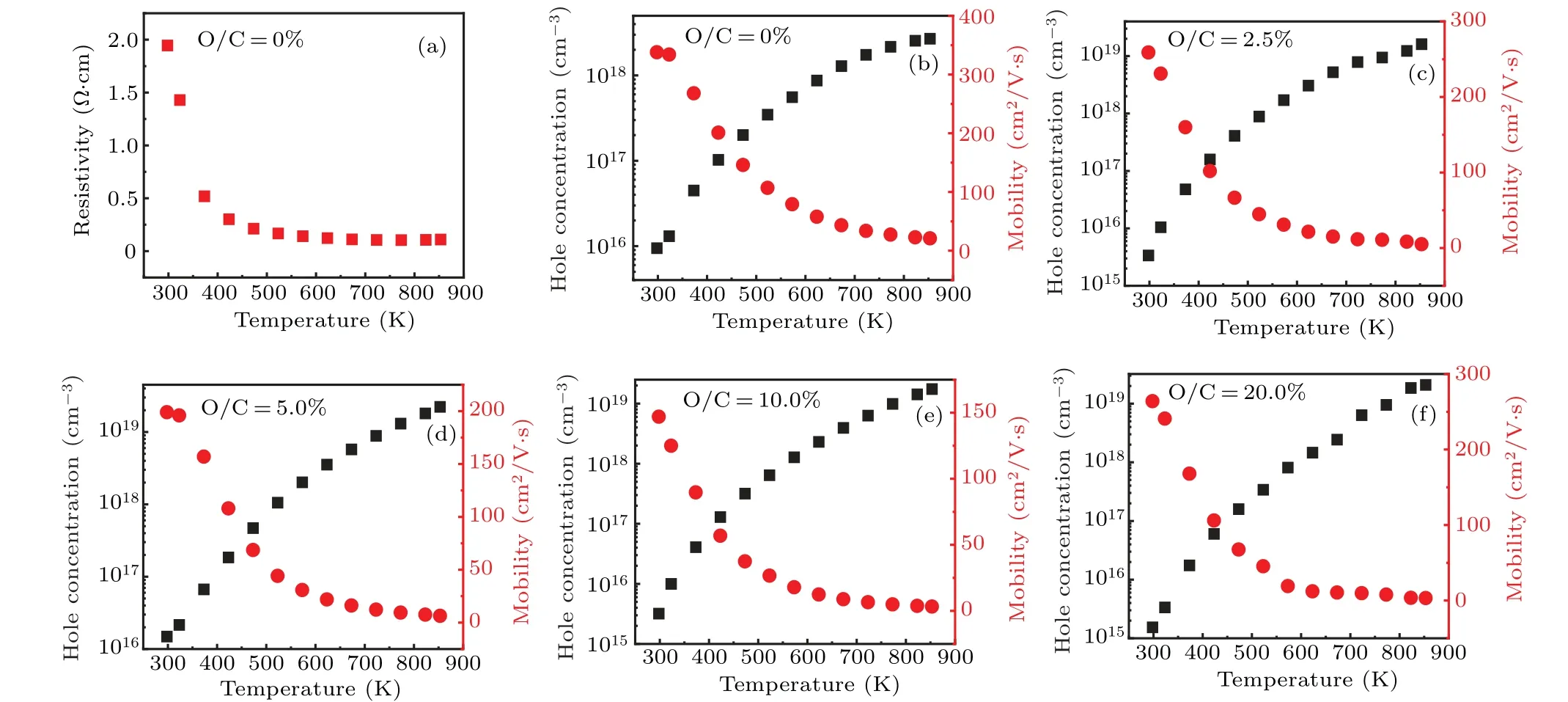
Fig.6. (a)Resistivity of boron-doped diamond(O/C=0%)versus temperature. (b)–(f)Hole concentration and mobility of boron and oxygen co-doped diamond versus temperature: (b)O/C=0%,(c)O/C=2.5%,(d)O/C=50%,(e)O/C=10.0%,(f)O/C=20.0%.

Fig. 7. (a) Fitting curve of hole concentration by the relation between hole concentration and temperature, (b) the fitted acceptor and donor concentration of diamond as a function of ratio of O/C,(c)the fitted ionization energy as a function of ratio of O/C.
As mentioned above, oxygen can act as a donor in diamond that compensates boron acceptors.Therefore,to investigate the compensation behavior of oxygen on the boron-doped diamond, the samples were investigated by Hall-effect measurement with measuring temperatures from 300 K to 850 K.As shown in Fig. 6(a), the resistivity of boron-doped diamond is decreased from 1.9 Ω·cm to 0.1 Ω·cm with increasing temperature from 300 K to 850 K, indicating a gradual thermal activation of the acceptors. Figures 6(b)–6(f) show the temperature variable hole concentration and Hall mobility of the samples. As illustrated in Fig. 6(b), the hole concentration of boron-doped diamond(O/C=0%)is increased from 9.5×1015cm−3to 2.7×1018cm−3and the mobility is correspondingly decreased from 338.0 cm2/V·s to 21.1 cm2/V·s with increasing the measurement temperature. With adding oxygen (O/C=20.0%), the hole concentration is increased from 1.5×1015cm−3to 2.1×1019cm−3,and the mobility is correspondingly decreased from 264.0 cm2/V·s to 3.3 cm2/V·s with increasing the measurement temperature.

Moreover, for a p-type semiconductor, the general description of the hole concentrationpat a temperatureTis given by the following equation:[25]Here,EiandkBare the ionization energy of acceptor and the Boltzmann constant,respectively;NAandNDare the acceptor concentration and donor concentration, respectively;MVandpare the effective density of state of the valence band and the hole concentration in diamond,respectively;gdis the acceptor degeneracy. Using the equation to fit the hole concentration data in Figs.6(b)–6(f), we can have the values ofNAandNDto be the ionization energy of the acceptor(Ei).
Figure 7(a) shows the hole concentration and the fitted curve for all the samples. It can be seen that the data could be well fitted by the theoretical model. The fitted results are drawn in Figs.7(b)–7(c)and also listed in Table 1. For borondoped diamond(O/C=0%),the fitted boron ionization energy,NAandNDare 0.22 eV,7.9×1018cm−3and 1.1×1018cm−3,respectively. The fitted ionization energy is closed to 0.23 eV as reported by Grotjohnet al.[26]With adding oxygen, the ionization energy of the acceptor (Ei) decreases. This could be due to the increased acceptor concentration as shown in Fig. 7(b). Grotjohnet al.[26]reported that the acceptor concentration increases from 1016cm−3to 1020cm−3,the activation energy decreases from 0.36 eV to 0.05 eV.TheNAandNDare both firstly increased then decreased while the ionization energy is firstly decreased then increased. The change of the acceptor doping concentration(NA)and the ionization energy of the acceptor(Ei)is consistent with each other,which double confirms the authenticity of the fitting treatment.

Table 1.The fitted ionization energy,acceptor concentration and donor concentration.
It is interesting to note that the compensated donor concentration(ND)does not continuously increase as we add more oxygen during growth.It otherwise shows an accordant evolution trend with the acceptor concentration.One obvious reason could be that the oxygen cannot have unlimited solubility in the diamond lattice. Thus, excessive oxygen addition would result in lower efficiency of the oxygen incorporation. The other reason could be that the form of the compensating donor could be B–O complex,[27]since the formation energy of B3O and B4O,acting as donor-like complexes, could be quite low in p-type diamond. In consequence, the compensating donor concentration would be constrained by the main acceptor concentration.
4. Conclusion
In summary,the regulation of oxygen on the properties of moderately boron-doped diamond is fully investigated. With adding a small amount of oxygen, the crystal quality of diamond is increased, and a suppression effect of residual nitrogen is observed. At the same time,boron and oxygen complex structures are formed and exhibit as shallow donor in diamond,which results in increment of donor concentration. With further increasing ratio of O/C,the inhibitory behavior of oxygen on boron leads to a decrease of acceptor concentration.
Acknowledgements
Project supported by the National Key Research and Development Program of China(Grant Nos.2018YFB0406502,2017YFF0210800,and 2017YFB0403003),the National Natural Science Foundation of China (Grant Nos. 61774081,61775203, 61574075, 61974059, 61674077, and 91850112),the State Key Research and Development Project of Jiangsu,China (Grant No. BE2018115), State Key Laboratory of Wide-Bandgap Semiconductor Power Electric Devices(Grant No. 2017KF001), and Anhui University Natural Science Research Project(Grant No.KJ2021A0037).
猜你喜欢
杂志排行
Chinese Physics B的其它文章
- Editorial:Celebrating the 30 Wonderful Year Journey of Chinese Physics B
- Attosecond spectroscopy for filming the ultrafast movies of atoms,molecules and solids
- Advances of phononics in 20122022
- A sport and a pastime: Model design and computation in quantum many-body systems
- Molecular beam epitaxy growth of quantum devices
- Single-molecular methodologies for the physical biology of protein machines
