Substrate tuned reconstructed polymerization of naphthalocyanine on Ag(110)
2022-01-23QiZheng郑琦LiHuang黄立DeliangBao包德亮RongtingWu武荣庭YanLi李彦XiaoLin林晓ShixuanDu杜世萱andHongJunGao高鸿钧
Qi Zheng(郑琦) Li Huang(黄立) Deliang Bao(包德亮) Rongting Wu(武荣庭)Yan Li(李彦) Xiao Lin(林晓) Shixuan Du(杜世萱) and Hong-Jun Gao(高鸿钧)
1Beijing National Center for Condensed Matter Physics and Institute of Physics,
Chinese Academy of Sciences,Beijing 100190,China
2School of Physical Sciences,University of Chinese Academy of Sciences,Beijing 100049,China
3CAS Center for Excellence in Topological Quantum Computation,University of Chinese Academy of Sciences,Beijing 100190,China
4Department of Physics and Astronomy and Department of Electrical Engineering and Computer Science,Vanderbilt University,Nashville,TN 37235,USA
5Songshan Lake Materials Laboratory,Dongguan 523808,China
Keywords: on-surface polymerization, reconstruction, scanning tunneling microscopy, noncontact atomic force microscopy
1. Introduction
Polymerization is an important chemical process that can synthesize organic chains, ribbons or networks with unique physical and chemical properties[1,2]which have potential applications in nanodevices. The design of the linkage structures between the monomers in a polymer must be carefully considered as these linkages can greatly influence the properties of the entire polymer.[3,4]The C-C single or double bonds,[5-7]C-metal coordinate bonds,[8,9]and heteroatom bridging[10,11]have been demonstrated as polymer linkages, which, in the meantime, induce rotatable bonds in the polymer and lead to folded or tangled amorphous morphology that hinders further investigations. Therefore,rigid linkages,such as carbon polygons,are highly desirable to construct robust polymers for better performance in electronic or optical applications.[3,4,12]
On-surface polymerization can synthesize highly ordered two-dimensional (2D) structures such as graphene nano-ribbons(GNRs)[13-16]and covalent organic frameworks(COFs),[17-19]and can be easily tuned with different substrates. We used high-resolution scanning tunnelling microscopy (STM) and non-contact atomic force microscopy(nc-AFM) to investigate the mechanism of on-surface polymerization at atomic level,[20,21]which further promotes the synthesis of new polymerized structures on surface. As model molecules, phthalocyanine (Pc) and naphthalocyanine(NPc) have been widely studied by STM and AFM for their adsorption,[22,23]self-assembly,[22,24-28]and electronic properties.[29,29-34]The outer periphery of both Pc and NPc is consisted of C-H bonds,which can yield C-C single or double bonds[35,36]and[4]-radialene like structures[37]in on-surface polymerization.
In this paper, we report the on-surface synthesis of NPc oligomers with dihydropentalene linkage structure on Ag(110)surface.While most of the polymerization products are singlebond connected, there is also a small portion of 6-5-5 membered ring connecting structure formed on Ag(110). The STM and nc-AFM images unambiguously unveil both of the linkage structures, which indicate the strain induced by the grooves on Ag(110)surface restricts the adsorption orientation of NPc molecules,and leads to the dihydropentalene linkage structure by atom rearrangement. Controlled experiment of NPc on flat surface Ag(100) results in only single-bond connected polymerized products,which further confirms that the dihydropentalene structure is induced by strain on trenched Ag(110)surface.
2. Results
Two types of linkage structures of polymerized NPc on Ag(110) surface. Ag(110) substrate has grooves along[1¯10]direction with width of 0.486 nm and depth of 0.289 nm,as shown in Fig. 1(a). The NPc molecules were sublimed at 620 K onto Ag(110) surface that was held at room temperature. The adsorption orientations of the as-deposited NPc molecules are along [1¯10] direction and 45°to [1¯10] direction (Fig. 1(b)). After annealing at 710 K for 20 min, some NPc molecules polymerized on the surface with the same adsorption orientations, as indicated in the STM image by red dotted circles in Fig.1(c).
There are two types of linkage structures in NPc oligomers as highlighted by the green (type I) and red (type II) boxes in the STM overview image (Fig. 2(a)). The type I oligomers (Fig. 2(b)) have larger units than the type II oligomers (Fig. 2(c)) as indicated by the superposed crossshaped contours with the same size where the two contours overlap for type II oligomers. Figures 2(d)and 2(e)show the center-to-center distance of adjacent monomers in type I and type II oligomers, in which type I is 1.93 nm, while type II is 1.86 nm. The adsorption orientations of type I oligomers are along[1¯10]direction and 45°to[1¯10]direction;while the type II oligomers are only found along[1¯10]direction.
The chemical bond resolved nc-AFM images obtained with CO-functionalized tips further unveiled the structure of the two kinds of oligomers. In type I oligomer,the linkage is a carbon-carbon single bond connecting the terminal phenyl of two monomers with a bond angle of 120°, as highlighted by the green circles in Fig. 2(f). For type II oligomers, the linkage has a 6-5-5 membered ring structure, which consists of one terminal phenyl of one of the NPc monomer reconstructed into a 5-membered ring, as marked in the red circles in Fig. 2(g). In addition, there is a carbon atom with bright contrast in each pentagon in the nc-AFM image, which indicates stronger repulsion on this carbon atom, most likely due to the out-of-plane C-H bond. Therefore, we could conclude that the type II linkage is a dihydropentalene structure. Since the dihydropentalene linkage can only form along the[1¯10]direction and is generated through complex reconstruction process,the surface assisted interactions,such as strains induced by the grooves, should play an essential role in the polymerization process.
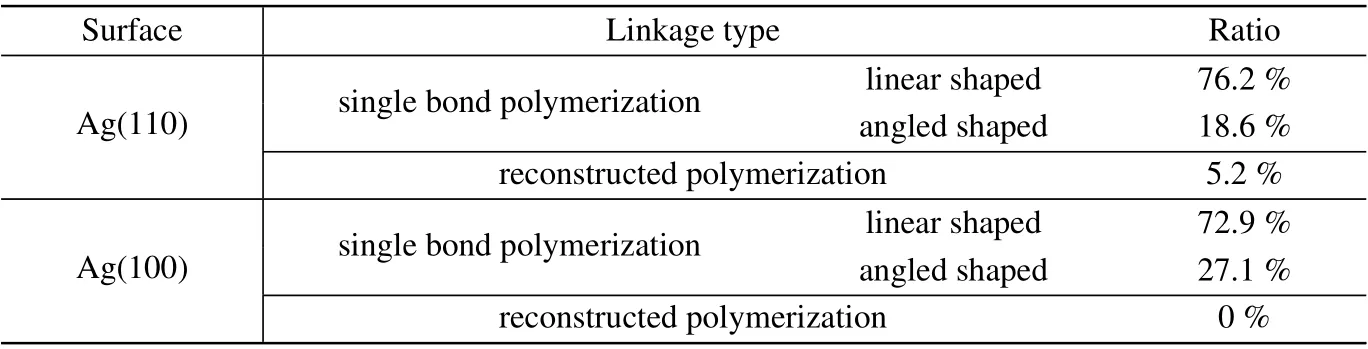
Table 1. The ratio of linkage types of naphthalocyanine oligomers on Ag(110)and Ag(100).
In addition, we also observed an angled linkage structure for NPc oligomers as marked by dotted green boxes in Fig.2(a), which is connected by a C-C single bond with two monomers adsorbed along different directions.The ratio of the two kinds of C-C single bond linkages and the reconstructed dihydropentalene linkage is displayed in Table 1, where the majority of the oligomers have linear shaped single bond linkages (76.2%), 18.6% oligomers are angled linked by single bonds, and only 5.2% have reconstructed into conjugated 6-5-5 membered rings.
Polymerization of NPc on Ag(100)surface. To further confirm the relation between the reconstructed dihydropentalene linkage and the strain from the substrate,a planar surface of Ag(100)was used for NPc polymerization as a comparison.As displayed in Fig.3(a), most monomers and oligomers adsorb along the high symmetry directions on the surface, and two kinds of oligomers,linear shaped and angled shaped,are formed on Ag(100), as marked by green and red rectangles.The zoom-in STM and nc-AFM images (Figs. 3(b) and 3(c))show that both oligomers are linked by single bond between monomers, with the two reacted lobes parallel in the linear shaped oligomers(Fig.3(d)),and 120°to each other in the angled oligomers(Fig.3(e)). The ratios of the linear and angled linkages of NPc oligomers on Ag(100)are 72.9%and 27.1%,respectively(Table 1). Notably,there are no naphthalocyanine oligomers formed on Ag(100)surface.
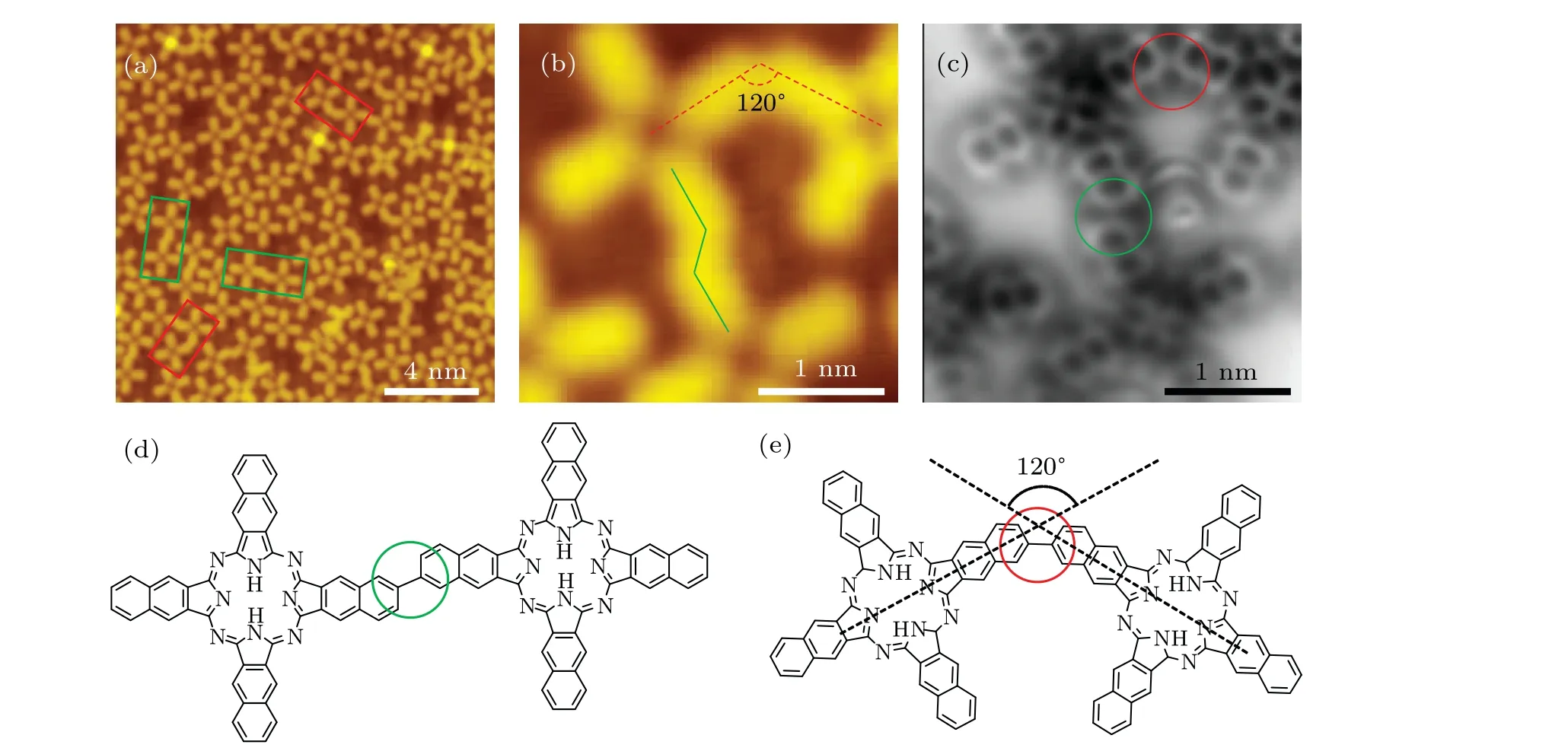
Fig.3. Polymerized NPc on the Ag(100)surface. (a)STM topography of NPc oligomers and molecules after annealing at 720 K for 20 min on Ag(100)surface. The linear shaped and angled shaped oligomers are highlighted by green and red boxes,respectively. (b),(c)Zoom-in STM image and corresponding nc-AFM image of two kinds of NPc oligomers. (d), (e) Sketches of the two kinds of linkage structures. Scanning parameters: (a)Vs=-0.3 V,It=10 pA;(b)Vs=-0.04 V,It=10 pA;(c)amplitude=100 pm.
3. Discussion
For two NPc monomers adsorb on Ag(110) surface, the relative positions of the terminal phenyls can have two configurations. In the first configuration as depicted in Fig.4(a),the terminal phenyls are corner to corner, and a C-C single bond with a bond angle of 120°to the terminal phenyls can be formed. For the second configuration as depicted in Figs.4(b)and 4(c),the terminal phenyls are side by side,which results in a C-C single bond of 90°bond angle formed between C1 and C1′. On the anisotropic Ag(110)substrate,the grooves along[1¯10] direction restrict the adsorption of the monomers and the phenyl ring cannot rotate freely to release the strain of the 90°bond angle (step 2 in Fig. 4(b)), which may result in the bond cleavage between C1 and C2 with two radicals(step 3).Then,new bonds between C2 and C2′,and C1 and C3 would form(step 4)along with H atoms at C2′and C3 transferring to C2 and C6, respectively (step 5). After the rearrangement of electrons and H atoms,the two phenyl rings connecting with a 90°angled C-C bond turn into a 6-5-5 membered ring shaped dihydropentalene linkage. On the other hand,for polymerization starting from the side-by-side configuration on Ag(100),as shown in Fig. 4(c), the strain of the 90°angled C-C bond can be released by rotating the monomers due to the small restriction from the substrate,which yields oligomers with linkage of 120°bond angle C-C bonding.
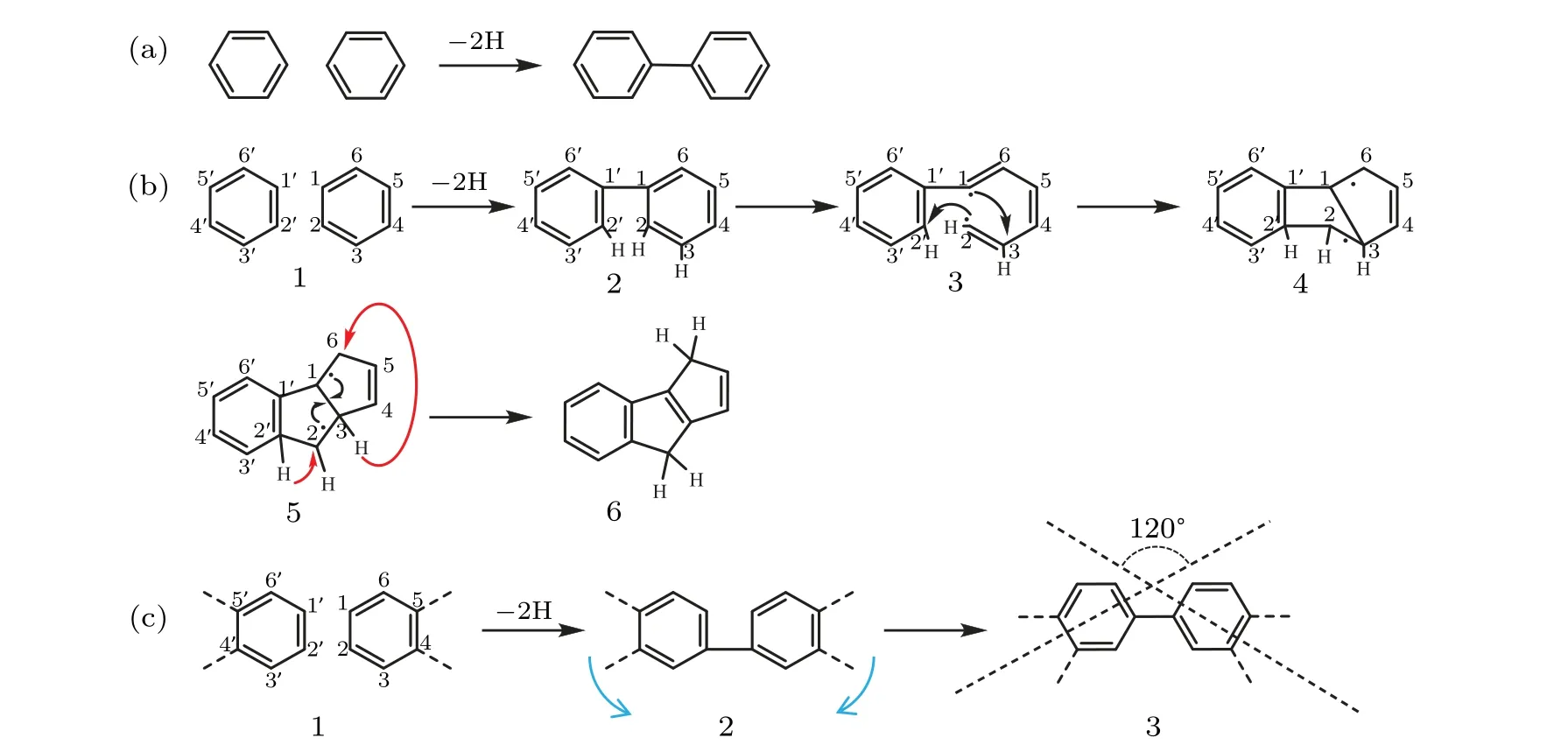
Fig. 4. Schema of the possible reaction routes for NPc polymerization on Ag(110) and Ag(100). (a) Single bond formation with corner to corner configuration of the terminal phenyls. (b)Starting from side by side configuration of the terminal phenyls,the formation of the 6-5-5 membered ring dihydropentalene linkage structure with reconstructions. (c)Starting from side by side configuration of the terminal phenyls,the formation of the single bond connection with rotation of the phenyl rings.
In conclusion, two kinds of linkage structures in NPc oligomers have been successfully synthesized by annealing at 710 K on Ag(110) surface. STM and nc-AFM images reveal that C-C single bond and dihydropentalene linkage structures can be formed on Ag(110). Some C-C single bonds at the linkage are restricted by the anisotropic substrate to a 90°bond angle between the monomers,which further reconstruct to the dihydropentalene linkage structures to release the strain.Our findings demonstrate strain-induced reconstructed polymerization on anisotropic substrate and provide new insights for on-surface synthesis.
4. Methods
4.1. Sample preparation
The 2,3-naphthalocyanine (NPc, Aldrich, purity>95%)in powder form was purified by a sublimation process with a homemade Knudsen cell (K-cell) evaporator in high vacuum for 5 hours, followed by degassing at 620 K for 60 min in the UHV chamber. The NPc was evaporated at 640 K during the experiment,while the substrate Ag(110)was kept at room temperature.
4.2. STM/nc-AFM measurements
STM and nc-AFM measurements were performed on a combined nc-AFM/STM system (Createc) at LHe temperature with a base pressure better than 2×10-10mbar. All measurements were performed using a qPlus tuning fork sensor in frequency modulation mode with a Pt/Ir tip. The resonance frequency of the sensor is 27.9 kHz, and its stiffness is about 1800 N/m. The STM measurements were performed by the same tuning fork sensor without excitation and the current amplifier is 109up to 1 kHz.
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No. 61888102), the National Key Research and Development Program of China (Grant Nos. 2018YFA0305800 and 2019YFA0308500), Chinese Academy of Sciences (Grant Nos. XDB30000000, YSBR-003, and 112111KYSB20160061), and the Fundamental Research Funds for the Central Universities,China.
杂志排行
Chinese Physics B的其它文章
- Superconductivity in octagraphene
- Soliton molecules and asymmetric solitons of the extended Lax equation via velocity resonance
- Theoretical study of(e,2e)triple differential cross sections of pyrimidine and tetrahydrofurfuryl alcohol molecules using multi-center distorted-wave method
- Protection of entanglement between two V-atoms in a multi-cavity coupling system
- Semi-quantum private comparison protocol of size relation with d-dimensional GHZ states
- Probing the magnetization switching with in-plane magnetic anisotropy through field-modified magnetoresistance measurement
