COVID-19 associated liver injury: A general review with special consideration of pregnancy and obstetric outcomes
2022-11-21KatherineCooperAlessandroCollettaAlisonAsirwathamTiffanyMooreSimasDeepikaDevuni
Katherine M.Cooper, Alessandro Colletta,Alison M.Asirwatham, Tiffany A. Moore Simas, Deepika Devuni
Abstract Liver injury is an increasingly recognized extra-pulmonary manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Coronavirus disease 2019 (COVID-19) associated liver injury (COVALI) is a clinical syndrome encompassing all patients with biochemical liver injury identified in the setting of SARS-CoV-2 infection. Despite profound clinical implications, its pathophysiology is poorly understood. Unfortunately, most information on COVALI is derived from the general population and may not be applicable to individuals under-represented in research, including pregnant individuals. This manuscript reviews: Clinical features of COVALI, leading theories of COVALI,and existing literature on COVALI during pregnancy, a topic not widely explored in the literature. Ultimately, we synthesized data from the general and perinatal populations that demonstrates COVALI to be a hepatocellular transaminitis that is likely induced by systemic inflammation and that is strongly associated with disease severity and poorer clinical outcome, and offered perspective on approaching transaminitis in the potentially COVID-19 positive patient in the obstetric setting.
Key Words: COVID-19 liver injury; Pregnancy; Perinatal liver disease; Systemic inflammation; Special populations
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease pandemic (COVID-19) is responsible for and upwards of 6.3 million fatalities worldwide[1]. The SARS-CoV-2 virus is a member of theCoronaviridaefamily, a diverse family of single-stranded positive RNA viruses[2]. Coronaviruses are frequently implicated in mild upper respiratory infections and cause 15%-30% of cases of the“common cold”[3,4]. However,Coronaviridaeviruses have also demonstrated an ability to infect the lower respiratory tract and cause severe lung disease associated with substantial mortality[5,6].
Mortality associated with COVID-19 is usually secondary to lung pathology that causes severe respiratory distress syndrome[7-9]. However, patients infected with SARS-CoV-2 often suffer other devastating end-organ injuries[10], suggesting the virus causes systemic infection and inflammation.These observations have prompted interest in the extra-pulmonary manifestations of COVID-19[11],including those in the heart[12,13], intestines[14,15], kidney[16], reproductive system[17,18], and the liver, where the effect of SARS-CoV-2 is poorly understood[19,20].
COVID-19 associated liver injury (COVALI) is a clinical entity encompassing any abnormal liver function test present in individuals positive for SARS-CoV-2[20]. Currently there are no specific or unique diagnostic criteria for COVALI relative to other causes of transaminitis[21] which complicates the process of synthesizing evidence from clinical studies. This is most salient when applying available data to those underrepresented in the literature, such as pregnant and birthing persons.
In the first section of this review, we will summarize clinical features of COVALI and the leading theories on the mechanism of liver damage in the general population. In the second section, we present existing literature on liver injury in SARS-CoV-2 positive pregnant persons, a topic not widely explored in the literature despite significant clinical relevance. Ultimately, we aim to synthesize data on COVALI in the general and perinatal populations and offer perspective on approaching this problem in the obstetric setting.
GENERAL POPULATION
Background
At the present time, COVALI is an umbrella term that applies to all patients with SARS-CoV-2 infection and transaminitis. Meta-analyses estimate that one in four patients with COVID-19 develop acute liver injury[22-24], but this figure is variable across studies and ranges from 14%-74%[25-27]. There seem to be no demographic factors to account for this variability, which may ultimately be due to differences in the study timeline or definition of liver injury[28]. Interestingly, only one of the three cited metaanalyses on COVALI included a study involving pregnant patients (n= 9)[29]. In the following sections we will review clinical and pathophysiologic considerations for COVALI in the general population.
Clinical Considerations
COVID-19 associated liver injury is a hepatocellular or mixed pattern liver injury with aspartate aminotransferase (AST) predominant transaminitis[28,30-33]. Most studies report mild liver injury with liver enzymes that peak at values less than five times the upper limit of normal[34-38]. Conversely,some reports suggest up to 25% of patients’ aminotransaminases exceed this threshold[39,40] and there is mounting evidence that liver enzymes can increase to the thousands (U/L) in patients with severe COVID-19[26,38,41-45]. The timeline of developing liver injury is not fully elucidated and has varied between studies[32,33].
Non-transaminase laboratory evidence of liver damage has also been identified, but is reported less consistently in the literature. Specifically, total bilirubin and alkaline phosphatase have been reported to be elevated in 1%-53%[46-48] and 0.3%-80.0%[48,49] of patients, respectively. This variability may be due to study timeline relative to the temporal course of laboratory changes in patients with COVALI.For example, it has been shown that alkaline phosphatase elevations begin and peak later in the disease course than aminotransaminases and may not be captured by studies that don’t follow laboratory data for extended periods[50,51].
The interest in COVALI is rooted in its association with disease severity and negative patient outcomes. First, patients with elevated liver enzymes at presentation or at hospital admission are more likely to develop severe COVID-19 lung disease[5,52-54]. Additionally, a large study by Guanet al[55]reported laboratory data from patients at over 500 hospitals and found patients with severe COVID-19 were more likely to have transaminitis compared to patients with non-severe COVID-19. Going further,Bloomet al[31] studied trends in aminotransaminase levels from time of admission to peak in patients hospitalized for COVID-19 and found that in addition to higher mean AST and alanine aminotransferase (ALT), there was a greater change from baseline to peak transaminases in patients with severe compared to non-severe COVID-19. A small single center study found that elevated AST was observed more often in patients who required intensive level care compared to those who did not require intensive care[56]. Further, in a cohort of 1611 hospitalized patients across 11 Latin American countries,abnormal liver enzymes conferred a 2.6-fold risk for severe COVID-19 pneumonia and a 1.5-fold risk of death[37].
Within the umbrella of COVALI, AST has been shown to have specific prognostic value[29,37,57]. For example, the numeric value for serum AST has been incorporated in clinical calculators created to predict progression from mild or moderate to severe COVID-19 disease[57]. Moreover, elevated AST has been found to be independently associated with increased risk of death, apart from other markers of hepatic dysfunction[29,34,50,58,59]. In a study including 206 patients across 26 institutions in Brazil,AST level greater than twice the upper limit of normal significantly increased the risk of in-hospital mortality when adjusted for age and biologic sex[29]. However, is important to note that when elevated,bilirubin may be a stronger predictor of death than AST in some cohorts[34].
Given the association with poor patient outcomes, identifying potential risk factors for COVALI is imperative. We found one meta-analysis that sought to define predictors for the development of COVALI. In this study Harapanet al[60] pooled data from 16 studies (n= 6253) to assess whether any of the following were associated with development of severe liver injury in patients infected with SARSCoV-2: Age, biologic sex, body mass index (BMI), diabetes mellitus, coronary artery disease,hypertension, underling liver disease, white blood cell count, lymphocyte count, and neutrophil count.They observed significant association between male sex, higher BMI, presence underlying liver disease,elevated white blood cell, and elevated lymphocyte counts with development of acute liver injury. After controlling for bias introduced by the meta-analysis, they concluded male sex and lymphocyte count were found to be independent risk factors for COVALI[60]. Not evaluated in this meta-analysis, inflammatory markers have also been shown to be a risk factor associated with liver injury[61-64]. For example, multiple studies have inflammatory markers directly correlate with liver enzymes[64] and that liver injury can be predicted using inflammatory markers such as ferritin and C-reactive protein[61,62].
Professional societies recommend clinically relevant work up for other causes of liver injury in patients who develop COVALI[21,65-68]. The American and Asian Pacific Association(s) for the Study of Liver Diseases suggest ruling out other causes of viral and toxin-mediated hepatitis in all COVID-19 patients with liver injury[21,66]. More nuanced suggestions include considering cytokine-syndrome,myositis, or cardiac injury in patients with disproportionally elevated AST, and primary sclerosing cholangitis in critically ill patients with cholestatic liver injury[21,65,66]. They advise trending liver enzymes of patients hospitalized with COVID-19, those with known chronic liver disease diagnosed with COVID-19 and of those receiving anti-retroviral medications for treatment of COVID-19 pneumonia[66]. Patients with chronic hepatitis B may be at particularly high risk both due to risk of severe infection and viral reactivation when receiving immunosuppressive therapy[66,69]. However,they do not recommend changing management and offer no specific intervention for liver injury in most cases of COVALI. They endorse targeting the viral illness in the acute setting is sufficient for liver injury and encourage work up for chronic liver disease when illness is resolved.
Pathophysiology
The underlying mechanism(s) of liver injury in COVID-19 are not fully understood. While there is increasing literature on this topic, the absence of explicit diagnostic criteria has resulted in heterogeneity in clinical studies and has impeded recognition of specific mechanisms of injury. There is consensus that COVALI is likely multifactorial and due to a combination of exacerbation of underlying liver disease,direct cytotoxicity, hypoxic liver injury, drug induced injury and systemic inflammation with immune dysregulation[28,70].
Early theories focused on exacerbation of underlying liver disease and toxicity from pharmacologic agents used to treat severe COVID-19 infection as a sources of liver injury. It is true that patients with cirrhosis are at risk for developing severe pneumonia and hepatic decompensation during SARS-CoV-2 infection[21]. Likewise, some antiviral medications used to treat COVID-19 have hepatotoxic properties and have been associated with abnormal liver function during the pandemic (e.g., lopinavir/ritonavir)[27,40,61,71]. In a combination of these, SARS-CoV2 infection treated with corticosteroids or tocilizumab has been showed facilitate reactivation and accelerate liver injury in patients with chronic hepatitis B[72]. However, these two factors are unable to explain most of this phenomena as: (1) Over 90% of patients with COVALI have no evidence of underlying liver disease; and (2) transaminitis is often present at baseline prior to administration of medications[73]. While it is possible that liver injury during SARS-CoV-2 infection may be exacerbated by these factors, COVALI is likely a distinct clinical entity.
Diverse studies have demonstrated direct viral infection of the liver can occur during COVID-19 infection. In a study including 156 autopsy samples, postmortem hepatic tissue evaluation revealed typical coronavirus particles in hepatocyte cytoplasm with associated mitochondrial swelling and endoplasmic reticulum dilatation in patients who died with COVID-19[74]. Other reports have shown SARS-CoV-2 nuclear material in liver tissue, including RNA in hepatocytes of live patients who underwent liver biopsy during SARS-CoV-2 infection[75]. Some of the most convincing data comes from a recent paper by Wanneret al[76] who demonstrated SARS-CoV-2 can be detected in up to threefourths of post-mortem liver biopsies using reverse transcriptase-polymerase chain reaction. Ultimately,there is irrefutable histologic evidence that SARS-CoV-2 directly infects hepatocytes, providing strong evidence that SARS-CoV-2-mediated cytopathy plays a role in COVALI. It is thought that angiotensin converting enzyme 2 and/or its receptor (ACE-2) may mediate cytopathy by enabling viral access to the liver[76,77]. However, the understanding of SARS-CoV-2 hepatotropism of is still evolving.
Epidemiology-based correlates support direct ACE-2 mediated entry into hepatocytes based on data that shows groups at increased risk of COVALI also have increased hepatic ACE-2 expression. For example, ACE-2 Levels are higher in males than females[78] and ACE-2 is upregulated in decompensated cirrhosis[79]. Interestingly, it has been shown that ACE-2 is dominantly expressed in cholangiocytes relative to hepatocytes and that infection of cholangiocytes may occur more often than infection of hepatocytes[80]. While this may seem to contradict direct cytotoxicity, it is possible that cholangiocyte infection can still result in direct viral access to the liver. In-vitro infection with SARS-CoV-2 has been associated with decreased expression of the cholangiocellular tight junctions that usually protect parenchymal liver cells from toxins in the biliary tree[81]. It has been further speculated that reduced barrier function of cholangiocytes during active COVID-19 infection may lead to hepatic injury through leakage of toxic bile into the adjacent liver parenchyma[81]. Lastly, it is known that ACE-2 can mediate viral entry into endothelial cells[82]. Viral infection of the portal systems and vascular cells in the liver may contribute to the endothelitis, microvascular changes, and intravascular thrombosis visualized in post-mortem examination of hepatic tissue in patients who died from COVID-19[83].
Reduced blood oxygen, which can negatively affect the liver, occurs in up to 50% of patients with COVID-19 infection[84]. However, only a small percentage of patients have transaminitis to the degree expected in ischemic hepatitis[34-38]. While ischemia from low blood oxygen seems to have a limited direct role in COVALI pathophysiology, the relationship between hypoxia and inflammatory pathways is significant. Specifically, hypoxia can trigger and amplify immune dysregulationviainflammatory pathways mediated by hypoxia inducible factor and tumor necrosis factor[85]. This may explain the link between severity of lung disease with liver injury and provide support for and transition to the inflammatory hypotheses of COVALI[86].
There is substantial data suggesting systemic inflammation and associated immune dysregulation,endotheliopathy and thrombosis are central to the pathophysiology of COVALI[87,88]. It is well established that severe COVID-19 infection induces systemic inflammation and that concentrations of several clinically evaluated inflammatory markers are increased in patients with COVID-19, such as Ddimer, C-reactive protein, procalcitonin, ferritin, and interleukin-6 (IL-6)[89-92]. Inflammatory markers are also higher in COVID-19 positive patients with biochemical liver derangements compared to COVID-19 positive patients without such derangements across, suggesting a link between liver injury and inflammation[61,62,92-94]. For example, a large retrospective analysis (n= 800) showed patients with COVID-19 complicated by COVALI had higher levels of C-reactive protein, procalcitonin, Ddimer, and serum ferritin compared to patients without COVALI[61]. In a unique study, Diaz-Louzaoet al[86] used joint regression modeling to evaluate the temporal relationship between increases in markers of liver injury and inflammation. They found that elevation of inflammatory markers precedes elevation of liver enzymes. Ultimately they created a statistical model that implicates inflammation in causation of liver injury. The specific inflammatory markers increased during COVALI are known to be involved inin vivoendotheliopathy and hypercoagulability[95,96], as has been visualized in hepatic tissue of patients with liver injury secondary to COVID-19. Further, histologic findings of macrovesicular steatosis, mild acute hepatitis, portal inflammation and portal/sinusoidal thrombosis in hepatic tissue of patients who have direct viral infection of the liver support that even with direct cytopathy, inflammation may have a preceding role[83,97-100].
Interleukin-6 is an inflammatory cytokine associated with endotheliopathy and a hallmark indicator of severe COVID-19. It has been shown that IL-6 can activate platelets and precipitate endothelitis in multiple organs during systemic COVID-19 infection, particularly those with a predilection for intravascular clot formation (e.g., the liver)[95]. Due to its association with biochemical liver injury[85,101-103] and known function[85,101,102], IL-6 has received interest as a likely active contributor to development of liver injury in COVID-19[103]. Recent work by McConnellet al[102] found a potential mechanism for this in that activating a soluble form of the IL-6 receptor triggers downstream proinflammatory and pro-coagulation pathways in the liver[102,104]. Further, that IL-6 signaling induces a hypercoagulable state in liver sinusoidal cells[85,104], which may contribute to the known endothelitis and thrombosis in hepatic tissue of patients with COVALI. Similarly, increased staining of a well-known platelet marker (CD-61) has been identified within dilated sinusoids in COVID-19 patients with elevated liver enzymes, suggesting activated platelets and endotheliopathy are critical in liver injury during COVID-19[85]. These findings are consistent with studies showing portal or sinusoidal vascular thrombosis is present in hepatic tissue of up to 50% of patients with COVID-19[83]. In context of literature on inflammatory markers in COVALI, Il-6 shows true mechanistic potential and bolsters the theory that inflammation, endotheliopathy and thrombosis are at the crux of this clinical syndrome.
OBSTETRIC POPULATION
Background
Liver injury is a rare and potentially serious complication of pregnancy that is estimated to affect 3%-5%of birthing persons[105]. The differential diagnosis for hepatic dysfunction in this population includes specific pregnancy related (perinatal) liver diseases[106], such as pre-eclampsia/eclampsia, hemolysis elevated liver enzymes and low platelet (HELLP) syndrome, acute fatty liver of pregnancy and intrahepatic cholestasis of pregnancy (obstetric cholestasis), and non-pregnancy related liver diseases,such as auto-immune hepatitis, viral hepatitis, non-alcoholic steatohepatitis, and now COVALI[107-109]. Perinatal liver diseases are associated with significant mortality and often require prompt delivery of the fetus for safety of the mother (summarized in Table 1). Because liver injury can strongly influence decisions regarding delivery[107], COVALI during pregnancy is of serious clinical significance.
Clinical considerations
General clinical course: Clinical characteristics of COVID-19 during pregnancy given current knowledge are well represented in the literature, but there is limited data specific to the course of liver injury. In the obstetric setting, COVALI is an AST-predominant transaminitis that affects 13%-42% of COVID-19 positive pregnant patients[108,110-112]. While these statistics are comparable to the general population, a meta-analysis that included pregnant patients reported key differences. They found (1)higher prevalence of COVALI in pregnant patients compared to non-pregnant patients; and (2) more severely elevated liver enzymes in pregnant patients with COVALI compared to non-pregnant patients with COVALI[113]. This was confirmed in a study that directly compared laboratory values of COVID-19 positive pregnant patients with non-pregnant counterparts and found COVALI was more common in pregnancy[110]. The authors of this study cautioned that many of their observations were likely related to physiological changes of pregnancy, but they concluded the rate of COVID-19 positive pregnant individuals with acute liver injury was out of proportion to expected physiologic changes. This may indicate that COVID-19 confers an increased risk of liver injury specific to pregnancy.
Clinical Cases: Obstetric providers are tasked with differentiating liver disease that necessitates urgent delivery for the health and safety of the pregnant personvsthat which can be managed expectantly and will be stable or resolve without delivery. Multiple reports illustrate this dilemma through cases of pregnant patients with acute liver injury who are COVID-19 positive and have concurrent features of high-risk perinatal liver diseases[114-120]. We identified seven cases and classified them according to the pattern in which liver enzymes improved throughout the clinical course: A, improved without delivery; B, improved with delivery; C, other (no improvement within 72 h of delivery, no timeline of COVID-19 symptoms) (Table 2). We will discuss cases that improved without delivery and highlight features that favored COVALI relative to perinatal liver diseases.
In a case described by Azimiet al[115], a 27-year-old Gravida (G) 2 Para (P) 1 woman presented at 30-wk’ gestation with a headache and was found to have abnormal liver enzymes, low platelets, increased inflammatory markers (LDH, ferritin D-dimer), and chest radiograph showing diffuse ground glass opacities, concerning for autoimmune diseasevsHELLPvssystemic COVID-19. Pending extensive laboratory evaluation that was negative for autoantibodies and signs of hemolysis, the patient was noted to be improving with only supportive care. She was discharged at 33-wk’ gestation and underwent normal delivery at 39-wk’ gestation. The next case was that of a 35-year-old G2 P1 with prior obstetric cholestasis presenting at 28-wk’ gestation with progressive fever and cough who was found to have high ALT and elevated serum bile acids[114]. The patient denied pruritis and had normal labs at her 20-wk appointment which reduced the likelihood of obstetric cholestasis; she subsequently testedpositive for COVID-19 which was then thought to be the source of her liver injury. The final case is that of a 39-year-old G5P1 presenting at 26 wk’ gestation with progressive dry cough and dyspnea. She was found to have new hypertension (BP 152/132), severe transaminitis (AST 1154 U/L, ALT 864 U/L), and PCR proven COVID-19 infection, concerning for pre-eclampsia with severe featuresvssystemic COVID-19[116]. Based on high suspicion for pre-eclampsia, the patient received betamethasone and dexamethasone to assist fetal lung maturation. Surprisingly, the patient’s blood pressure was noted to be improving, inconsistent with pre-eclampsia which requires delivery to return to normotension. To further evaluate this, serum maternal placental growth factor was tested and normal. Normal maternal placental growth factor effectively ruled out pre-eclampsia and favored a diagnosis of COVID-19 with COVALI. This patient went on to deliver a healthy full-term fetus. In each case hypertension and liver injury improved with conservative management for COVID-19 and did not require delivery as is the case with perinatal liver diseases.
Cases in the latter two sections demonstrate complicated cases that are difficult to parse out based on clinical course. For example, in the case by Arslanet al[118], the patient’s proteinuria was concerning for pre-eclampsia and liver enzymes trended down as expected after cesarean delivery, though both maternal and neonatal outcomes were poor which complicates interpretation of the case. Similarly, in the case by Choudharyet al[111], hypoglycemia and elevated bilirubin were highly suspicious for AFLP,but liver enzymes remained elevated for multiple days after delivery.
Outcomes: COVID-19 associated liver injury correlates with worse clinical outcomes and increased mortality in the obstetric setting. A retrospective cohort study of 122 COVID-19 positive pregnant patients in Istanbul found acute liver injury conferred a 3.5-fold risk of becoming critically ill during hospitalization[112]. Maternal mortality is reportedly more common in pregnant patients who delivered while COVID-19 positive with acute liver injury than COVID-19 positive without liver injury[111].
The largest published study evaluating COVALI in pregnancy is a 249-patient prospective cohort study performed at large tertiary care hospital in eastern India[111]. Unlike in previous studies, patients with hypertensive disorders, diabetic disorders, or concern for intrahepatic cholestasis were not excluded. Overall, 107 (42.1%) had evidence of hepatic dysfunction, but liver injury was more commonin patients with perinatal hypertensive, diabetic, or cholestatic disorders (47/87, 54%) compared to those without (60/162, 37%). Although no statistical metric of significance was provided by the study, it appears that COVID-19 may increase risk of or exacerbate underlying obstetric conditions associated with liver injury. The primary aim of the study was to evaluate the relationship between liver injury in COVID-19 and obstetric outcomes. While no associations between liver injury and mode of delivery or neonatal outcomes were identified, those with liver injury tended to deliver pre-term and/or require cesarean delivery more often, both of which increase morbidity. Their key finding was that obstetric complications were significantly higher in COVID-19 positive pregnant patients with liver injury,despite no differences in maternal or gestational age[111]. Specifically, pregnant persons with COVALI were less likely to have a normal vaginal delivery than those without liver injury (18.7%vs30.3%).Further, postpartum hemorrhage, sepsis, and death were more common in those who delivered while COVID-19 positive with acute liver injury[111].

Table 2 Summary of case reports

CT: Bilateral diffuse GGO’s; differential: Not given ALT 300 Improved over hospitalization and LFTs trended down (no timeline given)Bilirubin 9.4 PLC 90 CRP 78.5 LDH 3100 Ferritin 734 Choudhary et al[120],202127 y/o G1P0, GA 35 wk, di-di twins CC: Cough, fever, abdominal pain;vitals: BP 142/94, HR 88, RR 20.SpO298%; chest X-ray: Bilateral basal opacities; differential: aHELLP vs PEC vs AFLP vs COVID 19 AST 728.5 Suspicion of aHELLP→Cesareansection ALT 473.2 POD 0: Hypo-glycemia, altered mentation, ↑ bilirubin→AFLP Bilirubin 4.9 Transfer to ICU + IV labetalol PLC 162 POD 8 discharged, normal LFT’s CRP 22 LDH 96.9 Ferritin 120 Gestational age is noted as weekd. Vitals reported as: BP: Blood pressure (mmHg); HR: Heart rate (beats per minute); RR: Respiratory rate (breaths per minute); Spo2: Oxygen saturation (%). Laboratory values are reported with the following standardized units: AST: Aspartate aminotransaminase (U/L);ALT: Alanine aminotransaminase (U/L); bilirubin (mg/dL); PLC: Platelet count (× 103/ mm); CRP: C-reactive protein (mg/dL); LDH: Lactate dehydrogenase (u/L); ferritin (ng/dL). PEC: Pre-ec clampsia; HELLP: Hemolysis, elevated liver enzymes, low platelets; Ahellp: Atypical HELLP; AFLP:Acute fatty liver of pregnancy; ICHP: Intrahepatic cholestasis of pregnancy/obstetric cholestasis; CT: Computed tomography; GGO: Ground glass opacities; GA: Gestational age; WNL: Within normal limits; LFTs: Liver function tests; HD: Hospital day; C-section: Cesarean section; POD: Post-operative day; ICU: Intensive care unit.
Pathophysiology
The pathophysiology COVID-19 is not well studied outside the general population and thus the pathophysiology of COVALI in pregnancy is not well understood. Studies comparing COVID-19 positive pregnant individuals with acute liver injury and COVID-19 positive pregnant individuals with normal liver enzymes are crucial to build understanding of disease mechanisms in this cohort.However, there are only a handful of published studies on this to date[111,112,121]. We first use these studies to establish that relationships relevant to pathophysiology in the general population also exist in the obstetric population.
Specifically: (1) What is the relationship between severe COVID-19 disease and COVALI in pregnant patients? Patients with severe COVID-19 are more likely to develop COVALI. A prospective cohort study found that 87.5% of pregnant patients with severe COVID-19 pneumonia during hospitalization developed abnormal liver enzymes after having normal liver enzymes at baseline[122]. A later study demonstrated pregnant patients with liver injury had more severe disease and two thirds of this cohort ultimately died due to COVID-19 lung disease[111]; (2) what is the relationship between COVALI and markers of inflammation in pregnant patients? COVALI during pregnancy has been associated with elevated markers of inflammation. COVID-19 positive pregnant patients with liver injury have higher serum ferritin than expected in normal pregnancy, where a state of physiologic anemia is to be expected[112]. Furthermore, a study by Denget al[121] evaluating liver chemistries in 37 COVID-19 positive pregnant patients found those with liver injury had higher inflammatory markers, such as procalcitonin and IL-6; and (3) what is the relationship between COVALI and systemic inflammatory manifestations of COVID-19? Research by Choudharyet al[111] showed that obstetric complications were found to be more common in patients with COVALI. Most of these complications were related to inflammation,endotheliopathy, and coagulopathy. For example, they found pregnant persons with liver injury had higher prothrombin time and were more likely to experience postpartum hemorrhage requiring blood transfusion. Further, systemic inflammation was more common in those who delivered while COVID-19 positive with acute liver injury, as evidence by increased risk of sepsis with multi-organ failure[111].
Overall, these studies suggest the relationships between liver injury and disease severity, patient outcomes, and inflammation identified in the general population persist in the obstetric population.While pathophysiology is likely stable across cohorts, considering the increased risk of COVALI during pregnancy could help further elucidate pathophysiology.
One potential link to the increased risk is the upregulation of ACE-2 to increased plasma levels above non-pregnant individuals, secondary to increase in estrogen production[123,124]. During pregnancy ACE-2 is highly expressed in the placenta and helps regulate blood pressureviasystemic vascular resistance. This suggests there is increased activity of ACE-2 in the endothelium of pregnant patients[125] leading to the placenta as a potential target for COVID-19 infection. The interruption of the physiologic function of ACE-2 in pregnancy has been postulated to be a major contributing factor to the development of complications[126]. Lower levels of ACE-2 have been detected in the placentas from COVID-19 positive patients, suggesting that COVID-19 infection may alter ACE-2 expression and its biologic function in both the placenta and more widely in maternal circulation[124], potentially causing endothelial dysregulation as seen in COVALI.
Based on clinical manifestations, it is also reasonable to consider that pathophysiology of COVALI resembles or amplifies that of obstetric hepatobiliary pathology. This is exhibited in the case reports narrating the difficulty of differentiating COVALI from obstetric disorders that cause transaminitis in the clinical setting. Overall, the greatest overlap occurs between severe pre-eclampsia and the extrapulmonary manifestations of COVID-19, and pre-eclampsia has been diagnosed more often in pregnant persons with COVID-19 compared to pregnant persons without COVID-19[127,128]. A potential link to the increased risk is alpha-1-antitrypsin, an enzyme that can inhibit SARS-CoV-2 infection and protects endothelial cells from oxidative stress during pregnancy, which is reduced in seen in pregnant patients with pre-eclampsia[129,130].
Work by Mendozaet al[122] sought to determine the prevalence of “pre-eclampsia findings” in 42 COVID-19 positive pregnant women. Eight women had severe pneumonia secondary to COVID-19 of which seven (87.5%) had elevated liver enzymes consistent with COVALI and five (62.5%) had hypertension meeting criteria for pre-eclampsia. However, sonographic evidence of placental hypoperfusion was only found in one patient who ultimately required delivery to prompt resolution of hypertension and liver injury. The remaining patients did not require delivery and instead, liver injury and hypertension improved in parallel with symptoms of pneumonia due to COVID-19. They measured ratio of soluble fms-like tyrosine kinase-1 (sFlt-1) ad serum placental growth factor (PIGF), which has been shown to be predictive of pre-eclampsia[131], and found sFlt-1/PIGF normal ratio in patients who did not require delivery compared to an elevated sFlt-1/PIGF ratio in the patient with evidence of placental hypoperfusion who required delivery. These findings suggest severe COVID-19 complicated by COVALI can mimic hypertensive disease of pregnancy and may represent shared disease mechanisms (Figure 1).
Literature that was published during the writing of this review directly compared the pathophysiology of pre-eclampsia and COVID-19. In this study, Palomoet al[132] compared endothelial inflammation and angiogenesis in pregnant patients with pre-eclampsiavsCOVID-19 pneumoniavsnormotensive controls. They measured circulating inflammatory markers in patient blood and found different biomarker profiles of coagulopathy, endothelial inflammation, and angiogenesis. Both COVID-19 and pre-eclampsia had increased vascular cell adhesion molecules expression relative to controls and increased markers of innate immunity. Fortunately, there were multiple factors helpful in differentiating pre-eclampsia and COVID-19: (1) COVID-19 had higher von Willebrand factor and soluble tumor necrosis factor-receptor but lower PIGF; and (2) Pre-eclampsia had higher soluble tumor necrosis factorreceptor and sFlt-1but lower von Willebrand factor. They observed altered sFlt-1 to PlGF ratio was predictive of pre-eclampsia, consistent with findings of Mendozaet al[122] In the latter part of their study they observed how sera from each patient cohort induced change when applied to human dermal microvascular cells. Despite different angiogenic and endothelial profiles, sera from both cohorts activated a common downstream pathway associated with endothelial inflammation, potentially indicating a shared end-pathway. While liver injury was not specifically evaluated in this study, these findings can be interpreted as evidence supporting endothelial dysfunction and inflammation as drivers of systemic manifestations of COVID-19 that are also present in pre-eclampsia, such as liver injury.Shared histologic findings in COVALI and pre-eclampsia including microvascular changes and signs of platelet activation, further support this theory[106,133].
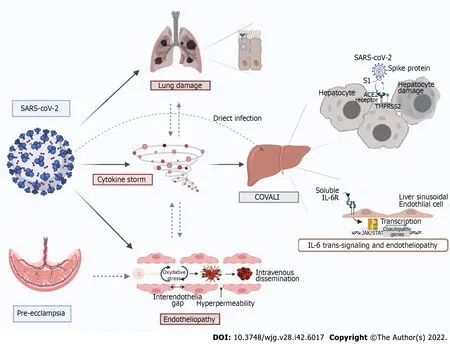
Figure 1 Mechanisms of COVID-19-associated liver injury: Inter-organ crosstalk. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)enters host cells via interaction of its spike protein with the receptor angiotensin converting enzyme 2 in the presence of TMPRSS2 in many tissues. Proposed mechanisms for SARS-CoV-2-mediaded liver injury include: (1) Direct viral cytopathic effect; (2) IL-6 trans-signaling in liver sinusoidal endothelial cells which leads to endotheliopathy; (3) cytokine storm-induced damage; and (4) hypoxemic injury. There is also a lung-gut crosstalk which promotes an increased inflammatory state as well as dysbiosis which increases intestinal permeability, thus facilitating viral entry. Furthermore, direct viral injury to the vascular endothelium leads to increased cytokine release, enhanced reactive oxygen species production and thrombo-embolic events involving both micro and macro circulation. In a similar fashion, preeclampsia spectrum syndromes cause inflammation and endotheliopathy that pre-disposes to liver injury and can be synergistic coronavirus disease 2019 (COVID-19) and COVID-19 associated liver injury. Original figure was created with BioRender.com.
CONCLUSION
In this paper we reviewed COVID-19 associated liver injury with a special focus on pregnancy. We demonstrated COVALI to be an inflammatory mediated AST-predominant transaminitis associated with COVID-19 disease severity and poorer patient outcomes. Emerging research in the general and obstetric populations supports inflammation and endothelial dysfunction as central to pathophysiology in systemic COVID-19 and COVALI. Figure 1 summarizes proposed mechanisms of COVALI and illustrates how some physiologic changes in pregnancy can pre-dispose or exacerbate processes of liver injury during COVID-19. There is significant opportunity to improve understanding of COVALI during pregnancy. At present COVALI appears to be independently associated with worse post-partum outcomes, though this has not been fully parsed on in the literature. Further research should be done to elucidate the relationship between post-partum outcomes and COVALI, relevant to short and long-term outcomes. There is also data supporting the use of specific of circulating biomarkers to differentiate systemic COVID-19 from other causes of transaminitis in pregnancy, but further research is required to define criteria that can guide management.
FOOTNOTES
Author contributions: Cooper KM conceptualized this article, completed research collection, and lead the writing and editing of the manuscript; Colletta A assisted in conceptualizing the article, wrote portions of the manuscript, edited the initial, and revised manuscripts; Asirwatham AM assisted in conceptualizing portions of the article and provided expert feedback in the area of Obstetrics and Gynecology; Moore Simas TA reviewed content, edited the initial and revised manuscript, and provided expert feedback in the area of Obstetrics and Gynecology; Devuni D reviewed content, edited the initial and revised manuscript, and provided expert feedback in the area of Hepatology.
Conflict-of-interest statement:Devuni D is an Associate Professor of Medicine at UMass Chan Medical School, she has received grant funding from Sequana Medical for a clinical trial unrelated to the present work; all other authors have no conflicts of interest to report.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Katherine M. Cooper 0000-0002-6030-4773; Tiffany A. Moore Simas 0000-0002-8356-6418.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
杂志排行
World Journal of Gastroenterology的其它文章
- Angiogenesis and immune checkpoint dual blockade: Opportunities and challenges for hepatocellular carcinoma therapy
- Clinical value of predictive models based on liver stiffness measurement in predicting liver reserve function of compensated chronic liver disease
- Novel management indications for conservative treatment of chylous ascites after gastric cancer surgery
- Computed tomography perfusion in liver and spleen for hepatitis B virus-related portal hypertension: A correlation study with hepatic venous pressure gradient
- Role of radiomics in the diagnosis and treatment of gastrointestinal cancer
