Bipolar Fluorenylcarbazole-based Host Materials for Efficient Red Phosphorescent Organic Light-emitting Diodes
2022-04-01LINGQiankunDONGBizhengCHENZhikuanWANGHuaWEITingweiLIXiangzhiZHAOBoLITianbaoCHENFei
LING Qian-kun DONG Bi-zheng CHEN Zhi-kuan WANG Hua* WEI Ting-wei LI Xiang-zhi ZHAO Bo LI Tian-bao* CHEN Fei
(1.Key Laboratory of Interface Science and Engineering in Advanced Materials,Ministry of Education,Taiyuan University of Technology,Taiyuan 030024,China;2.Ningbo Research Institute of Northwestern Polytechnical University,Ningbo 315199,China;3.Department of Chemical and Environmental Engineering,The University of Nottingham Ningbo China,Ningbo 315199,China)
Abstract:Achieving high-efficiency red phosphorescent organic light-emitting diodes(PhOLEDs) is still a challenge,which is largely related to the host material used in device fabrication.Two new bipolar hosts,2-(9H-carbazol-9-yl)-7,7-dimethyl-5-(4-phenylquinazolin-2-yl)-5,7-dihydroindeno[2,1-b]carbazole(FC-CZ-PQZ) and 2-(4-(9H-carbazol-9-yl)phenyl)-7,7-dimethyl-5-(4-phenylquinazolin-2-yl)-5,7-dihydroindeno[2,1-b]carbazole(FCBCz-PQZ),were designed and synthesised as host materials to fabricate red PhOLEDs.The two compounds exhibited excellent physical properties with high thermal stabilities and balanced charge transport.FC-BCz-PQZ has a better bipolar carrier transport compared to FC-CZ-PQZ.FC-BCz-PQZ-based device demonstrated good electroluminescence performance with the maximum current efficiency,power efficiency and external quantum efficiency of 13.5 cd/A,14.2 lm/W and 14.8%,respectively,suggesting its promising application as a host material for red PhOLEDs.
Key words:fluorenylcarbazole;quinazoline;red PhOLEDs;bipolar hosts
1 Introduction
Since the discovery of organic light-emitting devices (OLEDs) by Tanget al.[1],this technology has been eventually applied because of its low power consumption,high brightness,flexible and wide view angle[2-3].Unfortunately,the efficiency of red OLEDs is not sufficient to date.Therefore,numerous materials and device structures have been designed for improving the device performance.The red phosphorescent materials of iridium(Ⅲ) complexes are widely applied as emitters because they can harvest both singlet and triplet excitons to achieve 100% internal quantum efficiency[4-6].However,iridium(Ⅲ) complexes are usually longlived at their triplet excitons during the relaxation processes,resulting in remarkable roll-off due to triplet-triplet annihilation(TT-A) and exciton quenching.To solve this issue,iridium(Ⅲ) complexes have been used as low-concentration dopants along with appropriate host materials in the emitting layer[7-11].The bipolar host materials with balanced charge mobility have recently acquired wide research attention.An adequate bipolar host material for phosphorescent OLEDs(PhOLEDs) has to satisfy the following requirements:good thermal stability,highest occupied molecular orbital(HOMO)/lowest unoccupied molecular orbital(LUMO) levels for charge injections,high triplet energy and high charge mobilities[12-16].
In recent years,red phosphorescent host materials have been rapidly developed[17-34].Carbazole is a frequently used molecular fragment because of its several excellent properties,including high triplet energy level and chemical stability,which make carbazole-containing molecules as hole-transport materials in OLEDs[35-36].Besides,quinoxaline derivatives are widely used as electron-transport materials due to their high electron mobility[28,37-38].Hu designed four host materials with quinoxaline as the acceptor,in which the maximum external quantum efficiency (EQE) was only 12.2% due to their low electron mobility[39].Fan designed a bipolar host material for high-efficiency red PhOLEDs,which was composed of two 6H-indolo[2,3-b]quinoxaline units bridged by tetraphenylsilane linkage,which exhibited EQE exceeding 20%[38].In spite of these reports,it is still a challenge to design ideal host materials with a quinoxaline unit for red PhOLEDs with high efficiencies.Quinazoline is similar in structure to quinoxaline and has high electron confinement ability,thus,it has the potential to facilitate the A unit of D-A type materials.Li and his coworkers used quinazoline as the A unit to construct efficient quinazoline-based thermally activated delayed fluorescence materials,and the devices using these materials achieved high EQE of up to 20%[38].In this paper,the quinazoline derivative 4-phenylquinazoline(PQZ) was utilised as the A unit to facilitate electron mobility.
Taking the advantage of the carbazole and quinazoline,two host materials,namely,9H-carbazol-9-yl)-7,7-dimethyl-5-(4-phenylquinazolin-2-yl)-5,7-dihydroindeno[2,1-b]carbazole (FC-Cz-PQZ) and 2-(4-(9Hcarbazol-9-yl)phenyl)-7,7-dimethyl-5-(4-phenylquinazolin-2-yl)-5,7-dihydroindeno[2,1-b]carbazole (FCBCz-PQZ),were designed and synthesised.We introduced a quinazoline moiety as the acceptor to facilitate electron transport and phenyl rings to enhance molecular thermal stability.The structure of the carbazole is flat and easily causes intermolecular packing,whereas fluorene exhibits good rigidity.Hence,we introduced fluorenylcarbazole(FC) composed of carbazole and 9,9-dimethylfluorene as the donor to increase the thermal stability by enhancing the rigidity of the spiral structure and to avoid quenching of exciton concentration,which contribute to the improvement of luminous efficiency.Furthermore,carbazole was introduced at position 3 of FC to improve hole mobility.Besides,bridge benzene was introduced between carbazole and FC to investigate the effect of benzene on the material properties.Experimental results showed that the two host materials exhibited excellent physical properties and high thermal stabilities for red PhOLEDs.The red PhOLEDs based on the host material FC-BCz-PQZ achieved good electrolumiscence(EL) performance with the maximum current,power and EQE of 13.5 cd/A,14.2 lm/W and 14.8%,respectively.
2 Experiments
2.1 Synthesis
In this work,all solvents and reagents used for the synthesis of materials,and medicines were purchased without purification.The synthetic routes are shown in Scheme 1.
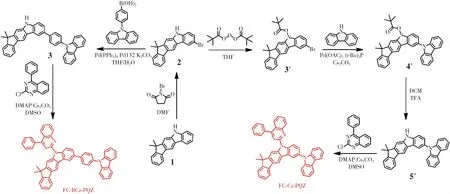
Scheme 1 Synthetic routes of FC-BCz-PQZ and FC-Cz-PQZ
2.1.1 FC-BCz-PQZ
(1)2-Bromo-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole (2)
A mixture of 7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole (10 g,42.0 mmol) andN,Ndimethylformamide(100 mL) was placed in a 250-mL flask.Then,N-bromosuccinimide (6.29 g,42.0 mmol) was dissolved in 7,7-dimethyl-5,7-dihydroindeno[2,1-b] carbazole(63 mL) and was slowly added into the mixture through a dropping funnel under nitrogen atmosphere and at a constant pressure.The reaction mixture was stirred for 30 min at 0 ℃,filtered under vacuum and washed with ethyl alcohol,affording the white solid intermediate product 2(10.6 g,yield 70%).1H NMR(500 MHz,CDCl3)δ8.32(s,1H),8.24(d,J=1.7 Hz,1H),8.05(s,1H),7.82(d,J=7.5 Hz,1H),7.48(dd,J=8.5,1.9 Hz,1H),7.45-7.41(m,2H),7.37(t,J=7.8 Hz,1H),7.29(t,J=7.2 Hz,2H),1.55(s,6H).13C NMR(126 MHz,DMSO)δ153.02,152.75,140.45,139.17,138.76,130.83,127.42,126.98,126.15,124.58,122.60,122.44,120.94,119.04,112.87,111.81,110.49,105.39,55.95,54.81,46.12,27.66,18.47.Mass(ESI,m/z):[M +H]+calculated for C21H16-BrN:362.26,found:362.17.
(2) 2-(4-(9H-Carbazol-9-yl) phenyl)-7,7-dimethyl-5,7-dihydroindeno[2,-1-b] carbazole (3)
A mixture of the intermediate product 2(10 g,27.7 mmol),4-(9-carbazole) phenylboric acid(7.95 g,33.2 mmol),tetrakis (triphenylphosphine)palladium(1.61 g,1.39 mmol),dichlorobis[di-tert-butyl(4-dimethylaminophenyl) phosphine]palladium(Ⅱ)(0.98 g,139 mmol) and potassium carbonate(9.42 g,69.25 mmol) was dissolved in a solution of tetrahydrofuran and water(100 mL,v/v=2∶1).Then,the reaction mixture was refluxed for 3 h under nitrogen atmosphere and was extracted with CH2Cl2.Afterwards,the solvent was removed by vacuum,and the white solid intermediate product 3(9.72 g,67%) was obtained and then purified by silica gel column chromatography.1H NMR (500 MHz,CDCl3)δ8.39(s,1H),8.38(d,J=1.6 Hz,1H),8.11(d,J=7.7 Hz,2H),8.04(s,1H),7.90-7.87(m,2H),7.78 (d,J=7.5 Hz,1H),7.68(dd,J=8.3,1.8 Hz,1H),7.62-7.58(m,2H),7.45(dd,J=8.3,2.7 Hz,3H),7.40-7.36(m,4H),7.31(dd,J=14.8,1.0 Hz,1H),7.26-7.20(m,3H),1.50(s,6H).13C NMR(126 MHz,CDCl3)δ152.39,152.17,140.28,139.97,139.29,138.69,138.51,135.05,131.17,130.99,127.54,126.37,126.08,125.32,124.93,123.95,123.26,122.36,121.91,121.55,119.29,118.86,118.27,117.74,110.41,109.99,108.89,104.07,45.64,26.99.Mass(ESI,m/z):[M +H]+calculated for C39H30N2:525.23,found:525.39.
(3)FC-BCz-PQZ
A mixture of the intermediate product 3(9 g,17.2 mmol),2-chloro-4-phenylquinazoline(4.95 g,20.64 mmol),4-dimethylaminopyridine(2.10 g,17.2 mmol) and cesium carbonate(11.2 g,34.4 mmol) was dissolved in dimethyl sulfoxide (90 mL).Then,the reaction mixture was refluxed for 2 h under nitrogen atmosphere and added with water.Afterwards,the reaction mixture was filtered under vacuum and washed with ethyl alcohol,affording the yellow solid FC-BCz-PQZ(12.9 g,69%).1H NMR(500 MHz,CDCl3)δ9.24(s,1H),9.20(d,J=8.6 Hz,1H),8.45(s,1H),8.44(d,J=1.7 Hz,1H),8.22-8.15(m,4H),8.04-8.01(m,2H),8.01-7.97(m,2H),7.94- 7.91(m,1H),7.88(d,J=7.4 Hz,1H),7.83(dd,J=8.6,1.9 Hz,1H),7.70-7.65(m,5H),7.54-7.50(m,3H),7.49(d,J=7.3 Hz,1H),7.47-7.43(m,2H),7.40(td,J=7.4,0.9 Hz,1H),7.34-7.29(m,3H),1.62(s,6H).13C NMR(126 MHz,CDCl3)δ169.63,155.32,153.82,153.46,152.77,140.96,140.83,140.17,139.61,139.46,137.20,136.33,134.32,134.26,134.21,130.41,130.28,128.72,128.58,128.04,127.37,127.31,127.09,126.77,126.67,125.95,125.90,125.45,125.42,123.40,122.65,120.31,120.21,119.90,119.66,117.74,117.14,111.55,110.54,109.91,47.02,27.99.Mass(ESI,m/z):[M +H]+calculated for C53H38N4:728.88,found:728.54.Anal.Calcd for C53H36N4(%):C,87.33;H,4.98;N,7.69.Found:C,87.36;H,4.94;N,7.67.
2.1.2 FC-Cz-PQZ
(1)2-Bromo-7,7-dimethylindeno[2,1-b]carbazol-5(7H)-ylpivalate (3′)
The intermediate product 2(10 g,27.7 mmol),4-dimethylaminopyridine(5.1 g,41.1 mmol) and tetrahydrofuran were thoroughly mixed in a 500-mL flask under nitrogen atmosphere.Using a dropping funnel,di-tert-butyl pyrocarbonate(9.1 g,41.6 mmol) was slowly added to the flask at room temperature and constant pressure.Then,water was added into the mixture and filtered under vacuum,affording a white solid product(10.9 g,85%).1H NMR(500 MHz,CDCl3)δ8.39(s,1H),8.17(s,1H),8.15-8.10(m,2H),7.80(d,J=7.4 Hz,1H),7.52(dd,J=8.8,2.0 Hz,1H),7.46(d,J=7.2 Hz,1H),7.38(td,J=7.4,1.1 Hz,1H),7.32(td,J=7.4,1.0 Hz,1H),1.78(s,9H),1.57(s,6H).13C NMR(126 MHz,CDCl3)δ154.45,153.73,150.81,138.74,137.52,135.09,129.32,127.84,127.14,127.10,124.08,122.68,122.25,119.81,117.77,116.15,111.06,110.64,84.29,53.41,47.10,28.38,27.76.Mass(ESI,m/z):[M +H]+calculated for C26H24BrNO2:463.10,found:463.45.
(2)2-(9H-Carbazol-9-yl)-7,7-dimethylindeno[2,1-b]carbazol-5(7H)-ylpivalate (4′)
The intermediate product 3′(10 g,21.7 mmol),palladium acetate(0.26 g,1.1 mmol),cesium carbonate(17.7 g,54.3 mmol) and xylene(100 mL)were thoroughly mixed in a 250-mL flask under nitrogen atmosphere and then heated in an oil bath at 140 ℃ for 8 h with vigorous stirring.The crude product was purified by silica gel column chromatography,affording a white solid product (5.95,50%).1H NMR(500 MHz,CDCl3)δ8.35(s,1H),8.27(d,J=1.8 Hz,1H),8.24(s,1H),8.20(d,J=7.8 Hz,2H),7.78(d,J=7.5 Hz,1H),7.61(t,J=8.1 Hz,1H),7.55-7.52(m,1H),7.51(s,1H),7.45(d,J=7.4 Hz,1H),7.43-7.39(m,3H),7.37-7.33(m,2H),7.32-7.27(m,3H),7.26(s,1H),1.58(s,6H).13C NMR(126 MHz,DMSO)δ153.47,153.33,141.72,141.40,139.82,131.31,128.31,127.52,126.65,126.59,124.74,124.21,123.15,122.90,122.30,120.92,120.09,119.58,119.32,112.73,112.55,110.17,106.00,55.37,46.71,28.29.Mass(ESI,m/z):[M +H]+calculated for C38H32N2O2:549.25,found:549.40.
(3) 2-(9H-Carbazol-9-yl)-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole (5′)
The intermediate product 4′(5 g,9.12 mmol)and methylene chloride(50 mL) were thoroughly mixed in a 100 mL flask under nitrogen atmosphere.Using a dropping funnel,trifluoroacetic acid(10 mL) was slowly added to the flask at room temperature and constant pressure,affording a white solid product(2.86 g,70%).1H NMR(500 MHz,CDCl3)δ8.42(d,J=8.9 Hz,2H),8.16(s,1H),8.14-8.07(m,3H),7.69(d,J=7.3 Hz,1H),7.54(d,J=8.7 Hz,1H),7.38-7.32 (m,4H),7.25- 7.22(m,3H),7.16(s,2H),1.76(s,9H),1.52(s,6H).13C NMR(126 MHz,CDCl3)δ153.54,152.80,150.01,140.54,138.25,137.79,136.82,134.22,131.86,126.45,126.14,126.09,124.95,124.83,124.80,123.74,122.33,121.65,119.32,119.29,118.83,118.80,118.42,117.28,116.55,110.21,109.75,109.53,108.78,83.36,46.15,27.46,26.79.Mass(ESI,m/z):[M +H]+calculated for C38H32-N2O2:449.20,found:449.35.
(4)FC-Cz-PQZ
The intermediate product 5′(2 g,3.07 mmol),2-chloro-4-phenylquinazoline(0.88 g,3.68 mmol),4-dimethylaminopyridine(0.37 g,3.07 mmol),cesium carbonate(2 g,6.14 mmol) and dimethyl sulfoxide(20 mL) were thoroughly mixed in a 100-mL flask under nitrogen atmosphere and then heated in an oil bath at 120 ℃for 1 h with vigorous stirring,affording a yellow solid product(1.38 g,69%).1H NMR(500 MHz,CDCl3)δ(10-6) 9.32(d,J=8.8 Hz,1H),9.27(s,1H),8.36(s,1H),8.32(d,J=2.1 Hz,1H),8.27-8.19 (m,4H),8.08-8.04(m,2H),8.00-7.95(m,1H),7.81 (d,J=7.4 Hz,1H),7.72-7.67(m,4H),7.60-7.56(m,1H),7.50(dd,J=11.7,7.8 Hz,3H),7.46-7.42(m,2H),7.39-7.35(m,1H),7.32(t,J=7.2 Hz,3H),1.63 (s,6H).13C NMR(126 MHz,CDCl3)δ(10-6) 169.92,155.30,153.92,153.77,152.78,141.68,140.30,139.27,138.95,137.16,134.46,131.95,130.53,130.28,128.80,128.09,127.43,127.14,126.79,126.15,125.92,125.47,124.86,123.24,122.66,120.38,119.75,118.19,117.83,111.59,110.74,109.98,47.08,28.00.Mass(ESI,m/z):[M +H]+calculated for C47H32N4:653.37,found:653.27.Anal.Calcd for C47H32N4(%):C,86.48;H,4.94;N,8.58.Found:C,86.36;H,4.63;N,8.47.
2.2 Measurements and Characterisations
1H NMR and13C NMR spectra were recorded on a Bruker AM 500 spectrometer.Molecular mass was measured using a Waters LCT Premier XE spectrometer.Thermogravimetric analysis (TGA) and differential scanning calorimetry(DSC) were carried out using a TGA5500 instrument at a heating rate of 10 ℃/min to 700 ℃/min and a DSC 2500 instrument under nitrogen atmosphere,respectively.UVvisible absorption spectra were recorded on a TU-1810 spectrophotometer with baseline correction.Photoluminescence(PL) spectra were recorded on an F-4600 fluorescence spectrophotometer.The energy levels of the materials were determined by the ionization photoelectron spectrometer(IPS).
2.3 Device Fabrication
An indium tin oxide(ITO)-glass substrate was cleaned using detergent,deionised water and ethanol in this order.Then,it was treated with oxygen(O2)plasma before loading into a 10-source evaporator with a base pressure of 5.0× 10-4Pa for device fabrication.Organic layers were deposited on the ITO-glass substrate by thermal evaporation.
3 Results and Discussion
3.1 Thermal Properties
The ideal thermal stability of organic light-emitting materials is vital for the better lifetime of OLEDs.Thus,the thermal properties of the two materials were investigated by TGA and DSC.As represented in Fig.1 and Tab.1,FC-BCz-PQZ and FC-Cz-PQZ exhibited high thermal decomposition temperature of 480 ℃ and 445 ℃,respectively,suggesting their good thermal stability.It was worth noting that two compounds showed similar endothermic glass transition temperature(Tg) of 173 ℃and 169 ℃ for FC-BCz-PQZ and FC-Cz-PQZ,respectively,which were higher than that of the widely used 4,4′-bis(N-carbazolyl)-1,1′-biphenyl(CBP)host material.From these results,FC-BCz-PQZ with the bridge benzene was more thermally stable than FC-Cz-PQZ due to its large van der Waals forces,originating from more electron delocalisation.Such excellent thermal stability was favourable to form stable film surface morphology[39].
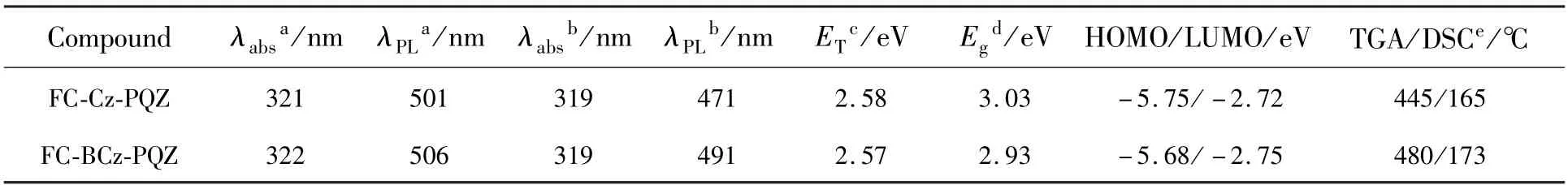
Tab.1 Physical properties of FC-Cz-PQZ and FC-BCz-PQZ
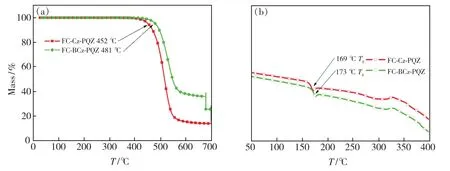
Fig.1 TGA(a) and DSC(b) curves of FC-Cz-PQZ and FC-BCz-PQZ
3.2 Photophysical Properties
The UV-visible absorption and emission spectra of FC-Cz-PQZ and FC-BCz-PQZ in toluene solution and in neat films at room temperature are shown in Fig.2.The detailed data are also summarised in Tab.1.FC-Cz-PQZ and FC-BCz-PQZ both exhibited strong absorption peaks at around 320 nm that are assigned to the π-π*transition of molecules,additional absorption peaks at 350 nm that are attributed to the n-π*transition of the carbazole moiety and weak absorption bands at 365-425 nm that are attributed to the intramolecular charge transfer effect of molecules.The absorption spectra of FC-Cz-PQZ and FC-BCz-PQZ displayed similar patterns,suggesting that the bridge benzene has no effect on the ground state.
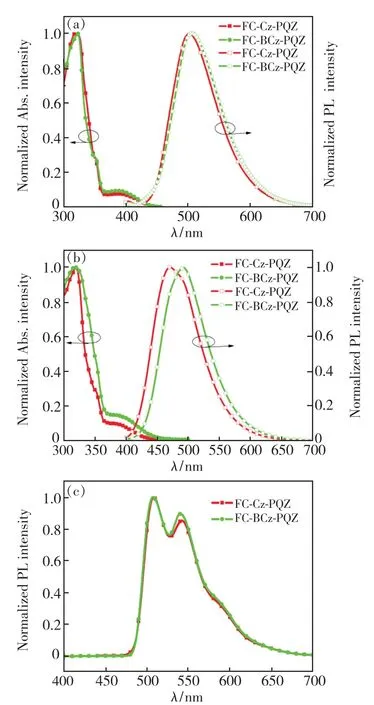
Fig.2 UV-visible absorption and fluorescence spectra of FCCz-PQZ and FC-BCz-PQZ in toluene solvents(a),and in films(b).(c)Phosphorescence spectra of FC-Cz-PQZ and FC-BCz-PQZ.
The maximum emission peaks of FC-Cz-PQZ and FC-BCz-PQZ in toluene solution were observed at 501 nm and 506 nm,respectively.Compared with FC-Cz-PQZ,the emission of FC-BCz-PQZ displayed a redshift of 5 nm due to the bridge benzene extended conjugation between carbazole and FC.It was noteworthy that the emission peaks of FC-Cz-PQZ and FC-BCz-PQZ in films exhibited large blueshifts of 30 nm and 15 nm compared to those in toluene solution,which are located at 471 nm and 491 nm,respectively.These large blueshifts of FC-Cz-PQZ and FC-BCz-PQZ in films are attributed to an increase in the spatial structure of the molecule caused by FC.The large spatial structure and torsion angle prevented intermolecular packing,weakened intermolecular interaction and created blueshifts,which avoided quenching of exciton concentration and contributed to the improvement of luminous efficiency.
The optical band gap(Eg) of FC-Cz-PQZ and FC-BCz-PQZ was calculated to be 3.03 eV and 2.93 eV,respectively,from the intersection between the absorption and emission spectra in films.Furthermore,the triplet energy(ET) estimated from the phosphorescence spectrum in toluene at 77 K was 2.58 eV for FC-Cz-PQZ and 2.57 eV for FCBCz-PQZ,suggesting that the bridge benzene has no effect on the triplet state.
3.3 Theoretical Investigation and Energy Level Structure
In order to further understand the electronic properties of FC-Cz-PQZ and FC-BCz-PQZ at the molecular level,frontier molecular orbital energy levels were calculated at the B3LYP/6-31G level by density functional theory.As shown in Fig.3,the LUMO levels of FC-Cz-PQZ and FC-BCz-PQZ were both mainly located at PQZ.On the other hand,HOMO levels of the two compounds were distributed among the electron-donating FC and carbazole and the 9-phenyl-9Hcarbazole moiety.Besides,the torsion angle between the carbazole group and the FC of FC-Cz-PQZ and between the 9-phenyl-9H-carbazole and the FC of FCBCz-PQZ was 120.93° and 36.94°,respectively.The smaller twist angle in FC-BCz-PQZ compared with FC-Cz-PQZ was due to the introduction of bridge benzene,expansion of the conjugation between FC and carbazole and reduction of steric hindrance.The FC moiety contributed to the effective separation of the electron density of the HOMOs and LUMOs,which induces a highET.Besides,the desirable separation of the HOMOs and LUMOs was beneficial for the transporting balance of holes and electrons.
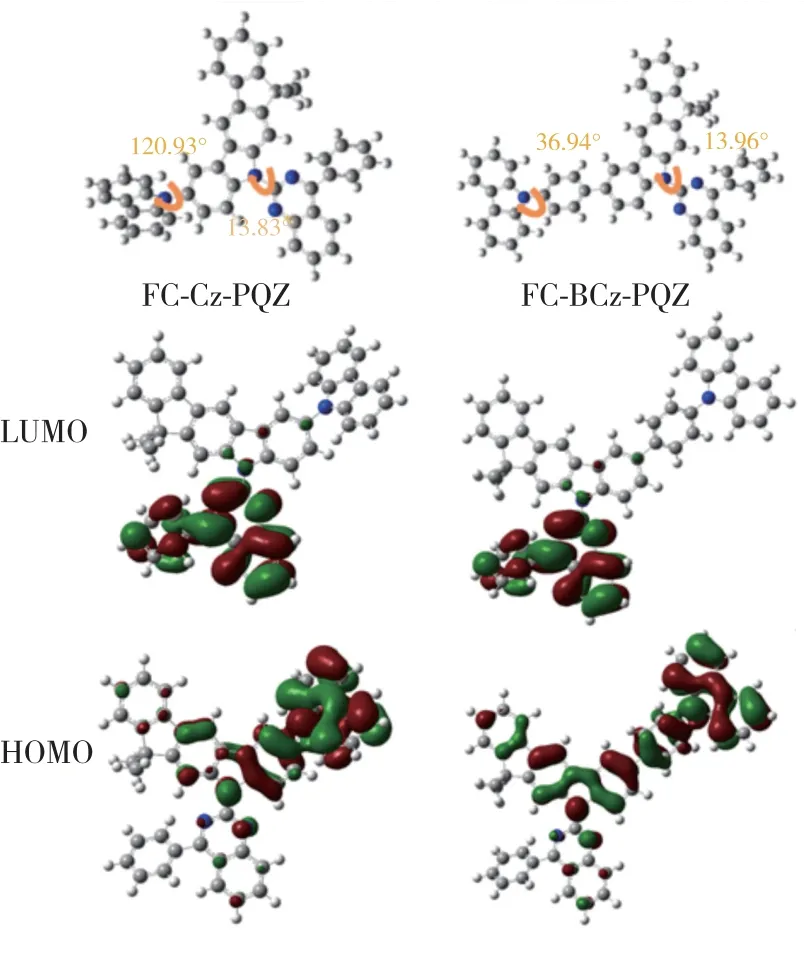
Fig.3 Molecular orbital surface of the HOMO and LUMO levels of FC-Cz-PQZ and FC-BCz-PQZ
The HOMO energy levels of FC-Cz-PQZ and FC-BCz-PQZ were measured by IPS.As shown in Fig.4,theEHOMOof FC-Cz-PQZ and FC-BCz-PQZ was 5.75 eV and 5.68 eV,respectively.The HOMO energy level of FC-BCz-PQZ was higher compared with that of FC-Cz-PQZ,which had bigger conjugation due to the FC and carbazole groups with a bridge benzene connection between them.The opticalEgof FC-Cz-PQZ and FC-BCz-PQZ was calculated to be 3.03 eV and 2.93 eV,respectively.According to the formula LUMO =HOMO +Eg,the LUMO energy level of FC-Cz-PQZ and FC-BCz-PQZ was 2.72 eV and 2.75 eV,respectively.The LUMOs of the two compounds are nearly identical due to their similar A units.
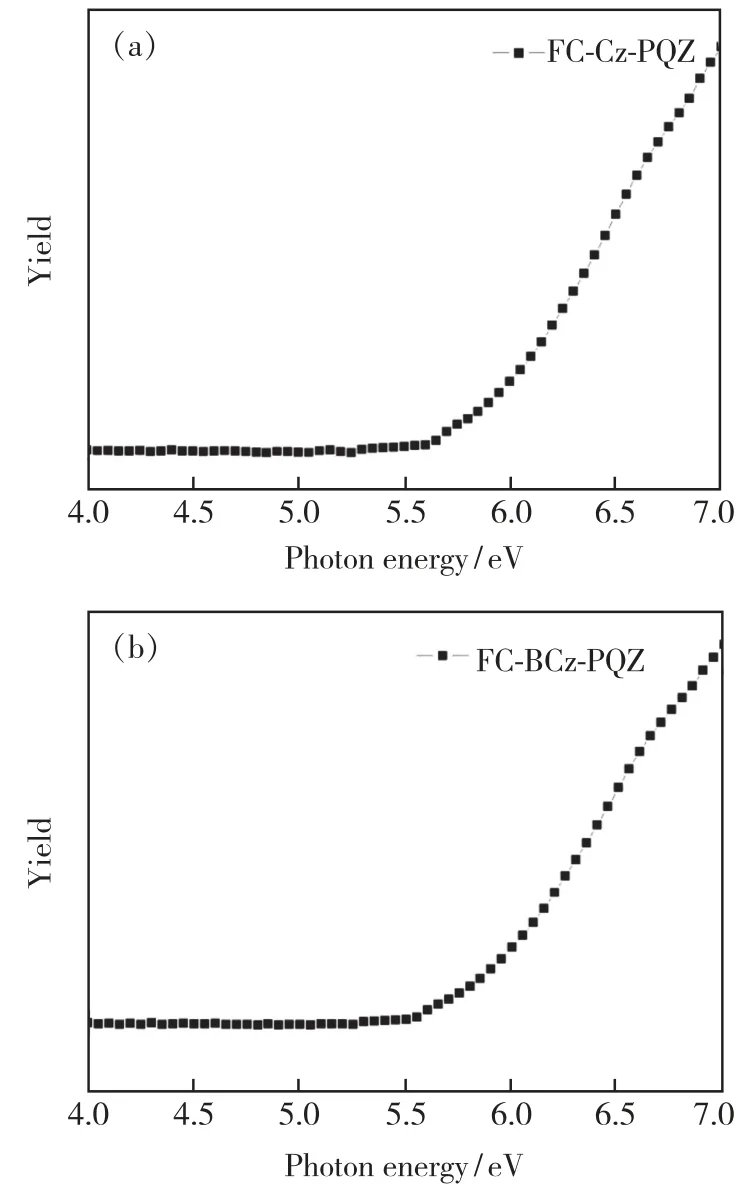
Fig.4 Energy levels of FC-CZ-PQZ and FC-BCz-PQZ were measured by ionization photoelectron spectrograph
3.4 Carrier Transport Properties
In order to verify the bipolar charge transport capabilities of FC-Cz-PQZ and FC-BCz-PQZ,holeonly(HOD) and electron-only(EOD) devices were fabricated with the following configurations:ITO/molybdenum oxide(MoO3,3 nm)/4,4′-bis[N-(1-naphthyl)-N-phenylamino]biphenyl(NPB,25 nm)/tris(4-(9H-carbazol-9-yl)phenyl)amine (TCTA,8 nm)/host(20 nm)/MoO3(3 nm)/Al(HOD) and ITO/host(20 nm)/1,3,5-tris(1-phenyl-1H-benzo[d]imida-ole-2-yl)benzene(TPBi,45 nm)/lithium fluoride(LiF,0.5 nm)/Al(EOD).In Fig.5,it can be seen that FC-Cz-PQZ and FC-BCz-PQZ exhibited different voltageversuscurrent density(V-J)curves in HOD and EOD.The hole current density of FC-Cz-PQZ was higher than the electron current density,which led to unbalanced bipolar transporting ability.Besides,FC-BCz-PQZ exhibited similar hole current and electron current densities in comparison with FC-Cz-PQZ,indicating its good and balanced carrier mobility that provided the evidence to support its better EL performance.
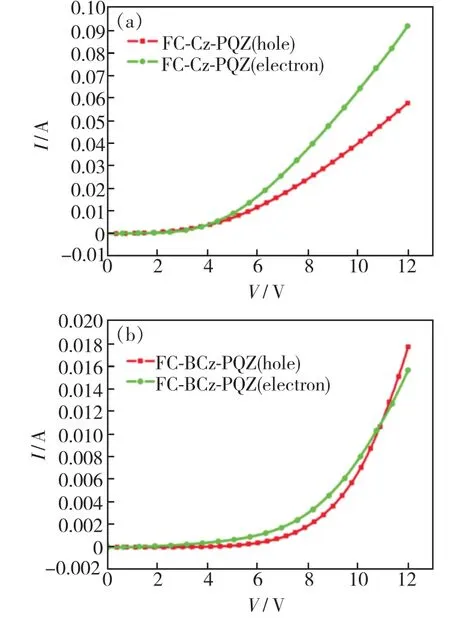
Fig.5 Current density-voltage curves of HOD(a) and EOD(b)
3.5 Electroluminescence of PHOLEDs
In order to evaluate the performance of FC-Cz-PQZ and FC-BCz-PQZ as host materials,three PhOLEDs with the following configuration were fabricated:ITO/MoO3(3 nm)/NPB(25 nm)/TCTA(8 nm)/host∶Ir(piq)2(acac)(20 nm,4%)/TPBi(45 nm)/LiF(1 nm)/Al(100 nm).The emitting layer was composed of 4% Ir(piq)2(acac) doped into the host materials(i.e.FC-Cz-PQZ in device A,FCBCz-PQZ in device B and CBP in device C).ITO and LiF/Al were used as the anode and cathode,respectively.MoO3was employed as the hole injection layer,NPB served as the hole-transporting layer,TCTA was used as the electron-blocking layer and TPBi was employed as the hole-blocking layer and electron-transporting layer.The device and the related HOMO and LUMO energy levels of these materials in PhOLEDs are displayed in Fig.6.As shown in Tab.2,the turn-on voltages(Von) of devices A and B are both 2.7 eV,which are slightly lower thanVonof device C(3.0 V).It is attributed to a smaller hole injection energy barrier (i.e.0.05 eV in device A and 0.02 eV in device B) betweenEHOMOof host andEHOMOof TCTA in devices A and B than that in device C (0.5 eV).The maximum luminance of devices A,B and C was 24 600,24 380,21 480 cd/m2,respectively.Compared with FC-Cz-PQZ,FC-BCz-PQZ had more balanced carrier transporting ability.Hence,in device B,the holes and electrons could recombine into excitons in the emission layer with high efficiency,resulting in higher luminance than in device A.Among the three PhOLEDs,device B exhibited the highest device efficiency with the maximum current efficiency(ηc),maximum power efficiency(η) and maximum EQE of 13.5 cd/A,14.2 lm/W and 14.8%,owingto the better bipolar charge transporting ability of FC-BCz-PQZ compared with that of FC-Cz-PQZ.As shown in Fig.7(d),the three PhOLEDs exhibited nearly identical EL spectra with red emission peaks.The devices only display the typical emission spectra from Ir(piq)2(acac) at 628 nm and 624 nm without any other peaks,indicating full exciton energy utilisation.
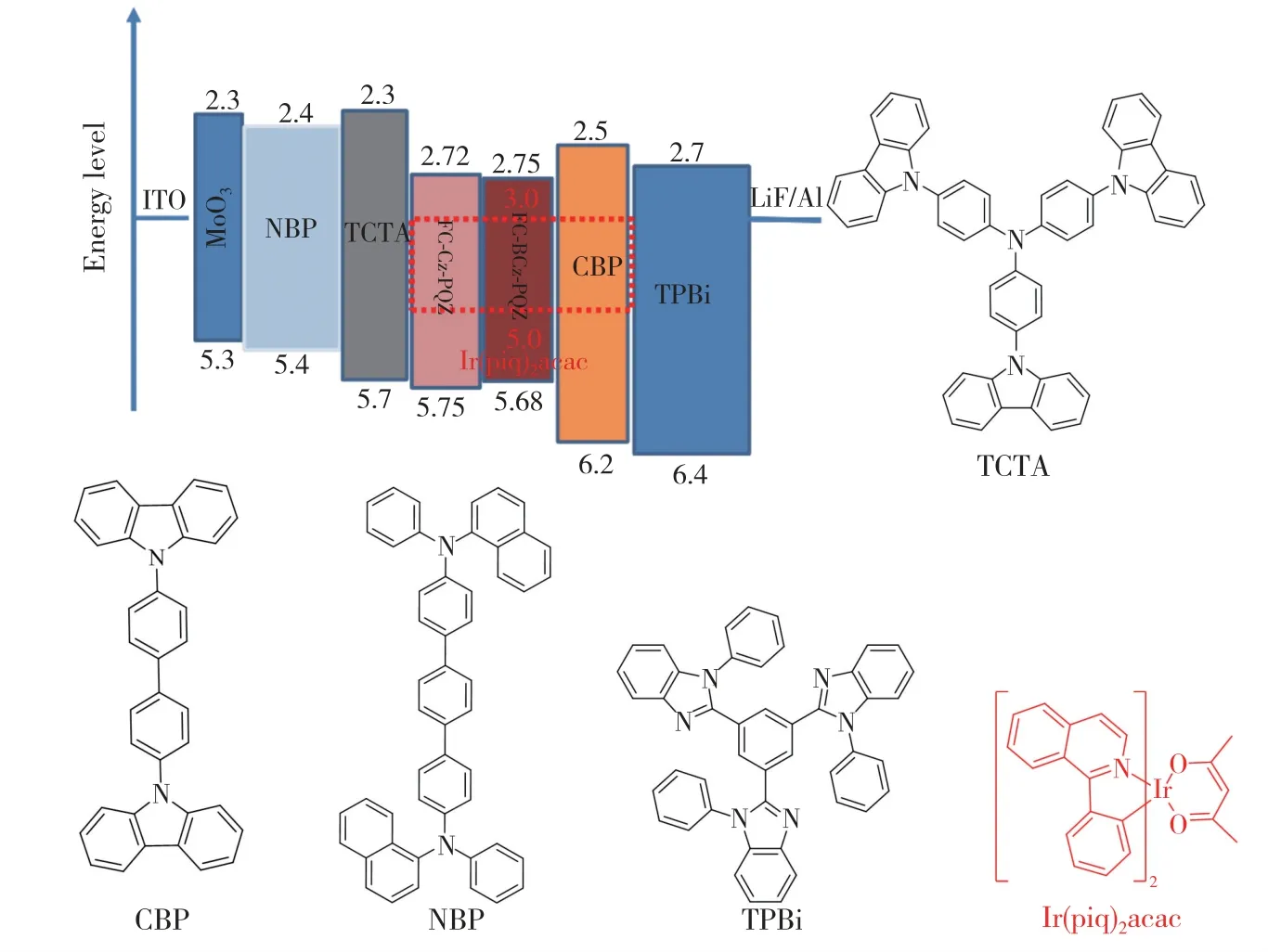
Fig.6 The energy level diagram of the materials in devices
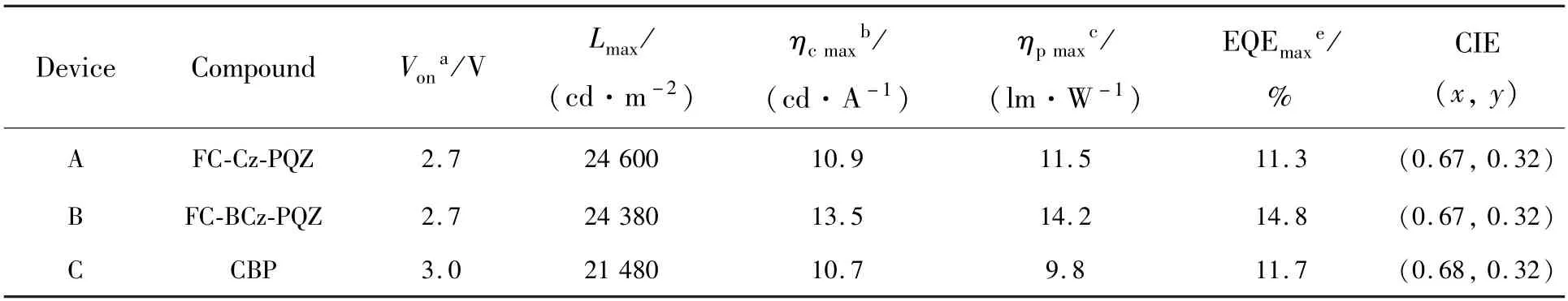
Tab.2 Characteristics of OLEDs with FC-Cz-PQZ and FC-BCz-PQZ host materials
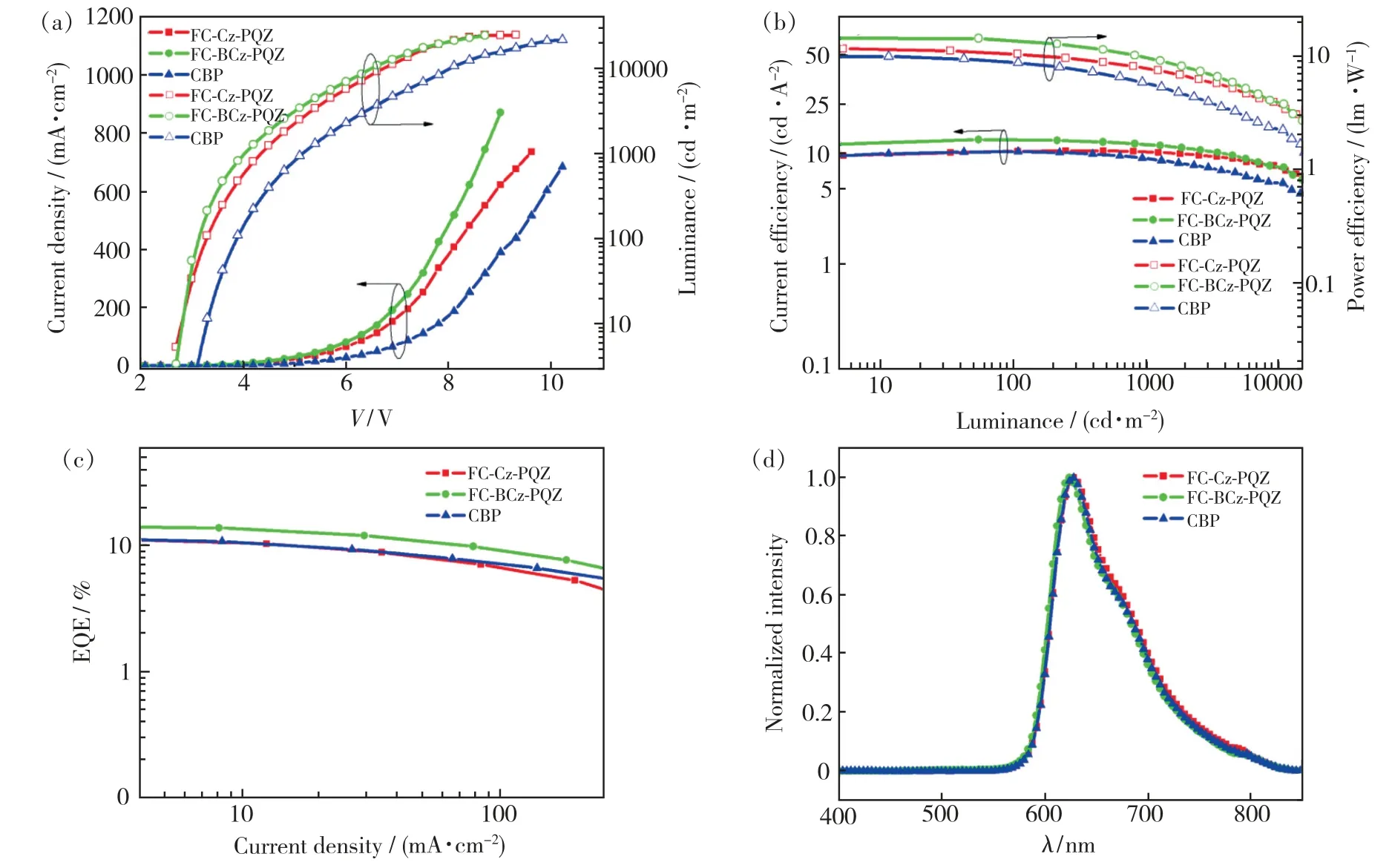
Fig.7 (a)Current density-voltage-luminance(J-V-L) characteristics.(b)Current and power efficiency.(c)External quantum efficiency(EQE) versus current density.(d)EL spectra at 4 V of red PhOLEDs.
4 Conclusion
In summary,two bipolar host materials,FCCz-PQZ and FC-BCz-PQZ,were synthesised and characterised.The fabricated red PhOLEDs using FC-Cz-PQZ and FC-BCz-PQZ as host and (piq)2Ir-(acac) as guest emitter realised high device performance.Compared with FC-Cz-PQZ,FC-BCz-PQZ demonstrated its promising potential as a desirable host material for red PhOLEDs with a highTdof 480 ℃.The red PhOLEDs based on FC-BCz-PQZ host material with the structure of ITO/MoO3/NPB/TCTA/host∶Ir(piq)2(acac)(4%)/TPBi/LiF/Al showed good electroluminescence performance and balanced hole-electron transport property with low turn-on voltage and maximum current,power and EQE of 13.5 cd/A,14.2 lm/W and 14.8%,respectively.We believe that this work could shed light on promising bipolar host materials with rational connection of quinazoline derivatives.
Supplementary Information and Response Letter are available for this paper at:http://cjl.lightpublishing.cn/thesisDetails#10.37188/CJL.20210397.
