Spectroscopic Properties of Ni2+Ions in Octahedral Complexes
2022-10-12BRIKMikhailKURBONIYONMekhrdodMAChonggeng
BRIK Mikhail G,KURBONIYON Mekhrdod S,MA Chong-geng*
(1.School of Optoelectronic Engineering & CQUPT-BUL Innovation Institute,Chongqing University of Posts and Telecommunications,Chongqing 400065,China;2.Institute of Physics,University of Tartu,Tartu 50411,Estonia;3.Faculty of Science and Technology,Jan Długosz University,PL-42200 Częstochowa,Poland;4.Academy of Romanian Scientists,Bucharest 050044,Romania)*Corresponding Authors,E-mail:mikhail.brik@ut.ee;macg@cqupt.edu.cn
Abstract:Based on an up-do-date literature data,we consider an empirical trend between the energy of the spinforbidden3A2-1E transition of the octahedrally coordinated Ni2+ions and a new nephelauxetic parameterβ1=(B,C(B0,C0)are the Racah parameters of Ni2+ions in a crysta(lfree state),respectively).It is demonstrated that the energy of the Ni2+1E state is a linear function of theβ1 parameter.These findings prove importance of a simultaneous consideration of reduction of both Racah parameters B and C due to the nephelauxetic effect.Such an approach is more accurate in estimating the energy position of the1E level.The commonly used nephelauxetic ratioβ=B B0,which completely ignores the reduction in the values of the Racah parameter C,is not accurate enough for this purpose.The collected in the present paper experimental data and their analysis can be useful for researchers working with the crystalline materials doped with Ni2+ions.
Key words:Ni2+;spin-forbidden transitions;covalency
1 Introduction
Theoretical and experimental studies of the transition metal(TM)ions spectroscopic properties in a free state and in solids are still actively being performed,which can be readily explained by numerous applications in science and technology[1-3].In particular,the TM ions with an unfilled 3d electron shell are of special importance.These ions can be stabilized in solids in different oxidation states and in different coordination,and this circumstance contributes to the variety and complexity of the optical spectra of these ions in various crystalline materials.Since the 3d electron shell is the outer one,its electrons strongly interact with nearest neighbors in the crystal lattice.The overall appearance of the absorption and luminescence spectra of these TM ions is determined by a combination of the spin-allowed broad emission bands and narrow spin-forbidden peaks.The former ones are used for getting tunable laser generation[4],whereas the latter ones are important for lighting[5-8],as well as for lasing(like ruby laser that operates on the sharp spin-forbidden2E-4A2emission transition of Cr3+ions).
One of those 3d TM ions is a divalent nickel,Ni2+,with its 3d8electron configuration.An interest to this ion has been revived recently because of its infrared emission in the second(1 000-1 350 nm)and third(1 550-1 870 nm)biological windows[9-11],which facilitates applications of these ions in optical thermometry and bioimaging.
The Ni2+electron configuration(3d8)in terms of the number of allowed states is equivalent to the 3d2electron configuration.Both configurations have 45 allowed microstates,which obey the Pauli exclusion rule.These 45 states produce five LS terms,whose properties are summarized in Tab.1.In that table the standard2S+1L notation is used,where S and L stand for the total spin and orbital momenta,respectively.These five terms make the energy level scheme of a free ion.
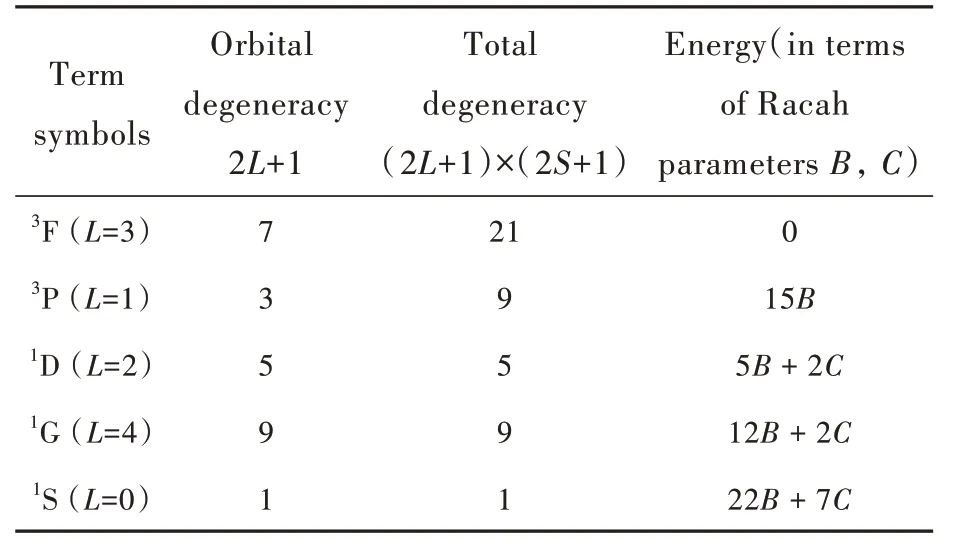
Tab.1 Symbols,degeneracy and energies of the LS terms of the 3d8 electron configuration.The ground term energy is taken as zero
The free ion's energy levels split,when such an ion is placed into a crystal field.The splitting pattern depends on the symmetry properties of the crystal lattice site occupied by an impurity ion.An analysis of the energy levels schemes of the TM ions in crystal field of cubic symmetry can be performed with the help of the so-called Tanabe-Sugano diagrams[12].Three main parameters are needed for this purpose:the crystal-field strength Dq(which describes the crystal field effects),and two Racah parameters B and C(which determine the energy intervals between the free ion terms due to the Coulomb interaction between the electrons in the unfilled electron shell).The horizontal axis in all such diagrams is the Dq/B ratio,the vertical axis is the energy E of the split states in terms of the Racah parameter B(or the E/B ratio),and the diagrams are plotted for a fixed C/B ratio.
Fig.1 depicts the Tanabe-Sugano diagram for a 3d8ion in an octahedral crystal field.
When Dq/B ratio is equal to zero,the free ion's energy level scheme is restored.When Dq/B>0,the energy levels are split,and the split levels depend on the Dq/B value.It can be noted,however,that the energy separation between the ground state spintriplet3A2and the first spin-singlet1E state is practically independent of the crystal-field strength.Moreover,at some Dq/B value the first excited spin-triplet state3T2and the first spin-singlet1E intersect with each other.This allows to consider two special cases:(1)a weak crystal-field,where the first excited state3T2originates from the same3F term as the ground state3A2and,(2)a strong crystal-field,where the first excited state is1E,which comes from the1D term of a free ion.The Dq/B value,at which the energies of the3T2and1E states are equal,is a separation between these two situations,as shown by a vertical dashed line in Fig.1.
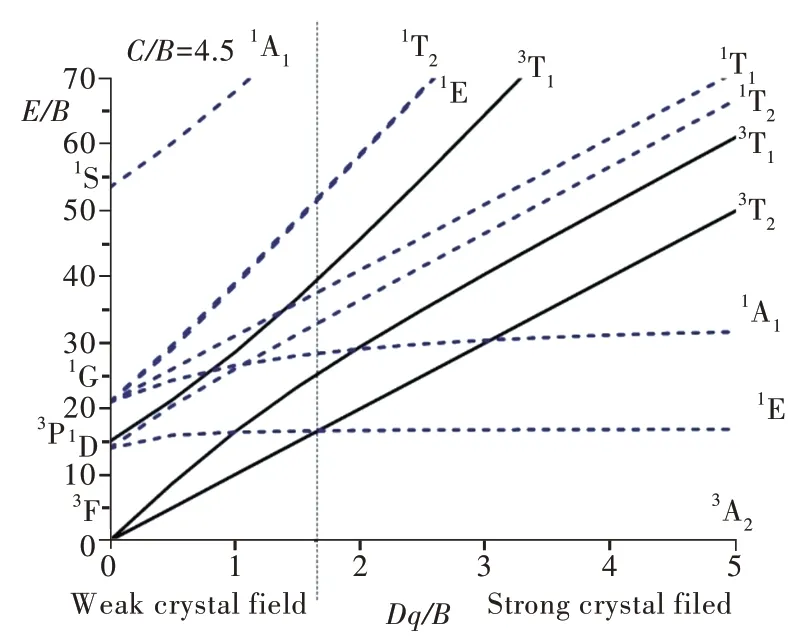
Fig.1 Tanabe-Sugano diagram for anion with the 3d8 electron configuration in an octahedral crystal-field.The spin-triplet and spin-singlet states are shown by the solid and dashed lines,respectively.
Since the energy of the1E state is very close to that of the1D free ion's term,it is possible to assume that only two Racah parameters B and C are needed to describe its energy position.The Racah parameters of the TM ions in crystals are considerably reduced relative to their“free ion”counterparts due to the so-called nephelauxetic effect[13];the degree of such a reduction depends on the peculiarities of the chemical bonds between the TM ions and ligands.The Racah parameters are reduced greatly in the covalent crystals,and their reduction is not so pronounced in the ionic compounds.As a result,the1E state will be lowered in covalently bonded systems and will be located higher in ionic crystals.
This observation allowed to introduce a new parameterβ1to the description of the spin-forbidden transitions[14-20].It has been shown that the energy of the spin-doublet2E of the Mn4+and Cr3+ions(or the1E state of the Ni2+ions)is a linear function of this new parameterβ1=(where B and C are the Racah parameters in the crystal and B0and C0are the corresponding free ion's values).In the present paper we give an extended overview of the Ni2+spectroscopic properties(in the octahedral coordination),based on the recent publications,and demonstrate this linear behavior.
The collected in the present paper experimental data on the Ni2+-doped solids provide a valuable source of reference information for the experimentalists,whereas the established linear trend between the1E level position and new parameterβ1allows for a meaningful estimation of the Ni2+spectroscopic properties in new hosts.
2 Analysis of Spectroscopic Data on The Spin-forbidden3A2→1E Absorption Transition of Ni2+Ions in Solids
Tab.2 contains the values of the Racah parameters B and C for Ni2+ions in various solids.In addition,the energetical positions of the Ni2+1E state(calculated from the crystal field theory)and measured experimentally are also listed,along with the corresponding literature references.

Tab.2 The main spectroscopic parameters related to the Ni2+3A2→1E transition in various crystalline solids.β1=images/BZ_148_381_2800_744_2889.png,B0=1 068 cm-1,C0=4 457 cm-1[21]AgBr AgCl Al2O3 β-BaB2O4 BaLiF3 708 807 900 850 1 062 2 615 3 141 4 250 3 500 3 865 0.885 27 1.033 25 1.272 56 1.118 08 1.319 39 10 223 12 206 15 009 13 351 15 504 10 700 12 470 15 840—15 504 10 765 12 411 15 072 13 354 15 593[22][23][24][25][26]Crystal B/cm-1 C/cm-1 β1 Position of the1E level/cm-1 Calculated Experimental Calc.,Eq.(1) Ref.

Tab.2(continue)
It is easy to see from the data presented in Tab.1 that all listed parameters vary in wide ranges.Thus,the value of B varies from 646 cm-1in NiI2to 1 062 cm-1in BaLiF3,the value of C changes from 2 615 cm-1in AgBr to 4 250 cm-1in Al2O3and the energy of the1E state is in between 10 700 cm-1in AgBr and 15 840 cm-1in Al2O3.
Such wide limits can be explained by differences in chemical bonding:in highly covalent iodides and bromides the nephelauxetic effect is strong and the Racah parameters are reduced considerably.At the same time,the highly ionic fluorides are characterized by a weaker nephelauxetic effect and,correspondingly,greater values of the Racah parameters.Therefore,the degree of covalency of the chemical bonds between the Ni2+ions and ligands is the primary reason for the observed variations of the spectroscopic parameters in Tab.1.The data in the“calculated”column correspond to the values obtained from the Tanabe-Sugano matrices in the cubic crystal field approximation.Since the Ni2+sites very often are characterized by a lower symmetry(trigonal or tetragonal),such calculated values can deviate from the experimental data.
Fig.2 shows the variation of the experimental positions of the Ni2+1E state against theβ1=parameter.
The data points were fitted to the linear function

the value of the correlation coefficient R2is rather high(0.903),which indicates a good quality of the fit.
To assess the quality of the fit and variance of the data presented,we calculated the energy of the Ni2+1E state using Eq.(1)in the various compounds that are listed in Tab.1 and then determined the rootmean-squared deviation

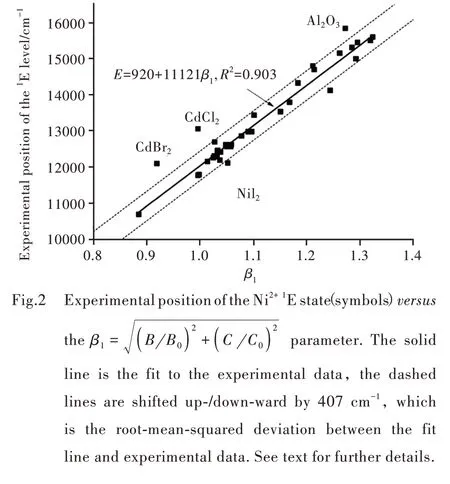
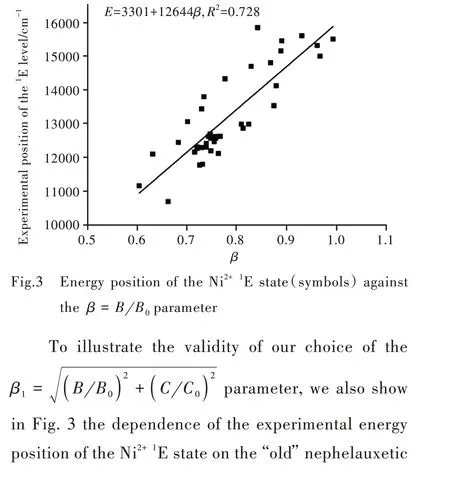
where Ei(exp.)and Ei(Eq.(1))are the corresponding experimental value and the calculated with the help of Eq.(1).The numerical estimations returned the value σ=407 cm-1.Two dashed straight lines in Fig.1 are parallel to the fit line(Eq.(1))and correspond to its upward/downward shift by the value ofσ.It should be noted that this value is of the order of magnitude of the phonon frequencies on solids and practically all data points in Fig.1 are within the±σinterval from the fit line.However,some data points,which correspond to NiI2,CdBr2,CdCl2,Al2O3compounds are outside of that area.The first three halides in this group are characterized by a layered structure and the chemical bonds are highly covalent.ratioβ=B B0,which for a long time was considered as a qualitative measure of covalency.It can be easily seen from Fig.3,that the data points are much more scattered than in Fig.2.The linear fit returns a much smaller value of the correlation coefficient(only 0.728).A similar result was obtained by us when considering the Mn4+ions and dependence of their2E level on the same parameter.Therefore,consideration of only one parameter B for the covalency description is insufficient,and both Racah parameters B and C have to be used when relating the energies of the spin-forbidden transitions of the TM ions chemical bonds covalency.Moreover,the present empirical finding has been confirmed and supported by our theoretical derivation based on the parameterized Tanabe-Sugano formula of1E energy level position of 3d8ions in octahedral complexes,as shown by Ref.[19].
We note that the linear relation between the1E level andβ1parameter holds true for the zero-phonon transitions.Quite often identification of the zero-phonon line(ZPL)position in the TM ions spectra is not an easy task,since the Stokes and anti-Stokes vibronic progressions that are observed in the experimental emission/absorption spectra can mask the true ZPL position.We consider this as an important factor that can cause deviation of some experimental data points in Fig.2 from the straight line determined by Eq.(1).In addition,such a linear relation can be also expected to be held for the case of 3d2ions in tetrahedral complexes because of the electron-hole complementarity between both 3d8and 3d2electronic configurations.
3 Conclusion
A thorough analysis of the recent publications on the spectroscopic properties of solids doped with Ni2+ions allowed to compile a database that collects the values of the Racah parameters B,C and energetic positions of the Ni2+1E level.We have re-examined an empirical trend between the lowest energy spinforbidden Ni2+3A2-1E transition and a new covalency parameterβ1=which allows to account for a decrease of both Racah parameters because of nephelauxetic effect.Theβ1parameter describes the covalent effects much better than the commonly used nephelauxetic ratioβ=B B0,which omits the second Racah parameter,C.It is hoped that the collected in the present paper data and their treatment will be useful for the description of the spectroscopic properties of Ni2+ions in solids.
Response Letter is available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20220243.
