Pressure dependence of the thermal stability in LiMn2O4
2022-01-23YanZeng曾彦HaoLiang梁浩ShixueGuan管诗雪JunpuWang王俊普WenjiaLiang梁文嘉MengyangHuang黄梦阳andFangPeng彭放
Yan Zeng(曾彦) Hao Liang(梁浩) Shixue Guan(管诗雪) Junpu Wang(王俊普)Wenjia Liang(梁文嘉) Mengyang Huang(黄梦阳) and Fang Peng(彭放)
1Institute of Atomic and Molecular Physics,Sichuan University,Chengdu 610065,China
2School of Science,Southwest University of Science and Technology,Mianyang 621010,China
Keywords: LiMn2O4,high pressure,x-ray diffractometer,decomposition reactions
1. Introduction
Spinel-type lithium manganese oxide (LiMn2O4, space groupFd-3m)is widely used as a cathode material for lithiumion batteries. LiMn2O4may be regarded as the most common alternative to lithium cobalt oxide(LiCoO2)because Mn is abundant in nature and can reduce the cost of lithiumion batteries.[1-5]Nowadays, LiMn2O4cathodes are commonly found in lithium-ion batteries used in sustainable energy vehicles.[6]The investigation of different properties of LiMn2O4under high temperature and high pressure is a promising strategy to rationalize the ideal synthesis conditions and thermal modifications of LiMn2O4. For LiMn2O4, there are many synthesis procedures available, including conventional solid-state synthesis, citric acid assisted sol-gel route synthesis and several novel techniques, such as the explosion or detonation method, which involves temperature and pressure.[7,8]The explosion process usually occurs instantly,so it is difficult to observe and develop a deeper understanding of this process. Nevertheless, by simulating the high temperature and high-pressure environment during synthesis,is possible to gain some insights into the synthesis process and the properties of the final LiMn2O4product.
The effects of high pressure and/or high temperature(HPHT) on the phase transition of LiMn2O4have been studied extensively in recent decades. Paoloneet al.[9]reported that spinel LiMn2O4will be distorted when the pressure rises above 10 GPa at room temperature,forming a metastable state,
whereas Yamauraet al.[10]showed that LiMn2O4undergoes a phase transition from the original spinel to the CaFe2O4(calcium ferrite)-type structure under 6 GPa at temperatures above 1100°C. Mukaiet al.[11]synthesized CaFe2O4-type LiMn2O4(CF-LMO) under high pressure (12.0 GPa) and studied its electrochemical properties and crystal structure.They reported that the LiMn2O4spinel converts into a CFLMO structure above 800°C.Linet al.[12]studied the related LiMn2O4compound. They demonstrated that LiMn2O4undergoes a reversible phase transition from a cubic structure to a tetragonal structure at 11.0 GPa(room temperature), where the bulk elastic modulus of the cubic structure was found to be 119(4)GPa. Darulet al.[13]showed that LiMn2O4undergoes a structural phase transition from cubic to tetragonal due to Jahn-Teller distortion at 4.0 GPa. Heating up to about 600 K under 4.0 GPa leads to the reverse phase transition, and the tetragonal phase transforms back to the cubic one. Furthermore, they showed that HP effects decrease the temperature of decomposition, where Mn3O4and MnO secondary phases appear. However, no explanation as to why this occurs was given.
To investigate the decomposition behavior of LiMn2O4,it is useful to study how HTHP affects the structural stability. This will help to rationalize the synthesis and modification of LiMn2O4cathode materials for lithium-ion batteries.In this work, we studied the decomposition of LiMn2O4at 2.0 GPa-5.0 GPa in a temperature range of 25°C-1000°C while using a cubic press (DS6×14 MN, China). The obtained products after HPHT experiments were then characterized byin-situx-ray diffraction(XRD)and scanning electron microscopy(SEM).
2. Materials and experimental methods
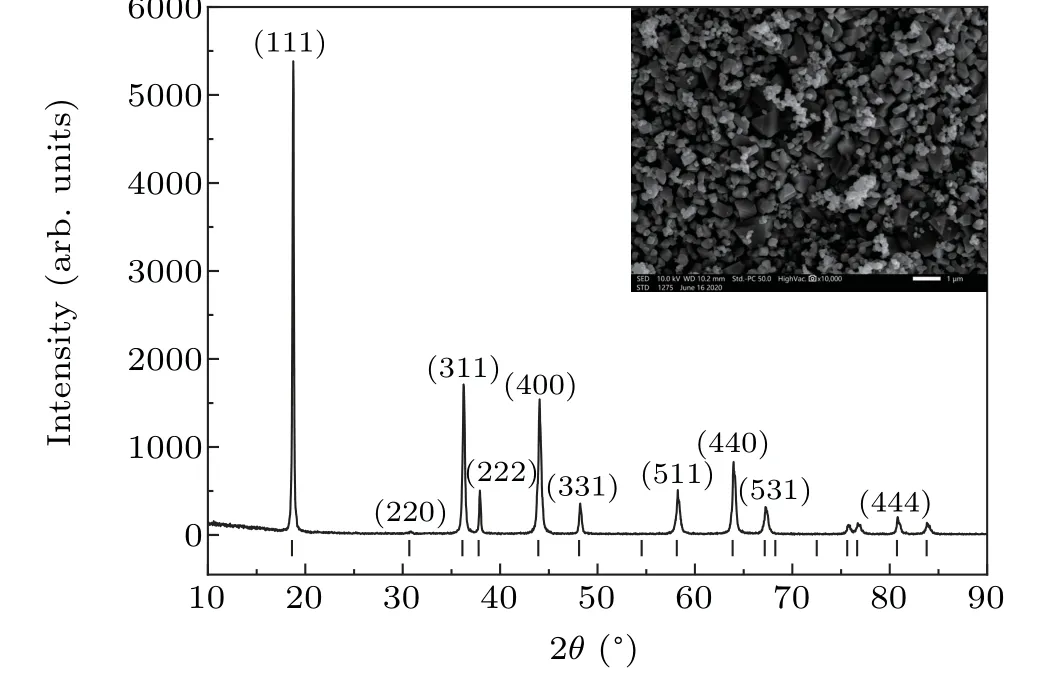
Fig.1. XRD pattern and SEM image of the initial LiMn2O4 powder.
LiMn2O4(Fd-3m)powder(purity>99.5%)with a grain size of~300 nm was purchased from Alfa Aesar. Figure 1 shows the XRD pattern and an SEM image of the initial powder. It can be clearly seen that the diffraction peaks are consistent with the standard card(JCPDS No.88-1030)of LiMn2O4.No impurities could be detected. The SEM image shows that the particles have an irregular shape and a flat surface with an average size of~300 nm.electrode. The closed cylindrical graphite tube was used as a heating source. The pyrophyllite was used as a pressuretransmitting medium for relative insertion of hydrostatic pressure.The pre-pressed specimen was surrounded by a tube containing NaCl pressure medium to provide the desired hydrostatic pressure environment.[15,16]The pressure range applied was 2.0 GPa-5.0 GPa,at a temperature range of 25°C-100°C.First,the oil pressure was set to the value corresponding to the cavity pressure. For example,when the cavity pressure needs to be at 4.0 GPa, the oil pressure is set at 30 MPa. When the actual oil pressure and the set value coincided, the press was placed into the pressure holding stage and the system began to heat. After reaching the set temperature,the holding time was 20 min,and the heating system was switched off.The pressure was released only after the temperature had dropped to room temperature(RT),and the sample could then be removed. The samples were embedded in bakelite powder using a XQ-2B metallographic sample setting machine and then polished on both sides in a Unipol-802 fine polishing machine for further testing and analysis.
HTHP experiments were carried out in a cubic press(DS6×14 MN, China). The structure of the six-sided top press includes a power distribution system, heating system,supercharger, hydraulic station, and the main machine (base and six groups of working cylinders). The six working cylinders are divided into three fixed cylinders (lower, rear, left)and three moving cylinders (upper, front, right). The actual picture and schematic diagram of the cubic press are shown in Figs. 2(a) and 2(b). Under the push of hydraulic oil, the corresponding top hammer of the six cylinders moves to the center and extrudes the assembly. The pressure and temperature are gradually increased by the setting program,which can assure HT and HP conditions at the same time. For HTHP sintering experiments, the density of the initial material has an important effect on the shrinkage and densification behavior of the sample. The lesser the volume shrinkage and the greater the pressure transfer efficiency results in closer intergranular bonding. In other words, the higher density of the initial powder can lead to a better densification effect under the same pressure and temperature conditions. Therefore,the initial LiMn2O4powder was pre-pressed to improve its initial density.[14]According to calculations, the sample’s relative theoretical density after pre-pressurization can reach 67%of the initial sample density,followed by the HPHT treatment described below.
The standard B assembly designed in our laboratory is shown in Fig. 2(c). The steel ring was used as a conductive

Fig.2. (a)Actual picture of the cubic press,(b)schematic diagram of the cubic press. (c)Experimental assembly structure schematic diagram(standard B)for the HPHT experiment.
XRD phase analysis was carried out using a Dandong square circle DX2700 x-ray diffractometer. The working conditions of the diffractometer were set as 30 mA,40 kV,Cu-Kαradiation(λ=0.15404 nm),the stepping mode was selected,the step size was set as 0.03°/s,and the Bragg-Brentano mode wasθ-2θ. The morphology, structure, and size of the prepared sample were observed by SEM(JSM-IT500HR,JEOL,Akishima,Japan).
3. Results and discussion
3.1. Phase identification and diffraction peak analysis
The XRD patterns from all samples obtained from the HTHP experiments at pressures of 2.0 GPa (normal pressure and temperature(NPT)-1000°C),3.0 GPa(NPT-700°C),4.0 GPa(NPT-700°C)and 5.0 GPa(NPT-700°C)are shown in Figs.3(a)-3(d).
At a pressure of 2.0 GPa (Fig. 3(a)), the structure of LiMn2O4was found stable at RT up to 600°C. Several additional diffraction peaks started to appear at~700°C,which were associated with orthorhombic Mn2O3,monoclinic Li2MnO3and tetragonal Mn3O4. It is indicated that Mn2O3appeared first.It should be noted that certain peaks in Fig.3(a)are from the sample holder.
Similarly, figure 3(b) shows the XRD patterns of the HPHT (3.0 GPa, RT-700°C) samples. The spinel structure was preserved up to 500°C, and decomposition into the same three additional phases as before started at 600°C.Some traces of Mn2O3,Li1.05Mn2O4,and Li2O2were also observed at 700°C. At a higher pressure of 4.0 GPa, a total of four decomposition products appeared at a lower temperature of~300°C, as shown in Fig. 3(c). These are orthorhombic Mn2O3,monoclinic Li2MnO3,tetragonal Mn3O4,and orthorhombic Li1.13Mn2O4with space groupFddd. It is worth noting that these decomposition products are not the same as before at 2.0 GPa and 3.0 GPa.

Fig. 3. XRD spectra at pressures of (a) 2.0 GPa (NPT-1000 °C), (b) 3.0 GPa (NPT-700 °C), (c) 4.0 GPa (NPT-700 °C), and (d) 5.0 GPa(NPT-700 °C).
Figure 3(d) shows the XRD patterns of the HPHT(5.0 GPa, RT-700°C) samples.Mn2O3, Li2MnO3,Li1.13Mn2O4, and Mn3O4are the four phases present at 700°C. Mn2O3appeared first at 200°C as the initial decomposition product. Below the decomposition temperature of 200°C,including at RT,the structure of LiMn2O4was stable at the maximum applied pressure of 5.0 GPa.
Based on the XRD patterns of all the HTHP experimentprepared samples,it can be concluded that the decomposition temperature decreases with increasing pressure from 700°C at 2.0 GPa, to 600°C at 3.0 GPa, to 300°C at 4.0 GPa and to 200°C at 5.0 GPa. Therefore, the high-pressure treatment leads to decomposition at lower decomposition temperatures.
Figure 4 shows a series ofin-situpowder XRD patterns collected at variable temperatures at ambient pressure. The structure of LiMn2O4was stable at RT up to 1200°C.When the temperature reached 1400°C,several decomposition products appeared. These are Li2MnO3with space groupC2/c,orthorhombic LiMnO2with space groupPmnmand tetragonal Mn3O4with space groupI41/amd. These results indicate that the decomposition temperature of LiMn2O4under ambient pressure is between 1200°C-1400°C.It should be noted that this is a higher temperature as compared to the 1000°C reported by Thackerayet al.[17]However, we had carefully calibrated our furnaces during all thein-situhigh-temperature experiments and muffle furnace high-temperature heat treatment experiments,and we can,therefore,confirm that the experimental results presented here are reliable.
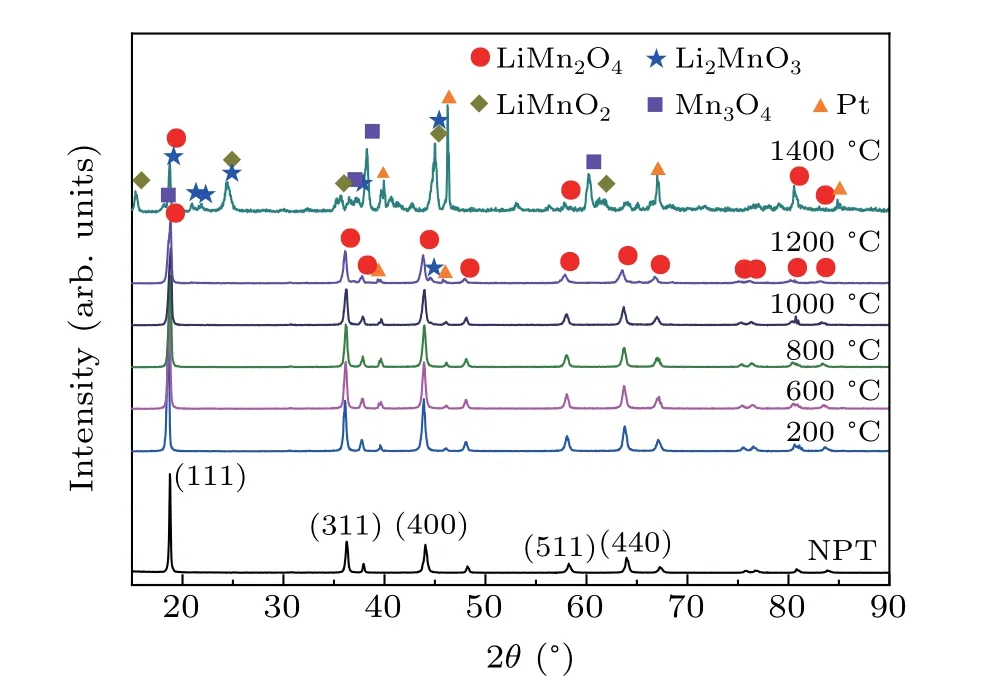
Fig. 4. In-situ XRD patterns of LiMn2O4 recorded at NPT to 1400 °C. If a small amount of sample is placed on the Pt substrate, some Pt diffraction peaks appear.
The variation of decomposition temperature of LiMn2O4with pressure is shown in Fig. 5. As mentioned before, the application of high pressure leads to the decrease of decomposition temperature,i.e.,the LiMn2O4phase is less stable under high pressure, at least at high temperatures. At 5.0 GPa, the decomposition temperature is only 200°C, which is 15% of the decomposition temperature at ambient pressure.
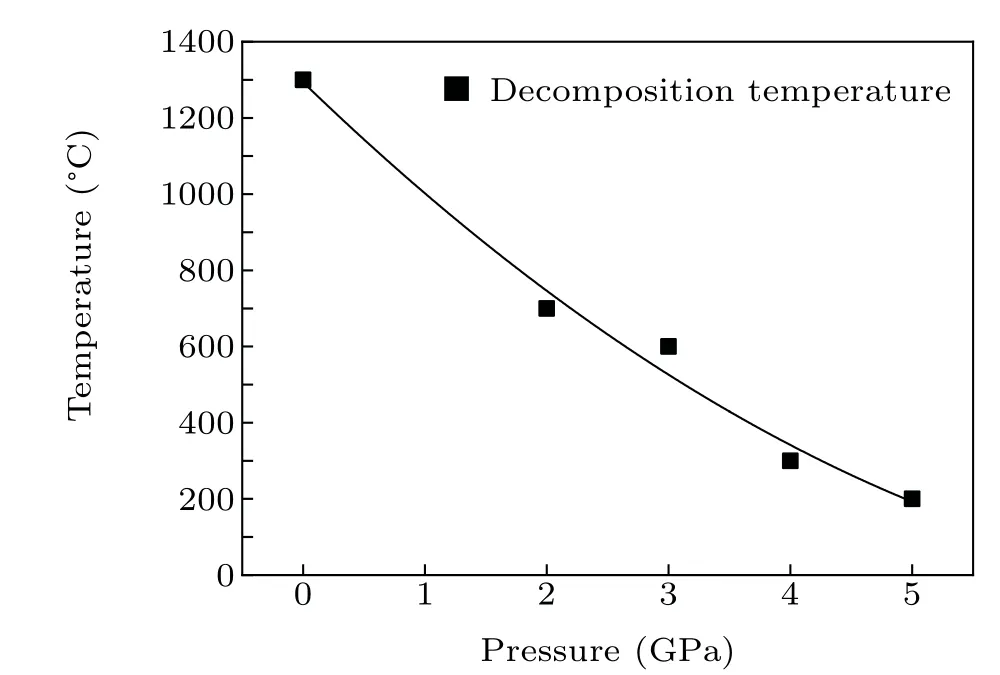
Fig.5. The decomposition temperature of LiMn2O4 versus pressure.
Since LiMn2O4could not decompose at room temperature under any pressure up to 5 GPa, additional experiments were conducted under 8.0 GPa (RT), but LiMn2O4still did not decompose. In addition,by comparing the decomposition products at each pressure in Fig.3,Mn2O3was always the first decomposition product that appeared above the respective decomposition temperature,except at ambient pressure. It may,therefore, be concluded that the occurrence of Mn2O3may be related to the stress-induced phase transition in the crystal structure during HP conditions.
The crystal structure of LiMn2O4is shown in Fig. 6(a).In the unit cell of LiMn2O4,Mn3+/Mn4+cations occupy 16doctahedral positions,whereas Li+cations occupy the 8aposition of the tetrahedron.From this structural diagram combined with the bond valence of LiMn2O4, it may be assumed that electrons move in the three-dimensional conduction pathways formed by Li+when LiMn2O4is charged and discharged. In the HTHP experiment,O2-anions changed from the original 32esite position to the 24cpositions, and part of the manganese cations moved to the 8aand 12bpositions. The MnO6octahedra are distorted due to high pressure and the Jahn-Teller instability of the Mn3+(d4)ions. Mn2O3is formed under this action,as shown in Fig.6(b). Therefore,it is plausible that MnO6octahedra may not distort and may not form Mn2O3in the absence of pressure. This argument may be supported by the fact that at ambient pressure,LiMn2O4decomposes at a high temperature of 1300°C,where the decomposition products are different as compared to the samples treated at high pressure,as mentioned above.

Fig.6. Crystal structures of(a)LiMn2O4 and(b)Mn2O3.
To ensure the accuracy and reliability of the SEM analysis,different areas of the sample were scanned by SEM.Representative images for the initial powder at ambient pressure(1400°C), HP (2.0 GPa, RT), HPHT (2.0 GPa, 1000°C),HP (3.0 GPa, RT), HPHT (3.0 GPa, 600°C), HP (4.0 GPa,RT),HPHT(4.0 GPa,300°C),HP(5.0 GPa, RT)and HPHT(5.0 GPa,200°C)were selected,as shown in Fig.7. The primary particles of the initial powder appeared smooth and flat with an average particle size of~300 nm (Fig. 7(a)). Figure 7(b) illustrates the microstructure of the sample with an average grain size of~30 µm when heated at 1400°C. At 1400°C, the grain size of the sample increased significantly as compared to the starting powder,as shown in Fig.7(a),and the grain surfaces exhibited pores resulting from gas production.
The particles became more clustered due to the HP environment at RT,as demonstrated in Figs.7(c), (e), 7(g), and 7(i). The grains became relatively dense after the cold pressing treatment. Smaller particles appeared evenly distributed around the large particles. This may be interpreted as a result of grains breaking into smaller ones under the action of pressure. This is supported by the notion that most of the grains are broken at the edges and corners, and the average size of the individual particles after high pressure is smaller than the initial particles. It should be further noted that the application of high pressure also leads to a particle agglomeration.However,the particle morphology and size drastically change with increasing temperature under various pressures,as shown in Figs. 7(d), 7(f), 7(h), and 7(j). In Figs. 7(d) and 7(j), the grain boundaries are blurred between the grains. The grains become denser under the action of pressure, while the grains grow with the increase in temperature. This grain growth process is clearly illustrated in Fig. 7(f), where such growth is accompanied by the precipitation of microcrystals on the surface. On the other hand,figure 7(h)also demonstrates that the grain surface has obvious growth striations.
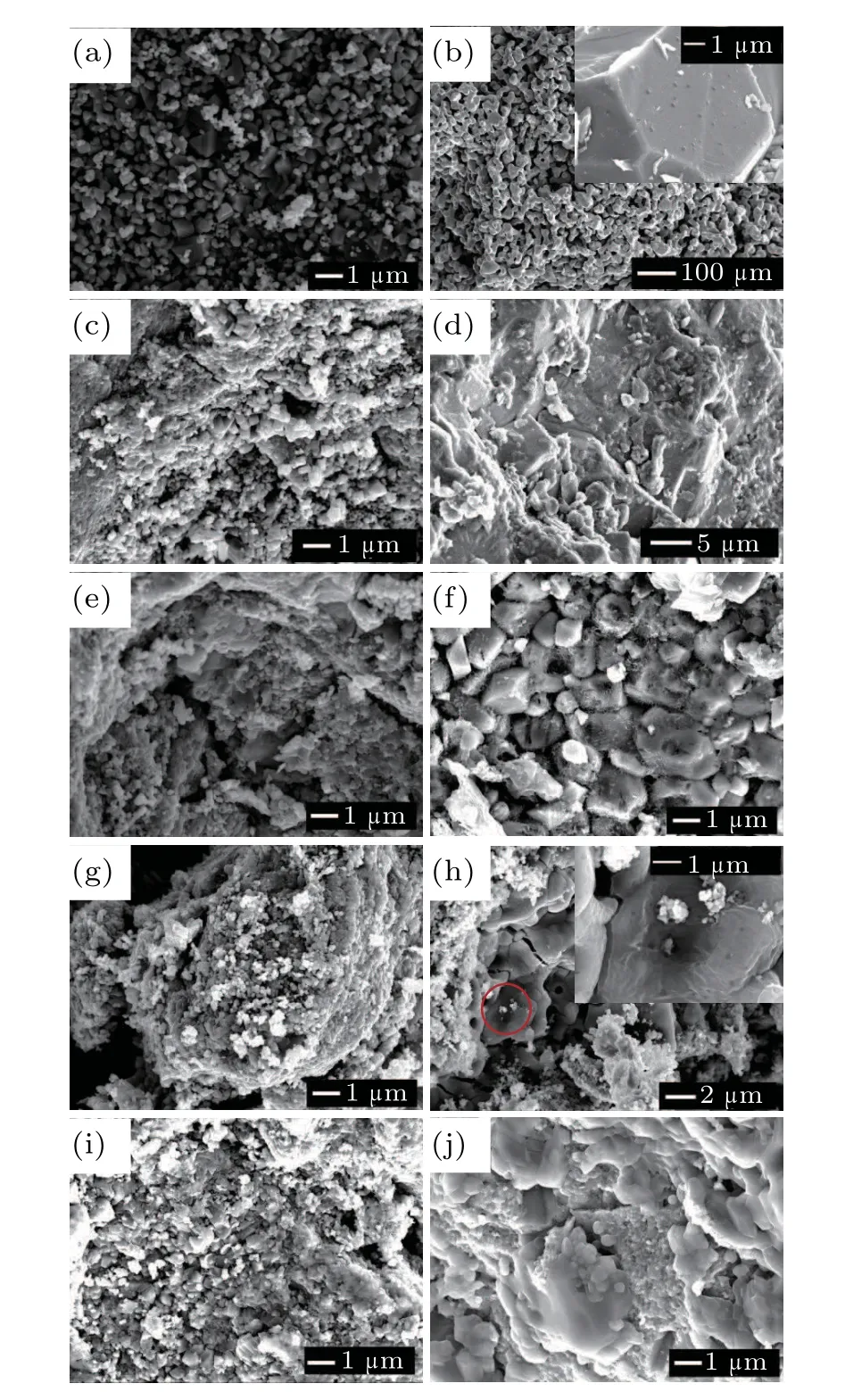
Fig.7. SEM images of LiMn2O4 (a)initial powder,(b)at ambient pressure(1400 °C), (c) HP (2.0 GPa, RT), (d) HPHT (2.0 GPa, 1000 °C), (e) HP(3.0 GPa,RT),(f)HPHT(3.0 GPa,600°C),(g)HP(4.0 GPa,RT),(h)HPHT(4.0 GPa,300 °C),(i)HP(5.0 GPa,RT),(j)HPHT(5.0 GPa,200 °C).
From these results, it can be concluded that the grains change significantly when approaching the decomposition conditions. Meanwhile, pressure and temperature change the size and morphology of the grains, which may well be relevant for future studies on the modification and application of lithium manganate.
4. Conclusion
In summary, we performed high pressure and hightemperature experiments on the LiMn2O4spinel phase.Insituhightemperature XRD was employed to investigate the influence of pressure on the decomposition temperature of LiMn2O4. The resulting products were characterized by conventional XRD and SEM to probe the microstructure evolution, phase composition and decomposition temperature of LiMn2O4under high temperature and high pressure. The results show that both the decomposition temperature and the decomposition products of LiMn2O4had a strong dependence on pressure. The decomposition temperature of LiMn2O4dropped sharply from 1300°C to 200°C when the pressure was increased from ambient to 5.0 GPa. This suggests that pressure may reduce the distance between atoms. On the other hand, the compound is stable at RT, even at a maximum pressure of 8.0 GPa. Therefore,it can be concluded that the decomposition of LiMn2O4is more sensitive to temperature than to pressure. Interestingly,high pressure can promote more complex decomposition products. In the case of Mn2O3,this can be attributed to Jahn-Teller distortions which are enhanced under high pressure.
Acknowledgments
Project supported by the National Natural Science Foundation of China (Grant No. 12074273) and the Doctoral Research Fund of Southwest University of Science and Technology(Grant No.20zx7136).
杂志排行
Chinese Physics B的其它文章
- Superconductivity in octagraphene
- Soliton molecules and asymmetric solitons of the extended Lax equation via velocity resonance
- Theoretical study of(e,2e)triple differential cross sections of pyrimidine and tetrahydrofurfuryl alcohol molecules using multi-center distorted-wave method
- Protection of entanglement between two V-atoms in a multi-cavity coupling system
- Semi-quantum private comparison protocol of size relation with d-dimensional GHZ states
- Probing the magnetization switching with in-plane magnetic anisotropy through field-modified magnetoresistance measurement
