桦木酸对人胃癌SGC-7901细胞自噬的影响
2022-07-07郭旭何孟奇张伟伟沈洋黄鑫孟令雪邵淑丽
郭旭,何孟奇,张伟伟,沈洋,黄鑫,孟令雪,邵淑丽
桦木酸对人胃癌SGC-7901细胞自噬的影响
郭旭1,何孟奇1,张伟伟1,2,沈洋1,黄鑫1,孟令雪1,邵淑丽1,2
(齐齐哈尔大学 1. 生命科学与农林学院,2. 抗性基因工程与寒地生物多样性保护黑龙江省重点实验室,黑龙江 齐齐哈尔 161006)
通过不同梯度浓度的桦木酸处理SGC-7901细胞,研究桦木酸对细胞自噬相关基因表达的影响,从而确定桦木酸在胃癌细胞内与自噬的关联.将人胃癌SGC-7091细胞分为5组,其中3组设置桦木酸的浓度梯度分别为10,20,30 mg/L,使用经典抗癌药物氟尿嘧啶(5-Fu)作为阳性对照组,使用0 mmol/L桦木酸为阴性对照组,每组3个复孔,处理细胞48 h,用qRT-PCR和Western blot方法检测桦木酸对人胃癌SGC-7091细胞自噬相关基因mRNA和蛋白表达的影响,使用免疫荧光法定位并检测SGC-7091细胞内的蛋白.与阴性对照组相比,梯度桦木酸处理组的细胞中-Ⅱ,mRNA和蛋白的表达均显著升高(<0.01),-Ⅰ蛋白的表达明显降低(<0.05).同时激光共聚焦的结果显示高浓度桦木酸诱导蛋白在细胞质内的聚集现象.在设置的梯度浓度范围内,桦木酸能诱导人胃癌SGC-7091细胞发生自噬,且呈剂量依赖性,浓度升高诱导效果也升高.
桦木酸;人胃癌SGC-7901细胞;细胞自噬
2020年全球新增癌症病例约1 930万例,癌症死亡近1 000万例(均不包括非黑素瘤皮肤癌),其中胃癌分别占新增和死亡病例数的5.6%,7.7%[1],其诱发因素多,复发率高[2],早期诊断极为复杂,绝大部分患者在胃癌早期无症状,出现腹痛等症状时已是晚期.外科手术及药物辅助治疗仍是早期胃癌最主要的治疗方式.由于大多数胃癌病例被诊断时已处于局部晚期甚至转移阶段,限制了根治性手术的适用性,且多半患者预后差,需化疗予以缓解[3].而目前的几种主流化疗药物(如氟尿嘧啶、顺铂等)毒副作用较大,在治疗肿瘤的同时对患者自身正常组织也造成伤害,且碍于胃癌的独特性,其对化学疗法普遍不敏感.因此研发特异性强、副作用小的化疗药物刻不容缓.
自噬是一种细胞营养匮乏导致的应激所诱导的分解代谢降解过程,大多数情况是一种细胞保护措施[4],指细胞在特定因素(如饥饿)刺激下,调动自噬相关蛋白分解自身物质,抵抗外来干扰,以维持内环境稳定的枢纽.但如果自噬过程调用频繁,破坏原本细胞的生理结构,则也会引发细胞凋亡.通过引发癌细胞自噬进而杀灭癌细胞是化疗药物的一种作用方式.研究表明,多种药物可通过诱导自噬引发细胞凋亡[5-7].
桦木酸(Betulinic acid,BA)是一种五环三萜类化合物,可从白桦树、三叶树和枣树中提取[8].桦木酸具有广泛的生物活性[9-13],在诱导细胞凋亡时也伴随着自噬.桦木酸会通过抑制人宫颈癌细胞[14]、口腔鳞状细胞癌[15]、乳腺癌[16]、白血病细胞[17]等的G0/G1期来抑制细胞增殖和诱导细胞凋亡,也会在骨髓瘤细胞[18]、大肠癌细胞[19]、肝癌细胞[20]中通过诱导自噬来介导细胞凋亡.
但目前桦木酸对SGC-7901细胞自噬方面的研究还未见报道.本研究在体外添加桦木酸后对自噬相关指标进行检测,完善桦木酸在胃癌自噬方面的研究,并为后续实验提供理论基础.
1 材料与方法
1.1 材料与试剂
人胃癌SGC-7901细胞株(中国医学科学院肿瘤研究所).
RPMI-1640培养基干粉(Gibico);胎牛血清(Biological Iudustries);桦木酸(北京汉博生物有限公司);Trizol(上海生工生物工程股份有限公司);反转录及RT-PCR Mixture(宝生物工程(大连)有限公司);,-Ⅰ,-Ⅱ一抗,兔二抗(北京博奥森生物技术有限公司).
1.2 细胞培养及药物处理
用含10%胎牛血清的RPMI-1640培养基在标准细胞培养箱内(37 ℃,5%CO2)培养人胃癌SGC-7901细胞,每1~2 d传代1次.细胞培养至对数生长期后,将细胞分为5组,设置3个梯度浓度,分别为10,20,30 mg/L,每组3个复孔,0 mg/L处理组作为阴性对照,70 μm/L的5-FU处理组作为阳性对照,处理48 h后收集细胞进行相应检测.
1.3 qRT-PCR检测自噬相关基因mRNA的表达
收集各组细胞,通过Trizol法提取RNA并进行反转录,随后使用cDNA作为模板,进行qRT-PCR检测,结果见表1.所有结果以2-△△Ct方法计算.

表1 荧光定量引物
1.4 Western blot检测自噬相关基因的蛋白表达
分别培养6瓶细胞,根据梯度浓度分别处理48 h后,收集细胞并提总蛋白,SDS-PAGE电泳后进行转膜,使用5%的脱脂奶封闭1 h,再按照蛋白大小进行剪膜,随后分别使用,-Ⅰ,-Ⅱ一抗(均按1∶500进行稀释)孵育对应的PVDF膜,4 ℃下孵育过夜;第2天回收所有一抗,TBST洗膜3次,弃净TBST后使用兔二抗(1∶1 000稀释)室温摇床下避光孵育1 h,再使用TBST洗膜3次,最后通过奥德赛扫膜仪扫描条带并分析处理.
1.5 免疫荧光检测人胃癌SGC-7901细胞自噬
分别培养6皿细胞,待细胞数合适时,根据梯度浓度设置处理细胞48 h,处理结束后使用甲醇进行固定处理.固定结束后清洗甲醇,标准封闭液封闭1 h.一抗4 ℃过夜,TBST洗3次;二抗避光孵育1 h,TBST洗3次.随后使用20 μL的DAPI孵育3~8 min,再使用PBST洗3次.清洗完毕后,滴加一滴抗荧光淬灭封片液,激光共聚焦显微镜下拍照.
1.6 统计学处理
2 结果
2.1 qRT-PCR检测桦木酸对自噬相关基因mRNA表达的影响
和的mRNA表达量见表2.由表2可见,与阴性对照组相比,随着桦木酸的质量浓度梯度升高,和的mRNA表达逐步升高,当桦木酸浓度为30 mg/L时,和的mRNA表达量最高,5-FU阳性对照处理组与10 mg/L处理组结果相似.结果表明,桦木酸上调了人胃癌SGC-7901细胞中的,基因mRNA的表达.
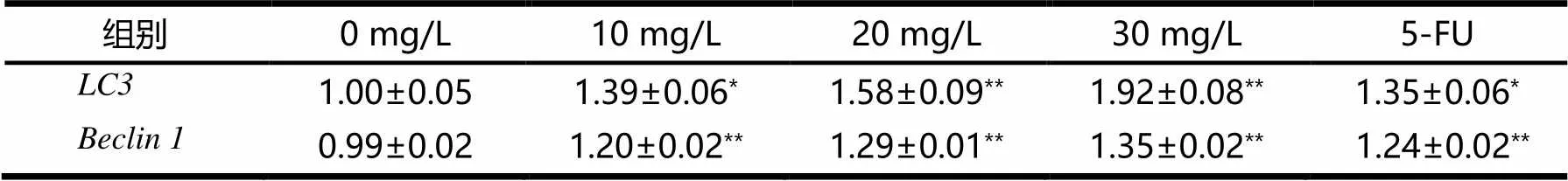
表2 LC3和Beclin 1的mRNA表达量
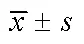
2.2 Western blot检测桦木酸对自噬相关基因蛋白表达的影响
不同浓度桦木酸对人胃癌SGC-7901细胞自噬相关,蛋白表达影响见表3和图1.由表3和图1可见,在3组梯度浓度处理组中-Ⅱ,蛋白表达水平梯度递增(<0.01),而-Ⅰ蛋白表达水平则随浓度梯度变化递减(<0.01),-Ⅱ/-Ⅰ比值随浓度梯度变化递增(<0.01).10 mg/mL桦木酸处理的效果与阳性对照组的效果相似.结果表明,桦木酸通过调控-Ⅱ,蛋白表达促进胃癌SGC-7901细胞自噬.

表3 桦木酸对Beclin 1,LC3-Ⅰ,LC3-Ⅱ蛋白水平的影响
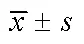

图1 桦木酸对Beclin 1,LC3-Ⅰ,LC3-Ⅱ蛋白水平的影响(n=3)
2.3 桦木酸对人胃癌SGC-7901细胞中LC3蛋白定位的影响
通过激光共聚焦显微镜观察蛋白在胃癌SGC-7901细胞内的定位及表达变化(见图2).鉴于30 mg/L的桦木酸处理细胞后细胞死亡过于明显,不利于镜下观察和染色,因此选择20 mg/L的质量浓度进行处理,48 h后蛋白在细胞质内的聚集现象显著,5-FU阳性对照组与20 mg/L处理组有相似结果.结果表明,桦木酸的处理诱导胃癌SGC-7901细胞发生自噬.
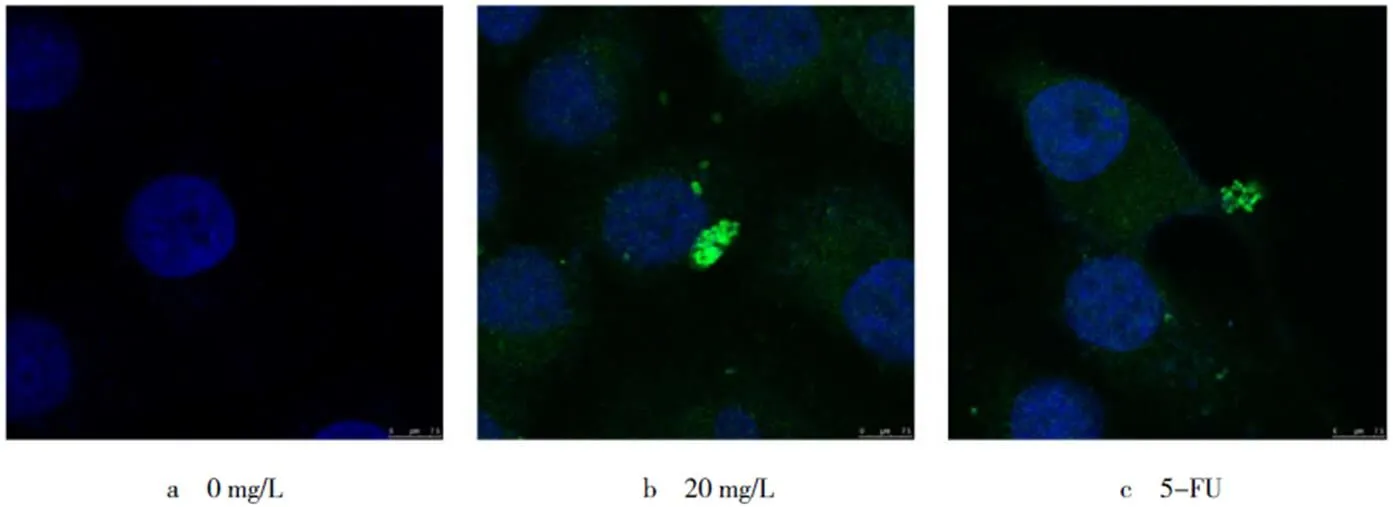
图2 桦木酸处理细胞48 h,LC3的细胞内定位(bar=100 µm,n=3)
3 讨论
细胞保护性自噬是应对化疗药物和放射治疗的重要反应.在大多数情况下,抗癌治疗中,自噬作用会支持癌细胞的存活;然而在某些情况下,它也会促进细胞死亡.因此,调节自噬有可能成为提高化疗和放疗疗效的策略之一.此外,自噬调节剂和传统治疗方法的结合可能使癌细胞对癌症治疗敏感.有越来越多的临床前证据表明,靶向诱导自噬,利用其细胞自身功能瓦解肿瘤,其毒副作用相对较小,在联合治疗中的作用也较为明显[21-22].如褪黑素对胃肠癌细胞的自噬具有调节作用,可通过联合用药增加胃肠癌细胞对药物的敏感性[23];脱落酸在胶质母细胞瘤细胞中可通过诱导肿瘤细胞自噬来抑制肿瘤的生长[24].桦木酸作为一种天然产物药物,已有研究表明其对人类肺癌、乳腺癌、结肠癌等均具有明显的细胞毒性[25-29].本实验研究证明,桦木酸可诱导人胃癌SGC-7901细胞自噬.
自噬的诱导途径有很多,其中自噬相关基因和是自噬过程的主要调节剂,许多自噬调控蛋白通过与的不同结构域或氨基酸发生直接或间接结合,形成蛋白复合体,进而调控自噬水平[30].而的功能主要参与了自噬小体的形成,饥饿时,核会被SIRT1(sirtuin 1)脱乙酰化,并与TP53INP2一起重新分布到细胞质中,以引发自噬[31].在自噬过程中,桦木酸可能通过触发细胞内的上游自噬信号(如PI3K/AKT/mTOR信号通路)来介导和的表达变化,同时桦木酸也可能通过对细胞内原本自噬信号的破坏,导致细胞自噬失调,进而引发肿瘤细胞的过度自噬行为,导致细胞死亡.
本实验5-FU为阳性对照组,证明随着桦木酸浓度的增加,自噬标志基因、mRNA和蛋白的表达量增加,但是-Ⅰ蛋白表达量减少.通过激光共聚焦显微镜观察蛋白在胃癌SGC-7901细胞质内形成点状聚集增多,证明桦木酸的处理诱导了胃癌SGC-7901细胞发生自噬.研究结果为桦木酸的开发利用提供了实验依据.综上所述,桦木酸通过对自噬标志基因和的调节,调控胃癌细胞SGC-7901自噬的发生.
[1] SUNG H,FERLAY J,SIEGEL R L,et al.Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].CA Cancer J Clin,2021,7(31):209-249.
[2] CHENG X J,LIN J C,TU S P.Etiology and Prevention of Gastric Cancer[J].Gastrointest Tumors,2016,3(1):25-36.
[3] FUJITANI K,YANG H K,MIZUSAWA J,et al.Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor(REGATTA):a phase 3,randomised controlled trial[J].Lancet Oncol,2016,17(3):309-318.
[4] LEVINE B,KROEMER G.Biological Functions of Autophagy Genes:A Disease Perspective[J].Cell,2019,176(1-2):11-42.
[5] DU Y,SHAO S L,JIAO K H,et al.Effects of hedyotis diffusa on mitochondrial membrane potential and expressions of apoptosis-related genes in human gastric cancer cell line MNK-45[J].,2020,36(2):171-175.
[6] XU J,PAN Y,LIU Y,et al.A review of anti-tumour effects of ginsenoside in gastrointestinal cancer[J].J Pharm Pharmacol,2021,72(10):1292-1301.
[7] JIN X,SUN P P,HONG Y,et al.Puerarin induces apoptosis in A549 cells[J].,2017,33(5):466-469.
[8] DUBEY K K,GOEL N.Evaluation and optimization of downstream process parameters for extraction of betulinic acid from the bark of Ziziphus jujubae L[J].Scientific World Journal,2013(1):e469674.
[9] COSTA J F,BARBOSA-FILHO J M,MAIA G L,et al.Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia[J].Int Immunopharmacol,2014,23(2):469-474.
[10] HONG E H,SONG J H,KANG K B,et al.Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus[J].Biomol Ther(Seoul),2015,23(4):345-349.
[11] LUO C,HUANG C,ZHU L,et al.Betulinic Acid Ameliorates the T-2 Toxin-Triggered Intestinal Impairment in Mice by Inhibiting Inflammation and Mucosal Barrier Dysfunction through the NF-kappaB Signaling Pathway[J].Toxins(Basel),2020,12(12):794.
[12] QIAN K,YU D,CHEN C H,et al.Anti-AIDS agents.78.Design,synthesis,metabolic stability assessment,and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus(HIV)agents[J].J Med Chem,2009,52(10):3248-3258.
[13] QUAN H Y,KIM D Y,KIM S J,et al.Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling pathway[J].Biochem Pharmacol,2013,85(9):1330-1340.
[14] XU T,PANG Q,WANG Y,et al.Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells[J].Int J Mol Med,2017,40(6):1669-1678.
[15] SHEN H,LIU L,YANG Y,et al.Betulinic Acid Inhibits Cell Proliferation in Human Oral Squamous Cell Carcinoma via Modulating ROS-Regulated p53 Signaling[J].Oncol Res,2017,25(7):1141-1152.
[16] FOO J B,SAIFUL YAZAN L,TOR Y S,et al.Induction of cell cycle arrest and apoptosis by betulinic acid-rich fraction from Dillenia suffruticosa root in MCF-7 cells involved p53/p21 and mitochondrial signalling pathway[J].J Ethnopharmacol,2015,166:270-278.
[17] CHEN Z,WU Q,CHEN Y,et al.Effects of betulinic acid on proliferation and apoptosis in Jurkat cells and its in vitro mechanism[J].J Huazhong Univ Sci Technolog Med Sci,2008,28(6):634-638.
[18] YANG L J,CHEN Y,HE J,et al.Betulinic acid inhibits autophagic flux and induces apoptosis in human multiple myeloma cells in vitro[J].Acta Pharmacol Sin,2012,33(12):1542-1548.
[19] WANG S,WANG K,ZHANG C,et al.Overaccumulation of p53-mediated autophagy protects against betulinic acid-induced apoptotic cell death in colorectal cancer cells[J].Cell Death Dis,2017,8(10):e3087.
[20] LIU W P,LI S L,QU Z L,et al.Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma[J].Am J Transl Res,2019(11):6952-6964.
[21] MOKARRAM P,ALBOKASHY M,ZARGHOONI M,et al.New frontiers in the treatment of colorectal cancer:Autophagy and the unfolded protein response as promising targets[J].Autophagy,2017,13(5):781-819.
[22] DJAVAHERI-MERGNY M,GIURIATO S,TSCHAN M P,et al.Therapeutic Modulation of Autophagy in Leukaemia and Lymphoma[J].Cells,2019,8(2):103.
[23] POURHANIFEH M H,MEHRZADI S,KAMALI M,et al.Melatonin and gastrointestinal cancers:Current evidence based on underlying signaling pathways[J].Eur J Pharmacol,2020,886:e173471.
[24] ZHOU N,WEI Z,QI Z,et al.Abscisic Acid-Induced Autophagy Selectively via MAPK/JNK Signalling Pathway in Glioblastoma[J].Cell Mol Neurobiol,2021,41(4):813-826.
[25] CHINTHARLAPALLI S,PAPINENI S,LEI P,et al.Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins(Sp)transcription factors[J].BMC Cancer,2011,11:371.
[26] CHINTHARLAPALLI S,PAPINENI S,RAMAIAH S K,et al.Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors[J].Cancer Res,2007,67(6):2816-2823.
[27] HSU T I,WANG M C,CHEN S Y,et al.Betulinic acid decreases specificity protein 1(Sp1)level via increasing the sumoylation of sp1 to inhibit lung cancer growth[J].Mol Pharmacol,2012,82(6):1115-1128.
[28] LI L,DU Y,KONG X,et al.Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer[J].Clin Cancer Res,2013,19(17):4651-4661.
[29] REINER T,PARRONDO R,DE LAS POZAS A,et al.Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis:possible role for inhibition of deubiquitinase activity[J].PLoS One,2013,8(2):e56234.
[30] KANG R,ZEH H J,LOTZE M T,et al.The Beclin 1 network regulates autophagy and apoptosis[J].Cell Death Differ,2011,18(4):571-580.
[31] SHIM M S,NETTESHEIM A,HIRT J,et al.The autophagic proteintranslocates to the nucleus and localizes in the nucleolus associated to NUFIP1 in response to cyclic mechanical stress[J].Autophagy,2020,16(7):1248-1261.
Effects of betulinic acid on autophagy of human gastric cancer SGC-7901 cells
GUO Xu1,HE Mengqi1,ZHANG Weiwei1,2,SHEN Yang1,HUANG Xin1,MENG Lingxue1,SHAO Shuli1,2
(1. School of Life Sciences,Agriculture and Forestry,2. Heilongjiang Provincial Key Laboratory of Resistance Gene Engineering and Protection of Biodiversity in Cold Areas,Qiqihar University,Qiqihar 161006,China)
SGC-7901 cells were treated with betulinic acid at different concentrations to study the effect of betulinic acid on the expression of autophagy related genes,so as to determine the association between betulinic acid and autophagy in gastric cancer cells.Human gastric cancer SGC-7091 cells were divided into 5 groups.The concentration gradients of betulinic acid in the three groups were 10,20,30 mg/L,respectively.The classic drug 5-fluorouracil(5-Fu)was used as the positive control group,and 0 mmol/L betulinic acid was used as the negative control group.Each group had three replicates and the cells were treated for 48 h,then qRT-PCR and Western blot were used to detect the effects of betulinic acid on mRNA and protein expression of autophagy related genes in human gastric cancer SGC-7091 cells.protein in SGC-7091 cells was localized by immune of luorescence.Compared with the negative control group,the mRNA and protein expressions of-Ⅱ andwere significantly increased(<0.01),and the protein expression of-Ⅰwas significantly decreased(<0.05).Confocal laser microscopy showed that high concentration of betulinic acid inducedprotein aggregation in cytoplasm.In the gradient concentration range,betulinic acid can induce autophagy in human gastric cancer SGC-7091 cells in a dose-dependent manner,and the induction effect increased with the increase of concentration.
betulinic acid;human gastric cancer SGC-7901 cells;autophagy
Q2
A
10.3969/j.issn.1007-9831.2022.06.013
1007-9831(2022)06-0075-05
2022-02-26
齐齐哈尔大学黑龙江省教育厅基本业务专项重点项目(135109104);黑龙江省高教强省优势特色学科——玉米“粮头食尾”重点项目(LTSW201737);黑龙江省省属高等学校基本科研业务费科研项目(YSTSXK201809)——植物性食品加工技术特色学科专项;2020年齐齐哈尔大学研究生创新科研项目(YJSCX2020046)
郭旭(1997-),男,黑龙江哈尔滨人,在读硕士研究生,从事肿瘤细胞基因表达调控研究.E-mail:757011379@qq.com
邵淑丽(1962-),女,黑龙江齐齐哈尔人,教授,博士,从事基因表达调控研究.E-mail:shshl32@163.com
