Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
2022-06-20ShnyoPnShnshnGuoJiruDiYnrongGuGuoxingWngYulongWngZhenghongQinLiLuo
Shnyo Pn, Shnshn Guo, Jiru Di, Ynrong Gu, Guoxing Wng,Yulong Wng, Zhenghong Qin, Li Luo,*
a School of Physical Education and Sports Science, Soochow University, Suzhou 215021, China
b School of Life Sciences, Fudan University, Shanghai 200032, China
c Department of Rehabilitation, Shenzhen Second People's Hospital, The First Affiliated Hospital, Shenzhen University School of Medicine, Shenzhen 518035, China
d Department of Pharmacology and Laboratory of Aging and Nervous Diseases, Jiangsu Key Laboratory of Translational Research and Therapy for
Neuro-Psycho-Diseases, College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, China
Keywords:
Exercise
Trehalose
Cognitive function
Autophagy
A B S T R A C T
Exercise is recognized as an effective strategy to delay brain aging, which is related to the activation of autophagy. Trehalose is a natural compound that can activate autophagy and exert beneficial effects in delaying brain aging. In this study, we investigated whether trehalose may exert neuroprotection similar to those of exercise in delaying age-related cognitive decline. Fifteen-month-old male C57BL/6 mice underwent swim exercise and/or were treated with 2% trehalose for 12 weeks. Trehalose, exercise and the combination of exercise and trehalose intervention improved the learning and memory of aged mice. They also improved the ratio of LC3-II/LC3-I, the protein level of LC3-II, Bnip3L, and Parkin respectively. Additionally, both exercise and trehalose increased the phosphorylation of AMPK. Exercise decreased cortical phosphorylation of mTOR and S6k, whereas trehalose did not change these cortical levels. These data indicated that exercise and trehalose might modulate autophagy through mTOR-dependent or mTOR-independent pathways,respectively. However, a combination of exercise and trehalose did not play a synergistic role in improving cognitive function and modulation of autophagy. Taken together, our findings suggest that trehalose exerts similar effects to those of exercise in delaying age-related cognitive decline and that it may thus represent an exercise mimetic to delay brain aging.
1. Introduction
Aging is often accompanied by progressive cognitive dysfunction,and accumulation of dysfunctional proteins/organelles is considered to play an important role in this process. The elimination of proteins and organelles in eukaryotic cells mostly depends on autophagic/lysosomal pathways, which have been shown to be dysregulated in the aging brain [1-4]. Moreover, restoring autophagic flux via genetic or pharmacological approaches can exert neuroprotective effects in the aging brain, thus reversing age-related memory decline [5-7].Hence, autophagic signaling is a promising target to prevent neurodegeneration and delay brain aging.
It has been revealed that physical exercise can ameliorate cognitive dysfunction by improving autophagic function in the aging brain. Kou et al. [8]demonstrated that swimming intervention reversed cognitive decline ofD-galactose (D-gal)-induced aging,with a concomitant improvement of miR-34a-mediated autophagy in the hippocampus of rats. We have also previously reported that 10-week swimming exercise improved autophagic-lysosomal degradation and mitochondrial quality control in the hippocampus to prevent aging-associated cognitive impairment [9]. Taken together,exercise-mediated conservation of cognitive function in elderly rats is associated with mitochondrial improvement in the hippocampus,relying on the autophagic-lysosomal degradation. There are also studies that have revealed exercise-induced activation of autophagy by exercise in other brain regions of elderly animals. Liu et al. [10]reported that aerobic exercise regulated the balance of autophagy and apoptosis via regulation of the adenosine 5’-monophosphateactivated protein kinase (AMPK)-sirtuin 2 (SIRT2) signaling pathway in the aged striatum. In another study, exercise activated autophagic/lysosomal pathways via upregulation of AMPK-SIRT1 signaling in the aged cortex [11]. However, Gusdon et al. [12]reported that exercise did not affect the autophagosomal marker, microtubuleassociated protein light chain 3-II (LC3-II), in the cortex or striatum of 24-month-old mice after 17 days of exercise. It is worth mentioning that these studies were conducted across multiple experimental models. Furthermore, the ages of the animals and the exercise protocols that were used also varied across these studies, which may have contributed to these seemingly inconsistent findings.
Considering that the intensity and frequency of physical exercise in the elderly may not reach sufficient levels for therapeutic benefits,mimicking the beneficial effects of exercise in elderly individuals via genetic and pharmacological approaches may represent a viable alternative to yield such benefits. Trehalose is a disaccharide that acts as an enhancer of chaperone-mediated autophagy [13]and is a mammalian target of rapamycin (mTOR)-independent inducer of macroautophagy [14,15]. Evidence from animal studies suggests that trehalose can reduce inflammation and improve antioxidant defenses in the brain, leading to an increased lifespan and health span in models of neuropathology [16]. Notably, trehalose exhibits prominent effects in improving clearance of autophagic vacuoles in neurons, thus enhancing clearance of protein aggregates and reducing neuropathology in animal models of neurodegenerative diseases [17-21].
Recently, several studies have investigated the effects of trehalose in brain aging. One-month oral administration of trehalose (2%,m/V)improved performance in motor learning and coordination tasks in C57BL/6N male mice aged 25 months, with increased cortical levels of LC3-II [22]. Trehalose (90, 180, 360 mg/kg) also ameliorated learning and memory impairments in aD-gal-induced aging mouse model [23]. Here, we hypothesized that trehalose may play a similar role to that of exercise in improving cognitive function and regulating autophagy pathway in the normal aging brains of mice, thus making it possible that trehalose may represent a novel exercise mimetic to delay brain aging.
In this study, we investigated if exercise and trehalose exert similar effects on improving learning and memory in aged mice, as well as in modulating autophagy signaling in aged cortex. Besides, we investigated whether a combination of exercise and trehalose play a synergistic role in improving learning/memory capacity in aged mice and modulation of autophagy in aged cortex.
2. Materials and methods
2.1 Animals and treatments
Young (3 months old, (28 ± 3) g) and old (15 months old, (42 ± 4) g)male C57BL/6 mice were obtained from the Experimental Animal Center of Soochow University. Mice were kept in individual cages at(22 ± 2.5) °C and were maintained under a 12-h light-dark cycle, with standard rat chow and drinking water providedad libitum. One week after arriving at the facility, all the mice underwent a 5-min swimming exercise, and the mice which could not swim would be eliminated from the experiment. Then the old mice were randomly distributed to 4 groups: (1) old sedentary control group (OC,n= 10); (2) 2% trehalose group (T,n= 10); (3) swim exercise group (E,n= 10);and (4) swim exercise plus 2% trehalose group (E+T,n= 10).Additionally, three-month-old mice were assigned as a young control group (YC,n= 10). The study protocol was approved by the Animal Care Ethical Committee of Soochow University. The experimental design is depicted in Fig. 1.

Fig. 1 Experiment design.
2.2 Exercise training protocol
Mice in the E group and E+T group acclimated to swim exercise during a one-week pre-training period (from week -1 to week 0, see Fig. 1). Free-style swimming was performed inside a plastic container(100 × 70 × 70 cm) with a water temperature of (30 ± 1) °C. From week 0 to week 12, the mice performed swimming exercise for 30 min/day, 5 days per week, for 12 weeks.
2.3 Endurance capacity
At 24 h after the last training session, all mice were subjected to a progressive load test to evaluate endurance capacity. The mice performed running-wheel exercise at a speed of 12 r/min, with a step increase of 3 r/min every 3 min until exhaustion. The time to exhaustion was recorded to be the endurance capacity of each mouse.
2.4 Y-maze task
At 48 h after endurance capacity test, all the mice were tested for their spatial learning and memory ability in the Y-maze. The apparatus was made of plywood and was painted black. Each arm of the Y-maze was positioned at an equal angle, including one arm with the light on and without electric shock (safe arm), and two arms with the light off and with an electric shock (unsafe arms). All the mice were allowed acclimate to the Y-maze before the formal test. Each mouse was placed at the end of one arm and allowed to move freely for 3 min to explore how to escape to the safe arm, and all the mice in this study were able to perform Y-maze test. After a delay of 1 h, the mice were reintroduced to the Y-maze for the formal test. Mice were randomly placed in an unsafe arm at the beginning of each trial, and the latency to find the safe arm was recorded. Each mouse was tested three times, with a 1-h rest between each trial.
2.5 Tissue harvesting
At 24 h after the Y-maze task, the mice were decapitated under anesthesia, and their cortices were removed and stored at −80 °C.
所有患者均顺利完成手术,无1例出现取活检相关的并发症,术中所取132份标本均符合病理科进行组织学检查的要求。90例术前诊断为骨质疏松性椎体骨折的患者,其中88例术后病理检查,未见到恶性肿瘤细胞,符合术前诊断;另外2例患者术后确诊为恶性肿瘤,误诊率为2.2%。术前诊断为肿瘤的15例患者术后有3例病理结果未见肿瘤细胞,其误诊率为20%。105例患者的132节椎体均完成PVP手术治疗,发生骨水泥渗漏9例,至末次随访均无临床症状;
2.6 Immunoblotting
Frozen samples were lysed and used for immunoblotting. Equal amounts (30 μg) of total protein extracts were separated via 8%-15% SDS-PAGE and were then transferred onto nitrocellulose membranes.After blocked by incubating membranes in Tris-buffered saline containing 0.05% Tween 20 (V/V) and 5% nonfat milk (V/V) for 1 h,the membranes were incubated with the following primary antibodies at 4 °C overnight: LC3 (1 : 1 000; Abcam), p62 (1 : 2 000; Abcam),NIP3-like protein X (Nix)/Bnip3L (1 : 500; Cell Signaling), Parkin(1 : 500; Abcam), p-AMPK (1 : 500; Cell Signaling), AMPK (1 : 500,Cell Signaling), p-mTOR (1 : 500; Cell Signaling), mTOR (1 : 500;Cell Signaling), p-S6k (1 : 500; Cell Signaling), S6k (1 : 500; Cell Signaling), andβ-actin (1 : 10 000; Sigma). The membranes were then washed three times and incubated with IRDye secondary antibodies(1 : 10 000; Li-Cor Bioscience) for 1 h at room temperature. The images of protein-antibody interactions were captured with an Odyssey infrared imaging system (Li-Cor Bioscience) and analyzed with ImageJ, usingβ-actin as a loading control for normalization.
2.7 Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The groups were compared using one-way analysis of variance (ANOVA),and Newman-Keuls test was used to determine significant differences among all groups viapost-hocpairwise comparisons. Differences were considered statistically significant atP< 0.05.
3. Results
3.1 Exercise training improves the endurance capacity of aged mice
The endurance capacity of each mice was determined by a progressive load running wheel test. Mice in the OC group exhibited significantly less time to exhaustion compared to that of the YC group (P< 0.01, Fig. 2). Comparing to that of the OC group, mice in the E group and E + T group exhibited longer time to exhaustion(P< 0.05;P< 0.01; Fig. 2). In contrast, there was no significant difference between the T group and OC group. Moreover, the time to exhaustion in the E+T group was longer than that in the T group or E group (P< 0.01;P< 0.05; Fig. 2), indicating that a combined exercise and trehalose intervention produced a greater effect at improving endurance capacity in aged mice compared to that of either intervention alone.
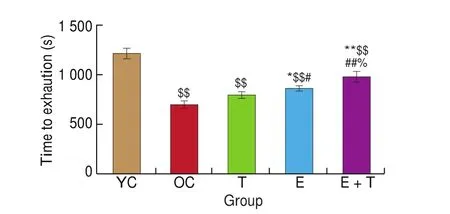
Fig. 2 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on the endurance capacity of the aged mice. The times to exhaustion in the progressive load test of YC, OC, T, E, and E+T groups were compared via one-way ANOVA analysis followed by Newman-Keuls post-hoc tests. Data are expressed as means ± SD. $$P < 0.01 as compared with the YC group; **P < 0.01 as compared with the OC group; *P < 0.05 as compared with the OC group; ##P < 0.01 as compared with the T group;#P < 0.05 as compared with the T group; %P < 0.05 as compared with the E group.
3.2 Trehalose, exercise and a combination of exercise and trehalose intervention improve the learning and memory capacity of aged mice
The latency to find the safe arm in the Y-maze for each mouse was analyzed as an indicator of learning and memory capacity. We found that this latency was significantly longer in the OC group than in the YC group (P< 0.01, Fig. 3), demonstrating a decline in learning and memory capacity with aging. To examine the effects of exercise and trehalose on learning and memory capacity in aged mice,we compared the latencies of age-matched groups after 12 weeks of interventions. The results showed that the latencies of the T, E, and E+T groups were not only significantly shorter than that of the OC group (P< 0.01;P< 0.01;P< 0.01; Fig. 3), but were also significantly shorter compared to that of the YC group (P< 0.05;P< 0.05;P< 0.05; Fig. 3). Collectively, these data indicated that exercise,trehalose, and a combination of exercise and trehalose exerted similar roles in alleviating learning and memory de ficits during aging.
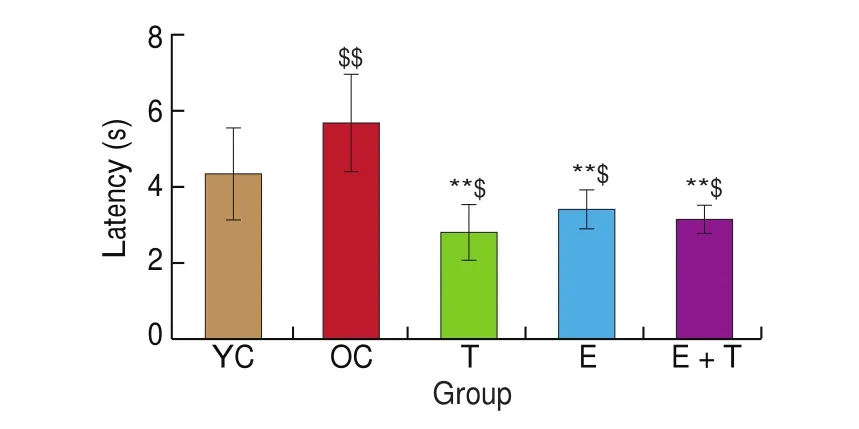
Fig. 3 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on the learning and memory ability of the aged mice in the Y-maze test. The latencies to reaching the safe arm in the Y-maze of YC, OC, T, E, and E+T groups were compared via one-way ANOVA analysis followed by Newman-Keuls post-hoc tests. Data are expressed as means ± SD.$$P < 0.01 as compared with the YC group; $P < 0.05 as compared with the YC group; **P < 0.01 as compared with the OC group.
3.3 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on autophagy-related proteins in the cortices of aged mice
Next, we evaluated cortical autophagy markers in each group to determine the effects of trehalose, exercise and a combination of exercise and trehalose intervention on cortical autophagy signaling in aged mice.
LC3 and p62 are the most widely-used autophagic molecular marker [24]. During the formation of early autophagosomes,cytoplasmic LC3 (i.e. LC3-I) will hydrolyze a small segment of polypeptide and transform into membranous LC3 (i.e. LC3-II);therefore, the ratio of LC3-II/LC3-I and the content of LC3-II are positively correlated with the number of autophagosomes. p62 participates in the degradation of autophagosome inclusions by lysosomes, indicating lysosomal degradation of autophagosomes [24].Our present results showed that the ratio of LC3-II/LC3-I and the content of LC3-II were both significantly lower in the OC group than in the YC group (P< 0.05;P< 0.05; Fig. 4A). However, the ratios of LC3-II/LC3-I in the T, E, and E+T groups were all significantly higher than that in the OC group (P< 0.01;P< 0.01;P< 0.01; Fig. 4A).Similarly, the content of LC3-II in the T, E, and E+T groups showed increased protein levels compared with that in the OC group (P< 0.01;P< 0.01;P< 0.01; Fig. 4A). In addition, there were no significant differences in the protein levels of p62 in the cortex among all the groups (Fig. 4B).

Fig. 4 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on the expressions of the autophagy markers of the aged cortex.Western-blot and quantitative analysis of protein levels of LC3-II and LC3-I (A), p62 (B), Bnip3L (C), and Parkin (D) in the YC, OC, T, E, and E+T groups. Data were compared via one-way ANOVA analysis followed by Newman-Keuls post-hoc tests. $$P < 0.01 as compared with the YC group; $P < 0.05 as compared with the YC group; **P < 0.01 as compared with the OC group; *P < 0.05 as compared with the OC group.
Mitophagy, a form of selective autophagy, refers to the degradation and elimination of damaged/redundant mitochondria via autophagy, maintaining the functional integrity of the mitochondrial network [25].When mitochondria are damaged and lose membrane potential, the kinase PTEN-induced putative kinase protein 1(PINK1) accumulates and recruits the E3 ubiquitin ligase Parkin from the cytosol specifically to the damaged mitochondrion and Parkin ubiquitylates mitochondrial proteins and causes mitochondria to become engulfed by isolation membranes that then fuse with lysosomes. NIX/BNIP3L has a WXXL-like motif, which binds to LC3 on isolation membranes and is thought to mediate the binding and sequestration of mitochondria into autophagosomes [26]. Our present results showed that there was no significant difference in the protein content of Parkin between the YC group and the OC group,but the protein content of Nix/BNIP3L in the OC group was lower than that in the YC group. Additionally, compared with those in the OC group, the protein levels of Nix/BNIP3L and Parkin were significantly upregulated in the T (P< 0.05, Fig. 4C;P< 0.01,Fig. 4D), E (P< 0.05, Fig. 4C;P< 0.01, Fig. 4D), and E+T (P< 0.01,Fig. 4C;P< 0.01, Fig. 4D) groups. Taken together, these results indicated that trehalose, exercise and a combination of exercise and trehalose intervention ameliorated autophagy dysregulation in aged cortex.
3.4 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on cortical AMPK/mTOR signaling in aged mice
In the autophagic activation pathway of neurons, serine/threonine protein kinase 1 (ULK1) participates in the formation of autophagosomes by forming complexes with focal adhesion kinase family interacting protein of 200 kDa (FIP200), autophagy-related gene 13 (Atg13), and autophagy-related gene 101 (Atg101), and enhancing the activity of the Beclin1 complex [27]. mTOR is the primary negative regulatory factor of autophagy by regulating the phosphorylation of ULK1ser757, while AMPK is the main positive regulatory factor of autophagy by regulating the phosphorylation of ULK1ser317/777. Furthermore, AMPK phosphorylation of ULK1 can also effectively activate mitophagy [28,29]. In addition, when mTOR phosphorylation level increases, it can inhibit the initiation of autophagy by inhibiting binding between ULK and AMPK; in contrast, when AMPK is activated, it can phosphorylate Raptor to inhibit mTOR, thus initiating autophagy [30].
Our results showed that the level of AMPK phosphorylation in the cortex decreased in the OC group as compared with that in the YC group (P< 0.05, Fig. 5A), while there were no significant changes in the phosphorylation of mTOR or S6k between these two groups.After 12 weeks of interventions, the levels of AMPK phosphorylation were increased significantly in the cortices of mice in the E, T, and E+T groups (P< 0.01;P< 0.01;P< 0.01; Fig. 5A). Furthermore, the ratios of p-mTOR/mTOR and p-S6k/S6k were both lower in the E group (P< 0.01, Fig. 5B;P< 0.01, Fig. 5C) and E+T group (P< 0.05,Fig. 5B;P< 0.05, Fig. 5C) as compared with those in the OC group.However, there were no significant changes in the phosphorylation levels of mTOR or S6k in the cortices of mice in the T group.
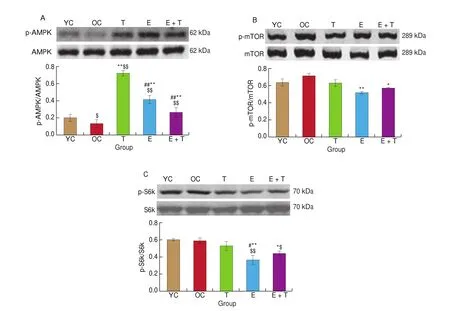
Fig. 5 The effects of trehalose, exercise and a combination of exercise and trehalose intervention on the AMPK/mTOR signaling pathway in the aged cortex.Western-blot analysis of protein levels of p-AMPK (A), p-mTOR (B), and p-S6k (C) and quantitative analysis of p-AMPK/AMPK (A), p-mTOR/mTOR (B), and p-S6k/S6k (C) in the YC, OC, T, E, and E+T groups. Data were compared via one-way ANOVA analysis followed by Newman-Keuls post-hoc tests. $$P < 0.01 as compared with the YC group; $P < 0.05 as compared with the YC group; **P < 0.01 as compared with the OC group; *P < 0.05 as compared with the OC group;##P < 0.01 as compared with the T group; #P < 0.05 as compared with the T group.
Thus, these results indicated that exercise might ameliorate autophagy dysregulation via the AMPK/mTOR signaling pathway,while trehalose might ameliorate autophagy dysregulation via an mTOR-independent pathway.
4. Discussion
In the present study, we found that both exercise and trehalose exerted similar effects in improving learning and memory ability in aged mice, and these effects were accompanied by amelioration of autophagy dysregulation in aged cortex. However, a combination of exercise and trehalose did not play a synergistic role in improving learning and memory capacity and modulation of autophagy.Additionally, exercise might modulate autophagy signaling in an mTOR-dependent manner, whereas trehalose might modulate autophagy signaling in an mTOR-independent manner.
It is particularly important to explore methods for preventing age-related cognitive decline, since such decline inevitably occurs during aging. In the present study, the spatial learning and memory capacity of mice was ascertained by the Y-maze task. The longer latency of finding the safe arm in the OC group than in the YC group con firmed that the learning and memory capacity of old mice decreased significantly compared to that of young mice. The latency of the E group was significantly shorter than that of the OC group,supporting that the exercise protocol used in our study effectively improved learning and memory capacity in old mice. According to the shortened latency in the T group as compared with that in the OC group, our findings provide evidence that trehalose exerted similar effects to those of exercise in alleviating age-related learning and memory de ficits.
Regular exercise has been proven to be an effective way to improve learning and memory. A previous study had demonstrated that 14-month-old mice starting exercise from three months old performed better in the Y-maze test compared to the matched controls who did not undergo such an exercise regimen [31]. Voluntary wheel running has also been demonstrated to be an effective way of enhancing hippocampal plasticity and thus improving retention of spatial memory in aging mice [32]. Moreover, growing evidence points out that the neuroprotective effects of exercise in the aging brain are closely related to improvement of autophagic/lysosomal pathways [8-11]. In the present study, 12-week swimming exercise restored the levels of cortical autophagy markers, including the upregulation of both autophagy-related proteins (i.e., the ratio of LC3-II/LC3-I and the content of LC3-II) and mitophagy-related proteins(i.e., the levels of Parkin and Nix/BNIP3L).
Trehalose has been recognized as an autophagy inducer,since it can mitigate aggregate-prone misfolded protein toxicity in neurodegenerative diseases via autophagic pathways. Krüger et al. [33]found that trehalose significantly reduced endogenous tau levels by activating autophagy in cortical neurons of a cellular model of Alzheimer’s disease (AD), as seen by elevated levels of LC3-II,reduced levels of p62, an increased number of autophagosomes,and stimulated autophagic flux. Direct evidence in favor of the degradation of tau aggregates via trehalose-induced autophagy has also been provided in mouse model of human tauopathy [34].Accumulation of α-synuclein (α-Syn) is a common pathology for different types of Parkinson’s disease (PD). Trehalose promotes the clearance of A53T α-Syn along with increased LC3 levels,lysotracker-RED-positive autolysosomes, and LC3-II levels, as well as yielding more autophagosomes in anin vitromodel of PD [35].Sarkar et al. [19]previously reported that trehalose enhanced the clearance of autophagic substrates, such as A30P and A53T mutants of α-Syn, associated with PD. In addition, trehalose treatment upregulated the expression of key autophagy-related genes, including LC3, Becn1, p62, and Atg5, at both mRNA and protein levels to ameliorate autophagic flux de ficits in motor neuronsin vitro[36]and in a mouse model of amyotrophic lateral sclerosis (ALS)in vivo[37].Furthermore, Liu et al. [38]reported that trehalose removed defective mitochondria damaged by Mn in striatal neuronal cells of mice through PINK1/Parkin-mediated mitophagy and the trehalose supplementation has been shown to restore the expression of Parkin and SIRT3, and upregulate the expression of mitophagy-related protein BNIP3 in a model of PD [39]. The present study showed that trehalose treatment upregulated the ratio of LC3-II/LC3-I, and the levels of LC3 -II, Parkin, and BNIP3L, which was in consistency with findings of Berry et al. [22]. Thus our findings suggested that 12 weeks of 2% trehalose intervention reversed autophagy dysregulation in the aged murine cortex.
The AMPK/mTOR/S6k signaling pathways play key roles in the regulation of autophagy in the brain. AMPK, the principal energy sensor in eukaryotic cells, has been shown to induce autophagy indirectly via reducing TOR signaling, and directly phosphorylating ULK1, the protein kinase that initiates autophagy. Ulgherait et al. [40]reported that neuronal AMPK activation inhibits TOR and induces autophagy in the brain and prolongs lifespan in drosophila. Activation of AMPK and inhibition of mTOR and its downstream protein S6k have been demonstrated to be effective ways for reversing autophagic decline in aging tissues [41]. In our study, the phosphorylation of AMPK was decreased in the aging cortex, which is consistent with previous studies [42], showing that activation of AMPK in the cortex decreases with age and may be involved in age-related decline of autophagy. Furthermore, we found that exercise increased the level of AMPK phosphorylation and decreased the levels of mTOR and S6k phosphorylation, indicating a role of AMPK/mTOR/S6k signaling in the activation of cortical autophagy in old mice.
The level of AMPK phosphorylation in the T group was significantly greater than that in the OC group. At the same time,the phosphorylation of mTOR and S6k, downstream targets of mTOR, did not change significantly, suggesting that trehalose might regulate autophagy via a mTOR-independent pathway, which is consistent with the findings of a previous study [37]. However, other mechanisms by which trehalose regulates autophagy may also exist.In both human and mouse neuroblastoma cell lines, Holler et al. [43]found that trehalose significantly increased LC3-II in a dose- and time-dependent manner, and further showed that activation of transcription factor EB (TFEB) was not responsible for trehalose-induced upregulation of autophagy. Thus, further studies are needed to fully understand how trehalose regulates autophagy in aged cortex.
In the present study, both exercise and trehalose improved learning and memory in aged mice with a concomitant amelioration of autophagy dysregulation, showing that trehalose may represent an exercise mimetic to slow brain aging. Previous studies have shown that pharmacological stimulation of brain-derived neurotrophic factor(BDNF) signaling successfully mimicked the beneficial effects of exercise in mouse models of down syndrome [44]and AD [45].Additionally, putative exercise mimetics such as the AMPK agonist, 5-aminoimidazole-4-carboxamide1-β-D-ribofuranoside(AICAR), the peroxisome proliferator-activated receptor-γ-coactivator 1α (PGC-1α) agonist, metformin, and the SIRT1 agonist, resveratrolhave been recognized to counteract cognitive decline in aging and neurodegenerative diseases in an exercise mimetic manner. Our present findings provide the first evidence that trehalose can rescue age-related learning and memory deficits, and these effects may be related to the modulation of autophagy.
We also investigated if exercise and trehalose exerted synergistic effects. The results showed that exercise and trehalose had no synergistic effects on learning and memory or cortical autophagy. A previous study showed that exercise and trehalose jointly activated autophagy within skeletal muscle in young female ICR mice, but did not further enhance autophagic activation compared with that of exercise or trehalose alone, which may have been due to the upper limit of autophagic activation under physiological conditions [46].Our present results are consistent with these previous findings,suggesting that there may be an upper limit for the improvement of learning and memory capacity and modulation of autophagy pathway following exercise and trehalose in aged mice.
Interestingly, in our study, exercise significantly improved the endurance capacity of old mice, and 2% trehalose had no significant effect on the endurance capacity of old mice. However, the time to exhaustion in the E+T group was not only longer than that in the OC group, but also than that in the T group and E group, suggesting that a combination of exercise and trehalose exert an addictive effect in improving endurance capacity in aged mice. It is seemingly that trehalose can only improve the endurance capacity of old mice on the premise of exercise’s effects. Further detailed studies should be done to determine the key factor for these discriminations. This opens the possibility for trehalose to be tested as a sports-nutrition supplement for the elderly nevertheless. It has been demonstrated that trehalose ingestion before prolonged exercise significantly increases total carbohydrate oxidation rates [47]and decreases lipid utilization [48]compared to those following drinking water.
Some limitations of this study should be pointed out. Firstly,although cortex is an integral part of spatial working memory [49],hippocampus is also very close to it. Further studies are needed to clarify the effects of trehalose on the aged hippocampus. Secondly,this study provide evidence that trehalose exerts similar effects as exercise in the improvement of cognitive function in the old mice and amelioration of autophagy dysregulation in the old cortex,however, genetic approaches will better clarify the role of autophagy in trehalose-induced neuroprotective effects. Thirdly, it is very puzzling that trehalose, exercise and the combination of exercise and trehalose intervention improved the ratio of LC3-II/LC3-I, the protein expression of LC3-II. However, there were no significant difference in the protein expressions of p62 among all groups. p62 is a multifunctional signaling hub which is well recognized as an ubiquitin sensor and a selective autophagy receptor. p62 plays crucial roles in myriad cellular processes including oxidative response [50],DNA damage response [51], aging/senescence [52], infection and immunity [53], chronic inflammation [54], and cancerogenesis [55],dependent on or independent of autophagy. Since exercise and trehalose can exert neuroprotection through multiple ways,including activation of autophagy, reduction of oxidative stress and inflammation [21,56-59], we propose that the reason why the p62 did not show significant difference among groups may be due to the complicated roles of p62 in the effects of exercise- and trehaloseinduced neuroprotection. Detailed studies, such as proteomics and transcriptomics may help to further elucidate the underlying mechanisms.
In conclusion, our present study demonstrated that exercise improved the learning and memory capacity of aged mice, and the effect might be associated with amelioration of autophagy dysregulation in the aged cortex via the AMPK/mTOR signaling pathway. Trehalose had similar effects to those of exercise in terms of improving cognitive function and regulation of autophagy in the aged cortex, but these effects may have occurred via an mTOR-independent pathway. Taken together, we propose that trehalose may represent a novel exercise mimetic to slow brain aging.
Conflict of interest
The authors declare that there were no conflicts of interest regarding either the publication of this article or the funding that they received to complete this study.
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (81771500).
猜你喜欢
杂志排行
食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells
