Changes in Ribose,AGEs and Transketolase in Female GK Rat,a Type 2 Diabetic Model
2022-05-23

GK大鼠(Goto-Kakizaki rats),常用于胰岛素抵抗,二型糖尿病及其并发症的相关研究。
Dear Editor,
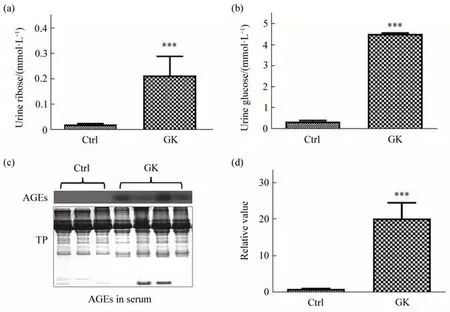
Fig.1 Urine ribose and serum AGEs in female GK ratsUrine ribose (a) and urine glucose (b) levels of 6-month old female rats (n=5) were measured through HPLC (Materials and Methods in Supplementary). (c)Advanced glycation end-products (AGEs) in serum were detected using Western blot, and the blot densities were statistically analyzed(d).TP,total protein.All values are expressed as mean±S.E.M.***P<0.001.
Type 2 diabetes is a chronic disease characterized by high glucose levels in the blood. Recently, it has been found that type 2 diabetic patients suffer from glucose and ribose dysmetabolism[1-3]. The Goto-Kakizaki (GK) rat is a nonobese, nonhypertensive model of type 2 diabetes,which,like humans,shares a susceptibility locus on chromosome 10[4-5]. The successive inbreeding of Wistar rats using glucose intolerance as a selection index developed the GK strain, meanwhile, it used the normal Wistar rat as control[6]. Glucose intolerance appears after 2 weeks of age and significant hyperglycemia is found as early as 4 weeks of age, and the animals show hyperinsulinemia and decreased pancreatic insulin stores. Although GK rats are widely employed in the study of hyperglycemic pathomechanism and pharmacology for diabetes, they have not received attention on ribose dysmetabolism. Here, we show that GK rats with high levels of urine ribose and advanced glycation end products (AGEs) are associated with changes in transketolase (TKT). As shown in Figure 1a, the urine ribose of female GK rats (n=5) is significantly higher (P<0.001) than that of female Wistar rats (n=3), and so is the urine glucose as the positive control (P<0.001, Figure 1b).Compared to Wistar rats, urine ribose levels (female,n=5) slightly increased with age of GK rats from 6-month to 8-month old (Figure S1a in Supplementary), however, urine glucose levels did not show marked changes under the same experimental conditions (Figure S1b in Supplementary). We also obtained a preliminary result that levels of urine ribose (Figure S1c in Supplementary) in male GK rats (n=4) was significantly higher than Wistar (n=5,P<0.05). Then,we determined the serum AGEs (Figure 1c) and observed a marked elevation of AGEs levels in the female GK rats (P<0.001) compared to Wistar rats(Figure 1d).
To reveal whether ribose metabolism is correlated with AGEs level in GK rats, we measured TKT, a key enzyme involved in ribose metabolism[7].First, we observed the expression of TKT protein in liver tissues. As shown in Figure 2a, TKT levels significantly increased (P<0.05) in the liver of GK rats (n=4), while AGE levels were not markedly different (P>0.05) compared with those of Wistar rats(n=3) under the same conditions (Figure 2e). Then,we examined the expression of TKT and AGEs in the kidney of rats. Unlike the results in the liver, TKT levels did not exhibit any significant increase in the kidney of GK rats (Figure 2b). However, AGE levels increased strikingly under the same experimental conditions (P<0.05, Figure 2f). From these data, it seems to be that ribose plays a role in the formation of AGEs in the liver and kidney of GK rats.
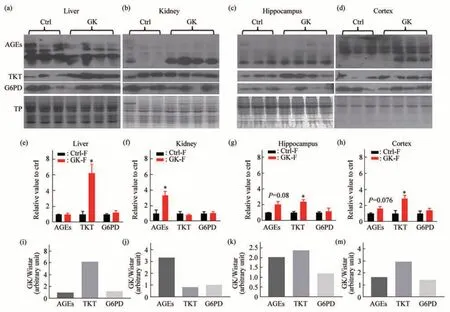
Fig.2 Levels of AGEs,TKT,and G6PD in different tissues of GK ratsAGEs, TKT, and G6PD in liver (a), kidney (b), hippocampus (c), and cortex (d) were measured by Western blot, respectively. Total protein (TP,Coomassie blue staining)was used as reference.Statistic blot densities for AGEs,TKT,and G6PD were shown for those 4 tissues(e-h).The average densities of AGEs,TKT and G6PD in GK rats(n=4)divided by those in Wistar rats(n=3)were shown in ratios of“GK/Wistar”in liver(i),kidney(j),hippocampus(k),and cortex(m),respectively.All values are expressed as mean±S.E.M.*P<0.05.
To verify whether ribose metabolism is also related to AGEs level in brain, we dissected GK and Wistar rats’ hippocampus and cortex to examine AGEs and TKT levels. As shown in Figure 2c, TKT levels increased markedly (P<0.05) accompanied by slight elevation of AGE levels (P>0.05) in the hippocampus of GK rats (Figure 2g). The cortex of GK rats also showed similar results as what the hippocampus did under the same experimental conditions (Figure 2d, h). In addition, no significant differences were observed in the weights of brain(Figure S2b inSupplementary), hippocampus(Figure S2c inSupplementary) and cortex (Figure S2d inSupplementary) except for those of body(Figure S2a inSupplementary) in comparison of GK with Wistar rats. Furthermore, we divided the average protein blotting density in GK rats by that in Wistar rats. The ratios for TKT of liver, kidney,hippocampus, and cortex were about 6.2, 0.85, 2.4,and 2.9, respectively. The ratios for AGEs of those organs were about 0.95,3.3,2.0,and 1.7,respectively,showing that the higher the TKT levels elevated, the lower the AGE levels decreased (Figure 2m). These data suggest that changes in AGEs are probably related to TKT in GK rats.
The pentose phosphate pathway (PPP) provides essential precursors for nucleic acids, and glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of PPP[7]. To clarify whether ribose comes from glycolysis, we examined G6PD levels in the liver, kidney, hippocampus, and cortex of GK rats(Figure 2). Nevertheless, we did not observe any notably different levels of G6PD in the organs of GK(P>0.05) compared to Wistar rats. It appears that the increased ribose may not result primarily from glycolysis in GK rats, at least compared with the Wistar rats under our experimental conditions.
As mentioned above,GK rats have high levels of AGEs in serum and kidney. AGEs are complex and heterogeneous group of compounds that have been implicated in diabetes-related complications[8]. We considered that ribose is involved in the formation of AGEs in GK rats. This viewpoint is based on these observations as the following. Firstly, ribose is much more active in its chemical characteristics than glucose[9]. Secondly, protein glycation with ribose is kinetically much more rapid than glucose, producing more AGEs[2]. Thirdly, TKT is involved in the regulation of ribose metabolism and transfers ribose to other compounds[7]. Lack of TKT causes a high level of ribose. Fourthly, Yu and his colleagues[10]found that the STZ-induced type 1 diabetic rat model has a high level of AGEs related to ribose levels in urine. Administration of TKT activator benfotiamine markedly decreased ribose levels in STZ-induced type 1 diabetic rats. Fifthly, gavage of ribose significantly impaired mouse kidney[11]. Finally, as shown in the current study, TKT is high in the liver with a low AGEs level,but not in the kidney,also suggesting that ribose plays a role in the formation of AGEs in GK rats,apart from glucose.
In 2015, Wu and her coworkers[12]gavaged C57BL/6J mouse with ribose for 6 months, led to Tau protein hyperphosphorylation in the brain(hippocampus and cortex).At the same time, Wei and her colleagues[13]observed brain Tau hyperphosphorylation of C57BL/6J mouse by intraperitoneal injection with ribose. The current project also detected Tau hyperphosphorylation in the brain of GK rats and Wistar rats as control. Tau phosphorylation significantly increased at Ser214(P<0.05), Ser396 (P<0.05) and slightly changed at AT8 (P=0.08) in the hippocampus of female GK rats(n=5) compared to female Wistar rats as control (n=3)(Figure S3a, c), but not those in the cortex (Figure S3b, e). No significant differences were observed in the phosphorylation levels of those aminoacids between female (n=5) and male (n=4) GK rats(Figure S3d,f inSupplementary). Whether ribose is related to Tau hyperphosphorylation and what kinases are involved in GK rats needs further experimental confirmation.
In summary, compared with Wistar rats, GK rats suffered from high levels of glucose and ribose in urine. AGEs levels correlate negatively with TKT in different organs of GK rats. However, G6PD did not show marked changes in GK rat. This work suggests that ribose plays a role in diabetes mellitus and provides new insight into understanding the path metabolism in diabetes. GK rats may be used as an animal model to study ribose as a potential target in diabetic treatment.
AcknowledgmentsThis work was supported by a grant from Beijing Brain Project(Z161100000217141). We thank Prof. XU Tao and Mr. HAO Qiang (Institute of Biophysics, Chinese Academy of Sciences) for providing GK and Wistar rats. Thanks to Ms. SHI Xiang and Mr. ZHOU Lei(Institute of Biophysics, Chinese Academy of Sciences) for their providing veterinary care,breeding, the management of laboratory animals and technical support.
CAO Xiao1)*,YU Le-Xiang2,4)*,WEI Yan2),QU Mei-Hua3),HE Rong-Qiao1,2,4)**
1)College of Basic Medicine, Southwest Medical University, Luzhou646000,China;2)Institute of Biophysics, Chinese Academy of Sciences, Beijing100101,China;3)Translational Medical Center, Weifang Second People’s Hospital,Weifang261041,China4)University of Chinese Academy of Sciences,Beijing100049,China
*These authors contributed equally to this work.
**Corresponding author.
Tel:86-10-64889876,E-mail:herq@ibp.ac.cn
DOI:10.16476/j.pibb.2022.0161
Received:April 12,2022 Accepted:April 12,2022
SupplementaryPIBB_20220161_Doc_S1.pdf is available online(http://www.pibb.ac.cn or http://www.cnki.net).
