基于叶绿素荧光参数评估蝴蝶兰的组织培养潜力
2022-06-01朱珈叶李丹丹陈倩印丽萍郭水良
朱珈叶 李丹丹 陈倩 印丽萍 郭水良
摘 要: 以蝴蝶兰( RcHb.F.)的“大辣椒”品种为对象,研究了不同高温胁迫处理时间下的蝴蝶兰形态、叶绿素荧光参数变化,并对处理过的蝴蝶兰材料进行了组织培养.结果发现:当蝴蝶兰“大辣椒”品种茎段的PSⅡ最大光化学量子产量(/)低于0.35时,其无法作为外植体进行组织培养.与蝴蝶兰叶片相比,茎段的/更能够指示蝴蝶兰组织培养的成功概率.
关键词: 蝴蝶兰; 茎段; 叶片; PSⅡ最大光化学量子产量(/); 组织培养
中图分类号: Q 945 文献标志码: A 文章编号: 1000-5137(2022)02-0251-06
Potential evaluation of tissue culture for by using chlorophyll fluorescence parameters
ZHU JiayeLI DandanCHEN QianYIN LipingGUO Shuiliang
(1.College of Life Sciences, Shanghai Normal University, Shanghai 200234, China;2.Shanghai Entry-Exit Inspection and Quarantine Bureau, Shanghai 200315, China)
“Dalajiao” is a common variety of The individuals of the variety were cultured at a high temperature of 35 ℃, 14 h light/d with different durations. Throughout the treatment, their morphological changes were observed and the chlorophyll fluorescence parameters including the maximum quantum efficiency of photosystem II (PSⅡ) photochemistry(/), electron transfer rate under different light intensities were determined. Their corresponding explants were cultured to evaluate the potential for successful reproduction . We found that there existed a positive correlation between / of the stems and the successful rate of tissue culture with corresponding stems. When / of stems was less than or equal to 0.35, the stems were unable to be cultured because of lacking vitality. The / of the stems is better than that of the leaves in indicating their potential as explants for tissue culture.
; stem; leaf; photosystem Ⅱ (PSⅡ) photochemistry(/); tissue culture
蝴蝶蘭( RcHb.F.)是兰科附生单茎性植物.由于其形态和结构上均与众不同,且外形具有娥蝶般的美丽,故以蝴蝶兰来命名.蝴蝶兰为多年生常绿草本,是热带/亚热带气生兰.中国云南南部、西藏南部、广东南部、海南及台湾为该属植物的自然分布的北界.蝴蝶兰在国内外鲜花市场上深受欢迎,具有很高的观赏价值和经济价值.
随着兰花种植业的发展,蝴蝶兰的组织培养发展迅速.早在1949年,人们利用蝴蝶兰花梗腋芽成功地进行了组织培养,正式提出了一种蝴蝶兰属植物营养繁殖的方法,实现了蝴蝶兰试管繁殖.蝴蝶兰的茎尖、茎段和幼叶、花梗腋芽、花梗节间、根尖等部位都可做离体组织培养的外植体.但是叶片的诱导成功率很低.蝴蝶兰不同外植体的成活率有差异,其中花梗侧芽的成活率最高,達75.0%,其次为花梗,成活率为62.5%,叶片和根尖最差,分别为12.5%和7.5%.
蝴蝶兰组培的基本培养基包括Murashige and Skoog (MS)培养基,1/2 MS培养基,Vacin and Went (VW)兰科植物培养基,Gamborg’s B-5培养基,Knudson C (KC)培养基,Kyoto so1ution花肥培养基,花宝及其改良型等.但不同品种的蝴蝶兰以及取用不同部位的外植体,最适培养基的选择也有所不同.1/2MS或改良MS较MS效果好,减少MS中大量元素和部分微量元素及有机成分,适当增加少量的叶酸,有利于原球茎的增殖生长.植物组织培养中常添加一些天然有机物.如椰子汁、马铃薯汁和香蕉汁可提高蝴蝶兰原球茎的增殖率.蝴蝶兰组培中常用的细胞分裂素为6-BA,质量浓度较高(1~8 mg∙L)的6-BA能促进蝴蝶兰原球茎增殖,质量浓度较低(0.1~0.5 mg∙L)的6-BA能促进原球茎分化.
蝴蝶兰在生长发育期对高温敏感,在高温胁迫下极易受到抑制和危害.其最适宜生长的温度是18~28 ℃.温度过高会导致蝴蝶兰细胞内活性氧(ROS)大量积累,使其代谢紊乱,从而导致植株生长缓慢甚至死亡.恒温30 ℃处理24 h能显著提高蝴蝶兰抗氧化酶活性,然而40 ℃处理会破坏抗氧化酶系统.关于蝴蝶兰高温胁迫方面的研究,主要集中在赤霉素、细胞分裂素与成花的关系,高温胁迫下蝴蝶兰的防御机制和伤害机理,蝴蝶兰的高温脱毒,以及耐热性的诱导及抗热害栽培等方面.
随着中国对外交流的扩大,园艺植物进出口频繁.目前国内外对蝴蝶兰的研究主要集中在组织快速繁殖体系的建立、灭菌方法、降低褐化,及对胁迫的生理响应等方面,但很少有关于环境胁迫对蝴蝶兰可组培性影响的研究.蝴蝶兰作为兰科珍贵的园艺种源,为防止其种源流失,同时也为利用境外蝴蝶兰种源,建立对蝴蝶兰植株活力的快速检测方法,具有实际应用价值.本研究分别以蝴蝶兰无菌试管苗为材料,研究了高温胁迫条件下蝴蝶兰的叶绿素荧光参数变化,及其与蝴蝶兰生长繁殖潜力之间的关系,旨在建立蝴蝶兰植株活力的快速检测方法.
1 材料与方法
培养材料为蝴蝶兰“大辣椒”品种3个月组培苗,购自潍坊市潍城区南关花卉市场.
选取株龄、株高、生势、叶色基本一致的3个月龄的蝴蝶兰“大辣椒”组培苗植株,放置在无菌瓶中,每瓶一株,于光照培养箱中培养.培养条件为:温度35 ℃,光照12 h·d,空气相对湿度70% RH,光照强度2 000 lx.对照的温度为(25±2)℃,光照12 h·d.
每隔2 d取出高温处理的蝴蝶兰幼苗植株,暗适应20 min后,采用便携式调制叶绿素荧光仪PAM,分别测定蝴蝶兰两个品种茎段和第二片叶的PSⅡ最大光化学量子产量(/).每个处理重复3次.同样每隔2 d取出高温处理的蝴蝶兰幼苗植株进行可组培性研究,切去根部和叶子,保留茎段部位进行组培.
接种培养基为:花宝1号(质量浓度3 g∙L)+6-BA(质量浓度5.0 mg∙L)+NAA(质量浓度0.5 mg∙L)+蔗糖(质量浓度30 g∙L)+椰汁(体积分数200 mL∙L)+琼脂(质量浓度6 g∙L)+活性炭(质量浓度1 g∙L)+pH值6.0.接种后的茎段放置在无菌培养室中培养,培养条件为(25±2)℃,光源为普通荧光灯,光照度约1 500~2 000 lx,光照时间为12 h∙d.每个处理设5次重复.培养75 d后观测蝴蝶兰成苗情况,以形成叶片、丛生芽为组培成功标准.
2 结果与分析
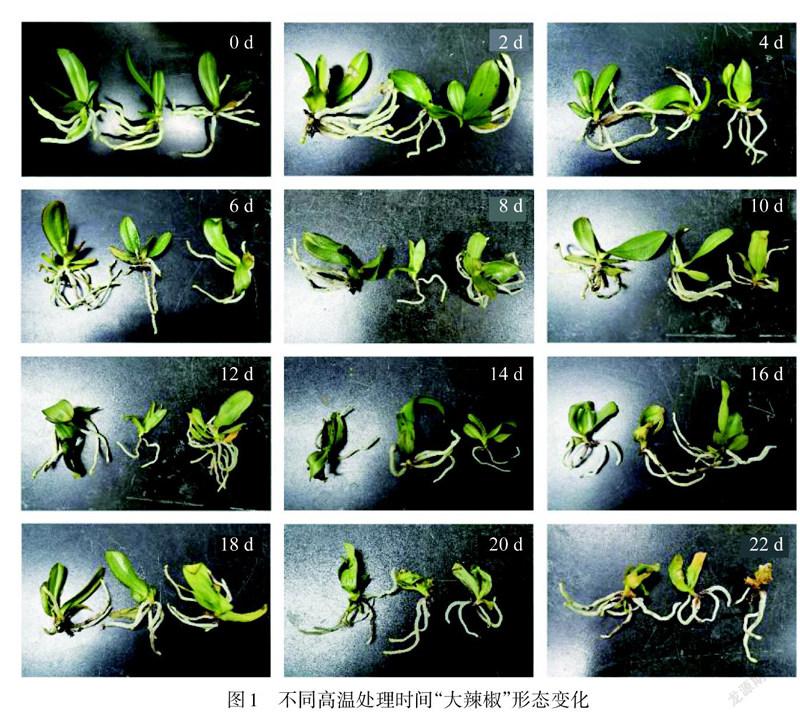
高温胁迫下蝴蝶兰“大辣椒”品种的形态变化
对照处理的蝴蝶兰植株,在实验期间均保持良好生长状态.高温处理下,“大辣椒”品种在第10天开始变黄,第22天大部分发黄枯萎(图1).
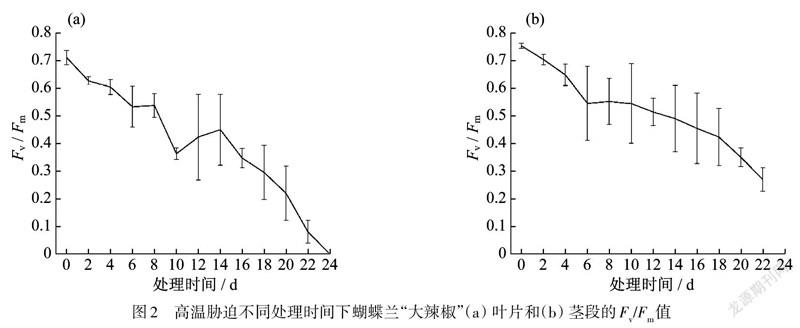
高温胁迫下蝴蝶兰“大辣椒”品种叶片和茎段/值
高温胁迫下,随着处理时间的延长,蝴蝶兰的叶片/值不断下降,第21天后,该指标急剧下降,到第24天时为0.与叶片相比,茎段的/值下降的相对平缓,到第24天时/值为0.35.
高温胁迫处理下,随着处理时间的延长,蝴蝶兰茎段/值整体呈不断下降的趋势,处理20 d后,/值为0.35,仍有一定活力.高温胁迫下,茎段的最大量子产量下降程度弱于叶片(图2).
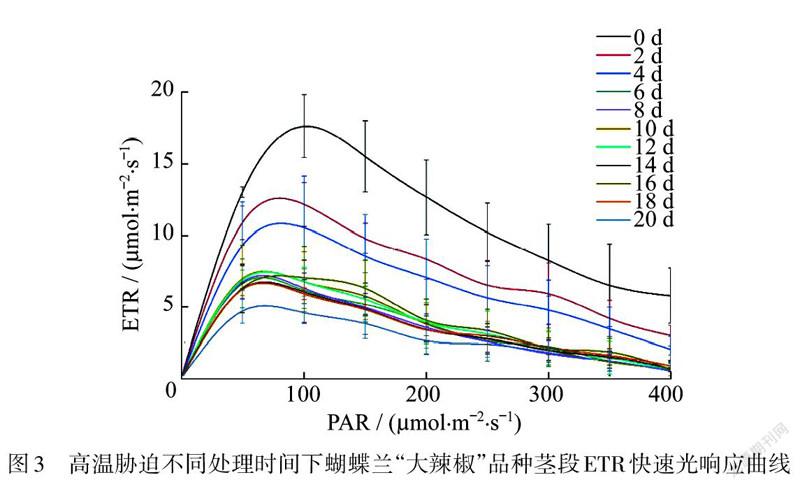
测定不同波长的光合有效辐射(PAR)下蝴蝶兰的光合电子传递速率(ETR),得到快速光响应曲线.发现在高温胁迫处理下,随着处理时间的延长,快速光响应曲线整体呈不断下降的趋势,第22天时光响应曲线降到最低,但是仍然有一定活性,整体趋势与高温胁迫下“大辣椒”茎段/值的变化趋势一致(图3).
高温胁迫下两种蝴蝶品种叶片/值与可组培相关性
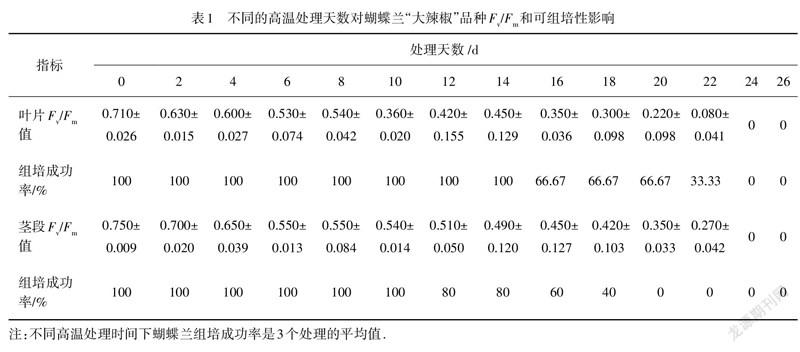
蝴蝶蘭“大辣椒”品种在高温胁迫环境下14 d后,外植体组培成功率都为100%;第16天时,组培成功率降到了67%;第22天时为33%;第24天以后为0.与“黄花红心”品种相比,“大辣椒”品种在高温胁迫下不管是/值变化,还是外植体的组培可繁育能力,相应指标下降的速率都更为缓慢.
高温胁迫环境下蝴蝶兰幼苗的可组培性与其叶片的/值正相关.由于测量/值的部位为叶片,进行可组培性探究的部位为茎段,茎段可组培性与其叶片的F/值对应性不是很强.
在高温处理12 d后,“大辣椒”品种茎段的/值大于0.5,组培成功率都在80%以上;/值低于0.5以后,可组培成功率依次递减;当/值小于0.35时,组培成功率均为0.由于组培部分用的是切掉根部和叶子的蝴蝶兰茎段作外植体,高温胁迫下蝴蝶兰茎段的v/值与可组培相关性更为直接.蝴蝶兰茎段是组培性探究的实验材料,直接测量茎段的叶绿素荧光参数,特别是/值,能够很好地指示蝴蝶兰的可组织培养成功率(表1).
3 讨论
高温胁迫下,蝴蝶兰幼苗PSⅡ反应中心出现了可逆或不可逆失活,降低了原初光能转换效率,对光合机构和光合色素产生光氧化破坏.不同的蝴蝶兰品种对高温胁迫处理的耐受性不同,由于“大辣椒”品种的叶片比较肥厚,蕴藏水分比较多,在高温胁迫下的耐受力比其他品种更强.今后将在更多的蝴蝶兰品种中开展实验研究,以评估不同品种对高温胁迫的耐受力.
随着高温胁迫处理时间的延长,蝴蝶兰茎段/值整体也呈不断下降的趋势,与高温处理蝴蝶兰叶片相比,茎段的/值变化较缓,而且其与蝴蝶兰的组培成功率之间的相关性更加紧密和直接.高温处理20 d后,蝴蝶兰“大辣椒”茎段的/值下降为0.35,此种情况下茎段已经失去进行扩繁的活力.因此,蝴蝶兰茎段/值0.35可以作为一个判别茎段是否能够成为外植体进行组培扩繁的阈值.研究蝴蝶兰幼苗在高温胁迫环境下的叶绿素荧光参数的变化与其可组培性的关系,不仅可以为蝴蝶兰规模化生产提供实质性的参考建议,更能为海关检验检疫局管控蝴蝶兰出入境情况提供帮助.
参考文献:
[1] MCWILLIAMS E L. Comparative rates of dark CO uptake and acidification in the Bromeliaceae, Orchidaceae, and Euphorbiaceae [J]. Botanical Gazette,1970,131(4):285-290.
[2] CHRISTENSON E A. : A Monograph [M]. Portland, Oregon (U.S.A.): Timber Press,2001.
[3] WANG L M, WANG S Q, YANG Y F. Germ plasm resources and breeding of orchids [J]. Journal of Anyang Institute of Technology,2005,2:4-10.
[4] LI Z H, JIA Y. Rapid Reproduction and Conservation of Rare Orchids [M]. Shanghai: Shanghai Publisher of Science and Technology,2006:3-5.
[5] ROTOR G J. A method of vegetative propagation of species and hybrids [J]. Proceedings American Society for Horticultural Science,1950,55:434.
[6] INTUWONG O, SAGAWA Y. Clonal propagation of by shoot tip culture [J]. American Orchid Society Bulletin,1974,93:893-895.
[7] TAN W C, DAI C G. Tissue Culture Methods for Ornamental Plants [M]. Beijing: China Forestry Publishing House,1991.
[8] PENG L X, WANG S, MENG G Y. Study on tissue culture and rapid propagation of [J]. Tianjin Agricultural Sciences,1999,5(2):27-29.
[9] LI J, HUANG M R, WANG M X. Plantlets regeneration from leaf explants of [J]. Journal of Nanjing Forestry University (Natural Sciences Edition),2005,29(3):28-32.
[10] TANAKA M, SAKANISHI Y. Clonal propagation of by leaf tissue culture [J]. American Orchid Society Bulletin,1977,46:73-737.
[11] LU X H, GUO W J, XU L H, et al. The preliminary research on the rapid propagation of by using the joint-points of flower-stems as explants [J]. Acta Horticulturae Sinica,2002,29(5):491-492.
[12] LI J J, LIAO J J, KE W L. Tissue culture using root segment for [J]. Plant Physiology Communications,2000,36(1):209.
[13] GU W M, CAO C Y, DING S M, et al. On tissue culture and rapid propagation for [J]. Journal of Shandong Forestry Science and Technology,2004(5): 47-48.
[14] LI C H. Study on micropropagation of species [D]. Yangzhou: Yangzhou University,2004.
[15] REN Q P. New technology of tissue culture and rapid propagation for [J]. Practical Forestry Technology,2007(12):9-13.
[16] PAN X F, WANG A S, LI H Z. A review on the progress of rapid propagation through tissue culture [J]. Tropical Forestry,2005,33(1):45-47.
[17] LI J M. Tutorials in Plant Tissue Culture [M]. Beijing: China Agricultural University Press,2002:4-5.
[18] ZHU Z Q. Plant Cell Engineering [M]. Beijing: Chemical Industry Press,2003.
[19] CHEN Z L, YE X L, LIANG C Y. culture of the inflorescence of [J]. Acta Horticulturae Sinica,2003,30(2):242-244.
[20] SUN L. Demonstration and application of rapid propagation technique in tissue culture of Orchidaceae [D]. Yangzhou: Yangzhou University,2009.
[21] LIU X Y, XIANG Q Y, LIU L L, et al. The effect of basic culture media and additional compounds on the propagation of PLB [J]. Seed,2005,24(6):18-20.
[22] HE S L, WANG X, LU L, et al. The effect of culture media and organic compounds on the differentiation of PLB [J]. Journal of Central South Forestry University,2003,23(5):11-13.
[23] CHEN Y, LIN K X, WANG J H. Studies on rapid propagation and large-scale planting of hybrids [J]. Journal of Zhejiang University (Sciences Edition),2004,31(1):84-88.
[24] WANG L, CHEN F D, CHEN F, et al. Effects of different media and additions on proliferation and growth of pedicel buds for [J]. Jiangsu Agricultural Sciences,2013,41(1):56-57,103.
[25] BHATTARCHARJEE S.Effect of growth regulating substance on seed germination of hybrid [J]. Indian Agriculturist,1999,43(1/2):79-83.
[26] YANG H G, YAN S L, CHEN H J, et al. Effect of exogenous methyl jasmonate, calcium and salicylic acid on the heat tolerance in seedlings under high temperature stress [J]. Chinese Agricultural Science Bulletin,2011,27(28):150-157.
[27] YANG H G, YANG Y M, LIAO H N, et al. The inducing effect of chitosan on heat resistance of seedlings [J]. Chinese Journal of Ecology,2015,34(12):3430-3437.
[28] ZHOU L L. Establishment of rapid propagation system for tissue cultures [D]. Hangzhou: Zhejiang Agriculture and Forestry University,2007.
[29] LIU L F, WANG J X. Study on rapid reproduction technology in hybrid species [J]. Journal of Anhui Agricultural Sciences,2013,41(29):11601-11603.
[30] ZENG D H, YU P Y, CHEN W Y, et al. Study on rapid propagation of Red Sky tissue culture [J]. Tropical Forestry,2014,42(1):32,46-49.
[31] CHEN L, PAN R Z, CHEN R M. Effects of media, growth regulators and dividing on the growth of protocorms cultured [J]. Journal of Tropical and Subtropical Botany,1999,7(1): 59-64.
[32] HUANG S Q, LI Y T, LV C T, et al. Changes in carbohydrates distribution in leaves and axillary buds during floral induction [J]. Acta Horticulturae Sinica,2007,34(6):1515-1519.
[33] HE J, WANG G D, WU Z. Effects of heat stress on morphological and antioxidant characteristics of seedlings [J]. Jiangsu Agricultural Sciences,2011(1):192-196.
[34] YANG H G. Effect of high temperature stress on chlorophyll and its fluorescence parameters in seedlings [J]. Chinese Agricultural Science Bulletin,2012,28(19):177-183.
(責任编辑:顾浩然)
