Behavioral and Physiological Responses of Sea Slug(Onchidium reevesii)to Low-Frequency Noise
2022-12-27TUZhihanLIChenqiJIAJingjingandSHENHeding
TU Zhihan , LI Chenqi JIA Jingjing and SHEN Heding ,
1)National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University,Shanghai 201306, China
2)International Research Center for Marine Biosciences, Ministry of Science and Technology, Shanghai Ocean University, Shanghai 201306, China
3)Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-Culture of Aquaculture Animals, Shanghai 201306, China
Abstract A large amount of marine noise pollution from coastal industrial construction and the water transportation industry affects the growth and development of marine life, which is a big issue threatening marine organisms. However, most studies have focused on vertebrates, such as marine mammals and fishes, and little is known about the effects of noise on invertebrates. Therefore,the impacts of low-frequency noise (100, 300, and 500 Hz)on behavioral and physiological responsesof the sea slug (Onchidium reevesii)were investigated. Under laboratory conditions, sea slugs were stimulated with low-frequency noise at 100, 300, and 500 Hz for 1 h. Then, hemolymph enzyme activities (glucose, albumin, triglycerides, superoxide dismutase, catalase, and malondialdehyde)were measured, and mRNA expression of the hsp70 gene was detected in hemolymph and the nervous system by quantitative realtime polymerase chain reaction (qRT-PCR)analysis, while expression of the hsp70 protein was analyzed by immunohistochemistry.The results demonstrated that sea slugs were away from the stimulus source under the influence of low-frequency noise compared to the control group. Enzyme activities, as well as hsp70 gene mRNA and protein expression levels, were significantly higher in the noiseexposed groups than those in the control group. Overall, these changes indicate that low-frequency noise caused oxidative stress in sea slugs in vivo, and the oxidative damage gradually increased when the noise frequency was increased from 100 to 500 Hz.
Key words ocean noise; Onchidium reevesii; low-frequency noise; biochemical stress; mollusks
1 Introduction
Many oceanic creatures have evolved the ability to ‘see with sound’ so that they can communicate with their species or detect preyers. After the Second World War, the world entered a period of rapid economic development.With the increasing development of marine industries, such as shipping, military, seismic survey, and offshore and port constructions, excessive industrial and human activities have generated considerable marine noise (Morettiet al.,2014; Edmondset al., 2016; Di Francoet al., 2020). Ocean noise has serious effects on the growth and development(De Sotoet al., 2013), metabolism (Penget al., 2016), and reproduction (Ruiz-Ruizet al., 2019)of marine animals.
Since the 21st century, there has been a global effort to develop green energy with the construction of a large number of facilities, such as offshore wind turbines and ports in coastal areas. However, offshore wind turbines and ship and ferry traffic generate a significant amount of low-frequency noise (Bassettet al., 2011; Penget al., 2015). Because of the longer wavelength of low-frequency sound waves, these waves propagate farther in the air and water,producing a greater range of longer-lasting noise and greater injury to marine animals. An increasing number of studies have reported that persistent low-frequency noise not only alters the hunting habits of marine animals (Schwarzet al., 1984)and affects their foraging activities (Waleet al.,2013), but also affects their physiology. Vazzanaet al. (2016)exposed mussels to different sound frequencies separately and found that biochemical parameters in the blood of mussels were significantly higher in the low-frequency group,and heat shock protein (HSP)70 expression and acetylcholinesterase activity in the mantle and gills were significantly higher than those in the other groups, indicating that mussels are more sensitive to low-frequency noise and that lowfrequency noise causes an oxidative stress responsein vivo.Spigaet al. (2016)reported that the noise generated by a pile driver increases the microalgal clearance of blue mussel(Mytilus edulis), indicating that pile driving influences mussel feeding. The changes in feeding activity were a reflection of elevated metabolismin vivo, suggesting that noise affects the blue mussel metabolism. Long-term exposure to seismic survey signals significantly increases mortality and hemolymph biochemical parameters change in scallop (Pecten fumatus), leading to an electrolyte imbalance, disruptions in homeostasis, and potential immune deficiencies that affect different cellular functions (Dayet al.,2017).
High-intensity noise is generated during the construction of offshore wind farms, but its effect is short-term,whereas the impact of a wind farm over 20 years of operation is long-term. Yanget al. (2018)measured and analyzed underwater wind turbine noise from the first offshore wind farm in China, and the spectral analysis results showed that the radiated turbine sound was mainly concentrated in the low-frequency domain from 300 to 500 Hz. Studies have shown that the noise generated during the construction of and power production by offshore wind farms has effects on the behavior and physiology of marine organisms,such as masking, temporary threshold shifts, and responsiveness (Thomsenet al., 2006; Bergströmet al., 2014).Most studies have focused only on marine mammals and fish, but mollusks are the most abundant and widely distributed invertebrates in the ocean and occupy a key position in the marine food web. Therefore, it is important to determine the effect of low-frequency noise on mollusks,as it can also affect the entire marine ecosystem. Exploring the effects of low-frequency noise on the sea slug reflects the effects of noise generated by human activities in coastal areas, such as wind turbines and industrial construction, on the entire coastal ecosystem.
Sea slug,Onchidium reveesii(Molluska, Gastropoda, Pulmonata, Systellommatophora, Onchidioidea), is a species of mudflat lung snail that lives in the high-tide zone of intertidal areas in southeastern China, such as semi-saltwater areas, river mouths, mangroves, and reeds. Wild sea slugs are widely distributed and are extremely sensitive to environmental changes in the intertidal zone, so they are often used for toxicological and ecological risk assessments(Guet al., 2019). In this study, we exposed sea slugs to laboratory-generated acoustic stimuli at different frequencies (100, 300, and 500 Hz)and measured physiological and biochemical indicators in the hemolymph and changes ofhsp70expression in the nervous system and hemolymph of these sea slugs. The results will reveal the potential effects of low-frequency noise on the behavior and physiology of marine invertebrates.
2 Materials and Methods
2.1 Study Species
The experimental animals were collected from mudflats in the East China Sea nearby Shanghai. The slugs were temporarily fed cornmeal every day (daily feeding rate of 5% of body weight)in the shellfish laboratory of Shanghai Ocean University. The feces were removed regularly,while seawater was sprinkled on the top of the sea mud to maintain the humidity based on the temporary feeding method of Shenet al. (2004). Under the experimental plan,36 animals (average weight 9.0 g ± 1.5 g)were randomly selected for the experiment.
All procedures were strictly carried out following the Regulations of the Experimental Animal Ethics Committee of Shanghai Ocean University and in compliance with regulations of the Institutional Animal Care and Use Committee.
2.2 Experimental Setup and Protocol
The low-frequency generator device is shown in Fig.1(Tuet al., 2021). The device consists of a swept-frequency signal generator (SA-SG030, SHIAO, Wuxi, China), a power amplifier (SA-PA010 100 W, SHIAO), and a loudspeaker (SA-JZ005, SHIAO). The experiments were conducted in a 3-m long, 0.6-m wide, and 0.5-m high tank with a 15-cm thick marine mud layer at the bottom of the tank and a loudspeaker at the other end. A sound level meter was placed on the wall of the tank to detect the decibel value of the sound produced. According to our sound pressure level measurements inside the box before the experiment, no significant difference was detected for the sound pressure level inside the box, and the error range was 3 dB.A thermometer and hygrometer were placed in the tank to monitor the temperature and relative humidity inside the tank at 26℃ and 90%, respectively. A sea slug was stimulated using a low-frequency generating device with sine waves of 100, 300, and 500 Hz and sound levels of 80 –107 dB for 1 h. The control and experimental groups were subjected to the same conditions. Each group was comprised of 9 biological replicates. At the end of the experiment, we performed biochemical testing on the hemolymph and nervous system of the sea slug.
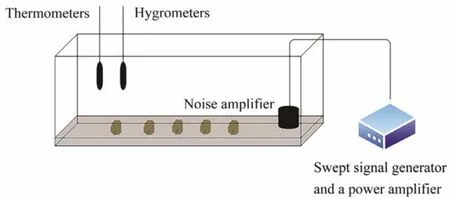
Fig.1 Schematic diagram of the experimental tank. A total of 9 sea slugs, a swept signal generator, a loudspeaker, and a power amplifier were placed in the tank. A thermometer and a hygrometer were located on the wall of the control tank.
2.3 Behavioral Experiments
We performed three replicates with nine individuals in each replicate for each experiment. The vertical distance from the slugs on the bottom and sides of the tank to the baseline was measured at the end of the noise stimulus, using the location of the loudspeaker as the baseline.
2.4 Hemolymph and the Central Nervous System(CNS)Sampling
Nine animals were randomly taken from the tank at the end of the noise stimulation and soaked in water containing MS-222 anesthesia. Hemolymph was drawn from the heart artery with a 1-mL sterile syringe moistened with sodium heparin solution (0.1 g of heparin in 100 mL of strokephysiological saline solution), placed in a 1.5 mL enzymefree centrifuge tube (containing heparin sodium dried at 60℃), and then stored at -80℃ until further analysis. The sea slugs were dissected with a DEPC-soaked scalpel, and the CNS was removed. The nervous system was stored in 4% paraformaldehyde and used for immunohistochemistry analysis. The hemolymph and CNS were stored at -80℃ before the quantitative real-time polymerase chain reaction (qRT-PCR)analysis.
2.5 Hemolymph Enzymatic Activities
To analyze the activity of superoxide dismutase (SOD),the samples (0.02 mL)were added to the working solution containing WST-1 and xanthine oxidase, incubated for 20 min at 37℃, and read at 450 nm using an enzyme-labeled instrument (Synergy 2, United States).
Catalase (CAT, U mL-1)was detected using the UV colorimetric method. Firstly, a pretreated sample (0.02 mL)was added to the bottom of the cuvette. Then, 3 mL of hydrogen peroxide solution, which had been pre-tempered to 25℃ with anODof 0.5 – 0.55, was quickly added to the cuvette. The absorbance value was measured immediately at 240 nm (OD1)using a UV spectrophotometer, and the absorbance was measured again immediately after 1 min(OD2). The calculation is then performed according to the formula:
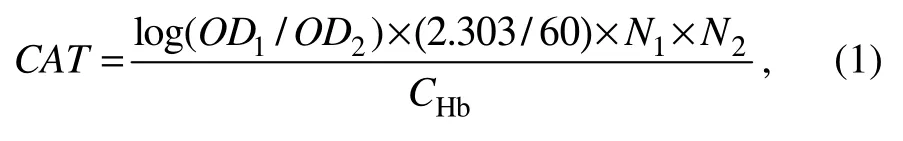
where 2.303 is the necessary multiplication when converting from natural log to common log;N1is the dilution multiple of the sample before testing;N2is the dilution multiple of the reaction system, herein is total reaction volume(3.02 mL)/ sample volume (0.02 mL);CHbis the sample hemoglobin concentration, 0.1508 g Hb mL-1.
Malondialdehyde (MDA)content was determined by the TBA enzymatic method. Hemolymph was added to the same volume of 1% thiobarbituric acid in a 95℃ water bath for 40 min. After cooling, the thiobarbituric acid reactive substance was centrifuged at 2000 ×gfor 10 min, and the absorbance of the supernatant was measured at 532 nm against a blank control consisting of 5% trichloroacetic acid mixed with 1% thiobarbituric acid.
Triglycerides (TGs)were measured by the GPO-PAP enzymatic method. The samples and working solution (Tris-HCl, ATP, lipases, and glycerol kinase)were mixed and incubated for 10 min at 37℃ and then read at 510 nm using an enzyme-labeled instrument.
Glucose (Glu)was measured using the glucose oxidase method. The samples were added to a working solution comprised of glucose oxidase, peroxidase, and 4-aminoantipyrine. The mixture was incubated for 10 min at 37℃, and absorbance was read at 505 nm using an enzyme-labeled instrument.
Albumin (ALB)was determined using a microenzymatic assay (bromocresol green and ALB binding changed from yellow to green), left at room temperature for 10 min,and then read at 630 nm using an enzyme-labeled instrument.
All enzymatic activities were determined using commercial kits following the instructions of the manufacture (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6 hsp70 Gene Expression Analysis with qRT-PCR
The CNS and hemolymph RNA was extracted using Trizol (Takara Biomedical Technology Co., Ltd., Beijing, China). The first-strand synthesis of cDNA was performed with a PrimeScriptTMRT reagent kit with the gDNA Eraser(Takara Biomedical Technology Co., Ltd.).
The open reading frame sequence of thehsp70gene was obtained from the nervous system transcriptome database and was used to design quantitative fluorescent primers using Primer Premier 5.0 software. The18SRNAgene was selected as the internal reference. The primers were designed and are listed in Table 1. The cDNA was used as a template with the Cham QTMSYBR®qPCR Master Mix kit (Vazyme Biotech Co., Ltd., Nanjing, China). qRT-PCR was performed to analyze the mRNA expression level of thehsp70gene in the CNS and hemolymph.

Table 1 PCR primers used for hsp70 gene expression
The comparative CTmethod was used to analyze the relative expression levels of thehsp70gene. The CTvalues of the targethsp70and18SRNAgenes were determined in all samples (amplification reaction procedure: 95℃pre-denaturation, 3 min; 95℃ denaturation, 30 s, 58℃ annealing, 30 s, 72℃ extension, 1 min, 34 cycles; 72℃ extension, 10 min). The difference between the CTvalue of the target gene and the CTvalue of the internal reference (ΔCT)was calculated to normalize the difference in the amount of template cDNA and determine qRT-PCR efficiency. ΔCTwas used as a calibrator for the control group. The difference between the experimental and control groups was ΔCT.hsp70expression levels were analyzed using the following equation:

The amount of change inhsp70gene expression in all samples was related to the control group.
2.7 Hsp70 Immunohistochemistry Analysis
The fresh CNS tissues from the control and 500 Hz noise treatment groups were fixed in 4% paraformaldehyde for 6 h, then kept in 20% sucrose overnight, and then transferred to 40% sucrose until the tissues sank to the bottom.After frozen sectioning (Leica CM1950; Tokyo, Japan), the slices were baked at 37℃ for 8 h. All sections were incubated with primary antibody (Hsp70 Rabbit Monoclonal Antibody, Beyotime Biotechnology, Shanghai, China)and secondary antibody (HRP-labeled Goat Anti-Rabbit IgG(H + L), Beyotime Biotechnology), following the instructions with the one-step immunohistochemistry kit (Key-GEN BioTECH, Nanjing, China)to complete subsequent experiments. A research-grade microscope (Nikon ECLIPSE 80i; Tokyo, Japan)was used to observe and take photographs of the CNS tissues.
2.8 Statistical Analysis
The statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA)and Duncan’s multiple range test were used to detect differences. Single-factor ANOVA was performed for the enzyme activity andhsp70expression analyses. AP-value<0.05 was considered significant.
3 Results
3.1 Effects of Low-Frequency Noise on Sea Slug Behavior
As shown in Fig.2, the behavior of the sea slugs did not change significantly at a frequency of 100 Hz (P> 0.05).The sea slugs were restless at a frequency of 300 Hz. The crawling distance tended to be farther in the noise-exposed groups than the control group, but not significantly (P>0.05). Crawling distance increased significantly when the frequency was increased to 500 Hz, compared to the control group (P< 0.05). We also observed that the sea slug coat membrane was upturned, and the granular glands on the coat membrane secreted a dark brown liquid with a strong odor.

Fig.2 Crawling distance results for the different groups. The crawling distance (mean ± SE)of the sea slugs exposed to low-frequency noise for 1 h. Different lowercase letters above the bars indicate a significant difference (P < 0.05).
3.2 Effects of Low-Frequency Noise on Biochemical Parameters
Sea slugs exposed to low-frequency noise (100, 300, and 500 Hz)for 1 h exhibited changes in several hemolymph biochemical parameters (Fig.3). The levels of Glu and ALB in the hemolymph of the experimental groups (300 Hz and 500 Hz)were significantly higher than those in the control group (P <0.05), while no significant difference was observed in 100 Hz group (P >0.05). Glu and ALB increased in the experimental groups, with the highest levels in the 500 Hz group (P <0.05). TG content was significantly lower in the experimental groups than in the control group (P<0.05)and showed a decreasing trend, with the lowest level observed at 500 Hz. TG content was lower in the control group compared to the 300 Hz and 500 Hz groups, but no significant difference was observed (P >0.05).

Fig.3 Effects of acoustic stimuli on hemolymph glucose (Glu), albumin (ALB), and triglycerides (TG)levels in sea slugs.Different lowercase letters above the bars indicate a significant difference (P < 0.05).
3.3 Effects of Low-Frequency Noise on Antioxidant Enzymes
Sea slugs exposed to 1 h of low-frequency noise (100,300, and 500 Hz)exhibited changes in several antioxidant enzymes in the hemolymph (Fig.4). SOD and CAT activities, as well as MDA content, were significantly higher in the experimental groups than those in the control group,and the highest values were detected in the 500 Hz group(P< 0.05). SOD and CAT activities, as well as MDA content, increased significantly when the sound wave frequency increased from 100 to 500 Hz (P< 0.05).
3.4 Hsp70 Expression
Based on the effects of the different acoustic stimulation frequencies, we further investigated thehsp70expression patterns in the hemolymph and CNS in response to acoustic stimulation (Fig.5). The results showed that expression of thehsp70gene after stimulation was significantly higher in the experimental groups than that in the control group(P< 0.05)and showed a gradually increasing trend in the experimental groups.
As shown in Fig.6, we performed immunohistochemical experiments to detect the Hsp70 protein in the CNS tissues. The experimental and control groups were positive for Hsp70 immunostaining. Moreover, the distribution and abundance of the Hsp70 protein were significantly higher in the experimental groups than those in the control group,as analyzed using Image J software (Fig.6C).

Fig.4 Effects of acoustic stimuli on hemolymph superoxide dismutase (SOD)and catalase (CAT)activities as well as malondialdehyde (MDA)content in sea slugs. Different lowercase letters above the bars indicate a significant difference (P < 0.05).

Fig.5 Effects of acoustic stimuli on the synthesis of hsp70 (mean ± SD)in the central nervous system and hemolymph of the sea slugs (N = 9). Different lowercase letters above the bars indicate significant differences (P < 0.05).

Fig.6 Neural ring immunohistochemistry (IHC)of the sea slug Hsp70 protein. A, control group IHC staining. B, IHE staining for neural ring exposure to low-frequency noise at 500 Hz. The yellow arrow represents the location of the Hsp70 protein. C, IHC optical density analysis of the central nervous system of the sea slug Hsp70 protein.
4 Discussion
Human activities in marine and inland waters have become more frequent, resulting in corresponding increases in noise. Among the different frequencies of noise, low-frequency noise is the most widespread source and has thegreatest biological impact (Edmondset al., 2016; Dayet al.,2019; Joneset al., 2020).
Many scholars have considered low-frequency noise to be a stress that causes a corresponding response in marine animals (Morleyet al., 2014; Vazzanaetal., 2020). Penget al. (2016)reported that sound stimuli induce an avoidance response in razor clam (Sinonovacula constricta),causing more active digging in the laboratory. Introducing an anthropogenic sound source to the clam (Ruditapes philippinarum)elicits a typical stress response (Solanet al.,2016). In the present study, similar behaviors were observed in sea slugs under the stimulation of low-frequency acoustic waves, such as moving away from the acoustic generator, accelerated crawling speed, and mud drilling. Sea slugs are mollusks that live in the intertidal zone and breathe with their lungs. Sea slugs escape the tides by burrowing into mud holes for extended periods without drowning. This behavior is considered a stress response to the surrounding environment, indicating that sea slugs are sensitive to changes in the low-frequency sound.
In this study, the physiological and biochemical parameters we measured are often used to describe the responses to external environmental stimuli in both invertebrates and vertebrates. These parameters reflect the health and physiology of aquatic animals (Jianget al., 2016; Andradeet al.,2018). Energy metabolic homeostasis plays a key role in the survival of aquatic animals under environmental stress conditions. Stress disturbs energy balance. Aquatic animals require additional energy to restore and maintain homeostasis if they are to survive (Sokolovaet al., 2012). Blood Glu is an indicator of the stress response (Arendset al., 1999;Rajendiranet al., 2016). As important components of the serum, lipids and proteins play important roles in the physiology and immunity of fishes (Leeet al., 2018). ALB acts as a nutrient carrier providing energy to the body, and is also involved in maintaining plasma colloid osmotic pressure (Khasaniet al., 2019). Mollusks increase their energy expenditure to maintain metabolism during the stress response (Borgeset al., 2004). In this study, Glu and ALB levels were significantly higher in the hemolymph of the experimental group than those in the control group, whereas TG levels were significantly lower than those in the control group. Glu and ALB contents in hemolymph increased gradually with increasing sound wave frequency in the experimental groups, while the opposite trend was observed for TG content. Low-frequency noise may be an environmental stress for sea slugs, causing increases in blood Glu and protein concentrations in the hemolymph to meet the energy demands for resisting this stress. However, the changes of TGs were exactly opposite to the changes of Glu and ALB in blood, and TGs are one of the main sources of energy for stress resistance in invertebrates (Voslooet al.,2002; Aparicio-Simónet al., 2010). Therefore, the decrease in TG content may have occurred because sea slugs moved faster to avoid low-frequency sound waves, and TGs provide energy for this behavior.
Many studies have shown that reactive oxygen species(ROS)increase when marine animals are exposed to external stress, leading to lipid peroxidation of cell mem0 branes, which damages DNA, proteins, and lipids (Muet al.,2017; Andradeet al., 2019). The antioxidant mechanism of marine animals is activated to produce antioxidant enzymes,including superoxide dismutase (SOD), catalase (CAT), and glutathione, against ROS (Farombiet al., 2007). SOD, CAT,and malondialdehyde (MDA)play a defensive role in the antioxidant enzyme system and are typical indicators of oxidative stress in animals. Trends in these antioxidants indirectly reflect the strength of the antioxidant capacity in an animal (Monteiroet al., 2019a, 2019b; Pintoet al., 2019).The expressions of antioxidant enzyme-related genes, such assod1,cat, andgpxin the liver, as well as ROS in the plasma, are significantly upregulated by short-term exposure of black porgy (Acanthopagrus schlegelii)to noise,and the fish exhibited a strong oxidative stress response(Changet al., 2018). Hanget al. (2021)reported that the liver MDA content in large-mouth bass (Micropterus salmoides)was significantly higher in a noise-exposed group,indicating a higher degree of oxidative stress in fish under noisy conditions. Similar phenomena were observed in mussels exposed to noise, in which oxidative damage occurred in mussel blood and gill epithelial cells, and DNA breaks occurred in the cells (Waleet al., 2019). In the present study, SOD and CAT activities, as well as MDA content,were significantly higher in the hemolymph of the experimental group than those in the control group, indicating that sea slugs have a high sensitivity to low-frequency sound waves, leading to the activation of antioxidant mechanismsin vivo. Moreover, SOD and CAT activities, as well as MDA content, increased gradually as the noise frequency was increased from 100 to 500 Hz, suggesting that the antioxidant defense in sea slugs increases as a defense against the production of ROS, which protects cells from damage.
Many scholars have identified the HSP protein family as one of the best bioindicators in invertebrates and vertebrates for environmental contamination (Moreira-de-Sousaet al., 2018; Muet al., 2019). According to the molecular weight, amino acid sequence homology, and function, HSPs are divided into four major families including HSP90,HSP70, HSP60, and the small HSP family (Noveret al.,1997). Hsp70 plays a role at all stages of protein synthesis and degradation; thus, it is essential for maintaining protein homeostasis and has a direct impact on human health (Rosenzweiget al., 2019). Expression of the Hsp70 protein is used as an indicator to detect environmental stress. Vazzanaet al. (2016)exposed Mediterranean mussel (Mytilus galloprovincialis)to noise and reported increased expression of the Hsp70 protein in the gills and mantle, indicating that Hsp70 could be used as a biological indicator in noise studies. Exposing sea urchin (Arbacia lixulato)to high-frequency noise results in a significant increase inhsp70gene expression in body cavity fluid cells, suggesting that sea urchins have a physiological stress response to noise (Vazzanaet al., 2020). We obtained similar results in this study. Thehsp70mRNA expression levels in the hemolymph and the CNS of the experimental group were significantly higher than those in the control group. Moreover,hsp70mRNA expression levels increased gradually with increasing frequency, reaching their highest level at 500 Hz,indicating that sea slugs are more sensitive to 500 Hz acoustic waves which produced a stronger stress response. The IHC results of the CNS showed that the abundance of the Hsp70 protein was significantly higher than that in the control group, indicating that sea slugs exhibited a stress response to low-frequency noise.
Sea slugs are a transitional aquatic-to-terrestrial species that feeds on unicellular algae and animal cells and plays key roles in the intertidal zone and energy cycle. Prolonged exposure to low-frequency noise leads to behavioral responses that alter the ways that species regulate ecosystem processes, thereby affecting important ecosystem functions,such as primary production. Anthropogenic noise affects survival during early life, which, in turn, affects population dynamics, resilience, and the community structure due to changes in selection pressure, which may, directly and indirectly, alter the ways that species regulate ecosystem functions (Nedelecet al., 2014; Celiet al., 2015; Hubertet al.,2018). These effects of low-frequency noise on sea slugs can alter their population biology, which can affect the health and service functions of coastal ecosystems. In future work,we will study whether low-frequency noise affects the early life stages (eggs and larvae)of the sea slug.
5 Conclusions
Low-frequency noise affected sea slug behavior, mainly in terms of accelerated movement and secretion of stimulating body fluid. Additionally, the changes in Glu, TGs,and ALB contents, as well as SOD, CAT, and MDA activities in hemolymph and the Hsp70 expression levels in hemolymph and neural tissues, indicate that low-frequency noise produced a stress response in the sea slugs, which affected their behavior and physiology. The physiological and biochemical parameters measured in this study, along with the stress response behavior of the sea slugs, could be useful as early detection indicators of noise contamination.
Acknowledgements
This study was supported by the Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture Animals (No. A1-3605-21-000202)and the Capacity Enhancement of Aquatic Germplasm Resources Research and Support Platform of Shanghai Ocean University (No. A1-3201-20-300206).
杂志排行
Journal of Ocean University of China的其它文章
- A Theoretical Model for the Microwave Emissivity of Rough Sea Surfaces
- Mechanism of Regional Subseasonal Precipitation in the Strongest and Weakest East Asian Summer Monsoon Subseasonal Variation Years
- Analytical and Experimental Studies on Wave Scattering by a Horizontal Perforated Plate at the Still Water Level
- Theoretical Prediction of the Bending Stiffness of Reinforced Thermoplastic Pipes Using a Homogenization Method
- Penetration Resistance of Composite Bucket Foundation with Eccentric Load for Offshore Wind Turbines
- Application of Converted Displacement for Modal Parameter Identification of Offshore Wind Turbines with High-Pile Foundation
